Abstract
Objective
To determine predictors of treatment failure and recurrence after surgical excisional procedures for CIN in HIV-infected women.
Methods
A retrospective cohort study was conducted in which 136 eligible HIV-infected women treated for CIN between 1999 and 2005 were included. Data were abstracted from charts and computer databases. Treatment failures were defined as the presence of CIN 1+ at initial follow-up. Recurrences were defined as the presence of CIN 1+ subsequent to initial normal follow-up.
Results
Treatment failure at initial follow-up was common, occurring in 51% of CIN 1 and 55% of CIN 2+. Most lesions detected at treatment failure were high grade (>70%), regardless of the grade of initial lesion. Significant risk factors for treatment failure were loop electrosurgical excision procedure (LEEP) compared to cold knife conization (RR=1.76; 95% CI: 1.15–2.64), and low CD4+ count (p = 0.04). Among those with an initial normal clinical evaluation, 55% eventually recurred. As with treatment failure, most lesions detected at recurrence were high grade. Risk factors for recurrence included use of LEEP (hazard ratio [HR] = 3.38; 95% CI: 1.55–7.39), higher HIV RNA level, and the presence of positive margins at treatment (HR = 6.12; 95% CI: 1.90–19.73).
Conclusions
Most CIN treatment of HIV-infected women studied either failed or resulted in recurrence. Of particular concern, many of these subsequent lesions were high grade. Conization, however, was associated with significantly less failure/recurrence than LEEP. Clinicians treating CIN in HIV-infected women should avoid raising expectations of cure and instead focus on the achievable goal of cancer prevention until there are better therapies for this patient population.
Keywords: Cervical intraepithelial neoplasia, HIV, Cervical conization, Loop electrosurgical excision procedure, LEEP
Introduction
Compared with immunocompetent women, HIV-infected women have high risk of cervical cancer [1] and cervical intraepithelial neoplasia (CIN) [2–5], as well as infection by human papillomavirus (HPV), the viral cause of cervical cancer and most CIN [6,7]. Further, the treatment of CIN in HIV-infected women often fails, and even after successful treatment of CIN in HIV-infected women, the lesions frequently recur [7–14]. This is in contrast to the high success of LEEP in immunocompetent women, where cure is common, not just cancer prevention. There are surprisingly limited data regarding the risk factors for CIN treatment failure and recurrence in HIV-infected women, and few data regarding the grade of neoplasia of the subsequent lesions after LEEP or cold knife conization in HIV-infected women.
In the few prior studies of HIV-infected women, the significant predictors of CIN treatment failure and recurrence included lowCD4+ T-cell count [10,13,15,16] and the presence of positive margins [4,13,16,17]. Only one study to our knowledge examined the grade of neoplasia detected at treatment failure and recurrence, and it reported that most of these lesions were low grade [8]. However, most of these early reports were based on observational or clinical cohorts, and the extent to which such data can be generalized to the broader population of HIV-infected women is unclear. In the current study, therefore, we conducted a retrospective study of unselected, sequential HIV-infected patients who presented for CIN treatment at either of two hospital systems, serving the same surrounding community, in the Bronx, NY.
Materials and methods
Study participants
After IRB approval, we searched electronic records to identify all HIV-infected women who had an extirpative procedure for CIN between 1999 and 2005, using the computerized database and informatics systems at Montefiore Medical Center (MMC) and Jacobi Medical Center (JMC); hospitals that serve an ethnically and racially diverse Bronx population. Relative to the United States as a whole, New York City has a high proportion of women infected with HIV [18], with similarly high rates of CIN and cervical cancer. Follow-up data were then obtained through further review of the medical records documented in the electronic medical records and patient charts.
Histopathologic, cytologic, and colposcopic follow-up data were recorded to assess associations with CIN treatment failure and recurrence. Abstracted data also included demographic, as well as known risk factors for cervical disease, such as smoking status, parity, use of hormone contraceptives, and diagnosis of other sexually transmitted infections. Use of highly active anti-retroviral therapy (HAART) was classified based on the medications listed in patients’ chart upon review.
CD4 T-cell count and HIV RNA level in our models were the most recent values documented within ±8 weeks from procedure date. Consistent with prior studies, we stratified CD4+ T-cell count >500 cells/µL, 201–500 cells/µL, and ≤200 cells/µL. HIV RNA level was also divided into three previously reported categories, namely, undetectable, detectable at 20,000 copies/mL, and detectable at >20,000 copies/mL.
The LEEP or cone specimens were used to define initial patient diagnosis. The choice of procedure, LEEP versus cone was based on provider preference and standard clinical factors such as lesion size and concerns for margins. Included subjects were all who had LEEP or cone biopsies during this defined time period. Currently, treatment guidelines for persistent CIN 1 in HIV are not clearly defined; there is some debate as to the appropriateness of treating CIN 1 in HIV-positive patients. However, in this patient population, treatment for persistent CIN 1 in HIV occurred and was thus included in this analysis. We conservatively defined treatment failure and recurrence as the presence of CIN 1+. Exclusions included women who underwent a hysterectomy and patients with a second procedure immediately following the first due to concerns for a microinvasive or invasive cervical cancer. Other exclusions included women who had no signs of CIN on procedure biopsy and women lacking follow-up data after the procedure. Also, women who required more aggressive management such as those diagnosed with adenocarcinoma in situ, invasive squamous cell carcinoma, or adenocarcinoma diagnosed from their LEEP or cone were excluded.
Statistical analysis
Log-binomial regression was used to evaluate the association of each patient characteristic (age, etc.) and risk factor (CD4 count, viral load, etc.) separately with treatment failure, in which treatment failure was conservatively defined as the presence of CIN 1+ at initial follow-up after the procedure [8,19]. In most cases, the time from initial follow-up to failure occurred within 1 year after the procedure.
Multivariate log-binomial regression was used to include multiple patient characteristics and risk factors in the regression model. The final multivariable model was constructed using variables that had an association of p<0.1 in the univariate analysis while controlling for age at procedure. Data are presented as relative risks with 95% confidence intervals (for categorical and ordinal variables) or as p values (for continuous variables). Ordinal variables (e.g., CD4+ T-cell strata) were evaluated for linear trend by parameterizing these strata as a continuous variable in the model. For example, 1 = CD4+ T-cell count >500 cells/µL, 2 = CD4+ 201–500 cells/µL, and 3 = CD4+ ≤200 cells/µL.
Recurrence of CIN was defined as the presence of CIN 1+ subsequent to an initial normal follow-up. The time to recurrence was calculated as the period between the date of CIN treatment and the date the recurrence of CIN 1+ was first detected. However, because patients were followed every several months, the exact time of the recurrence was not observed. We only know that the recurrence happened during a particular interval if recurrence was diagnosed on the latter of consecutive visits. To address this issue, a lifetime regression model allowing interval censoring and right censoring, in which patients who did not recur during follow-up were right censored, was used; assuming a Weibull distribution for time to CIN recurrence [20]. Covariates in recurrence analysis were assigned their value at time of procedure. A p<0.05 was considered statistically significant in all analyses. All statistical analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC).
Results
The electronic records included 191 HIV-infected women who had an extirpative procedure for CIN at either Montefiore Medical Center or Jacobi Medical Center during 1999–2005. Of these women, 136 were eligible based on our defined inclusion criteria. Exclusions included women who had a hysterectomy (n=13), women classified as having a normal histopathology at the time of their LEEP or cone (n=10), women with adenocarcinoma in situ, squamous cell carcinoma, or adenocarcinoma from their LEEP or cone (n=5), and patients who underwent a second procedure immediately following the first due to concern for microinvasive or invasive cancer (n=9). An additional 18 women with no documented follow-up were excluded from the analyses. Patient characteristics are shown in Tables 1a and 1b. A similar number of subjects were obtained from both medical centers, and the rate of CIN treatment failure was also similar at these two sites (p=0.45). The mean age at the time of CIN treatment was 39 years, with the majority of women classified as African American (47.5%). Of note, most of the women had previously reported having had a sexually transmitted infection (STI) other than HIV (55%).
Table 1.
| Table 1a Characteristics of 136 patients in our population-based study. | ||
|---|---|---|
| Characteristics | N/Total | % |
| Montefiore Medical Center | 57/136 | 41.9 |
| Jacobi Medical Center | 79/136 | 58.1 |
| Age at procedure | ||
| <30 | 24/136 | 17.7 |
| 30–45 | 80/136 | 58.8 |
| >45 | 32/136 | 23.5 |
| Race | ||
| Black | 56/118 | 47.5 |
| White | 10/118 | 8.5 |
| Hispanic | 36/118 | 30.5 |
| Other | 16/118 | 13.6 |
| Current smoker | ||
| Yes | 71/106 | 67.0 |
| No | 35/106 | 33.0 |
| Intravenous drug use | ||
| Yes | 21/99 | 21.2 |
| No | 78/99 | 78.8 |
| Parity | ||
| Multiparous | 44/48 | 91.7 |
| Nulliparous | 4/48 | 8.3 |
| Ever had an STD | ||
| Yes | 67/121 | 55.4 |
| No | 54/121 | 44.6 |
| Current hormone contraceptive use | ||
| Yes | 6/29 | 20.7 |
| No | 23/29 | 79.3 |
| Missed appointments | ||
| Rarely (0–3) | 39/89 | 43.8 |
| Frequently (3+) | 50/89 | 56.2 |
| Current HAART use | ||
| Yes | 57/111 | 51.4 |
| No | 54/111 | 48.7 |
| Table 1b Procedure and pathologic information for population-based study. | ||
|---|---|---|
| Characteristics | N/Total | % |
| First procedure type | ||
| LEEP | 93/136 | 68.4 |
| Cone | 43/136 | 31.6 |
| Procedure biopsy | ||
| CIN 1 | 61/136 | 44.9 |
| CIN 2 | 47/136 | 34.6 |
| CIN 3 | 28/136 | 20.6 |
| Margin status (endo- and ectocervical) | ||
| Both positive | 20/117 | 17.1 |
| Either positive | 49/117 | 41.9 |
| Both negative | 48/117 | 41.0 |
| CD4 count at time of procedure (±8 weeks) | ||
| ≤200 cells/µL | 38/114 | 33.3 |
| 201–500 cells/µL | 56/114 | 49.1 |
| >500 cells/µL | 20/114 | 17.5 |
| Viral load at time of procedure (±8 weeks) | ||
| Undetectable | 55/122 | 45.1 |
| Detectable –20,000 copies/mL | 35/122 | 28.7 |
| >20,000 copies/mL | 32/122 | 26.2 |
Initial treatment failure
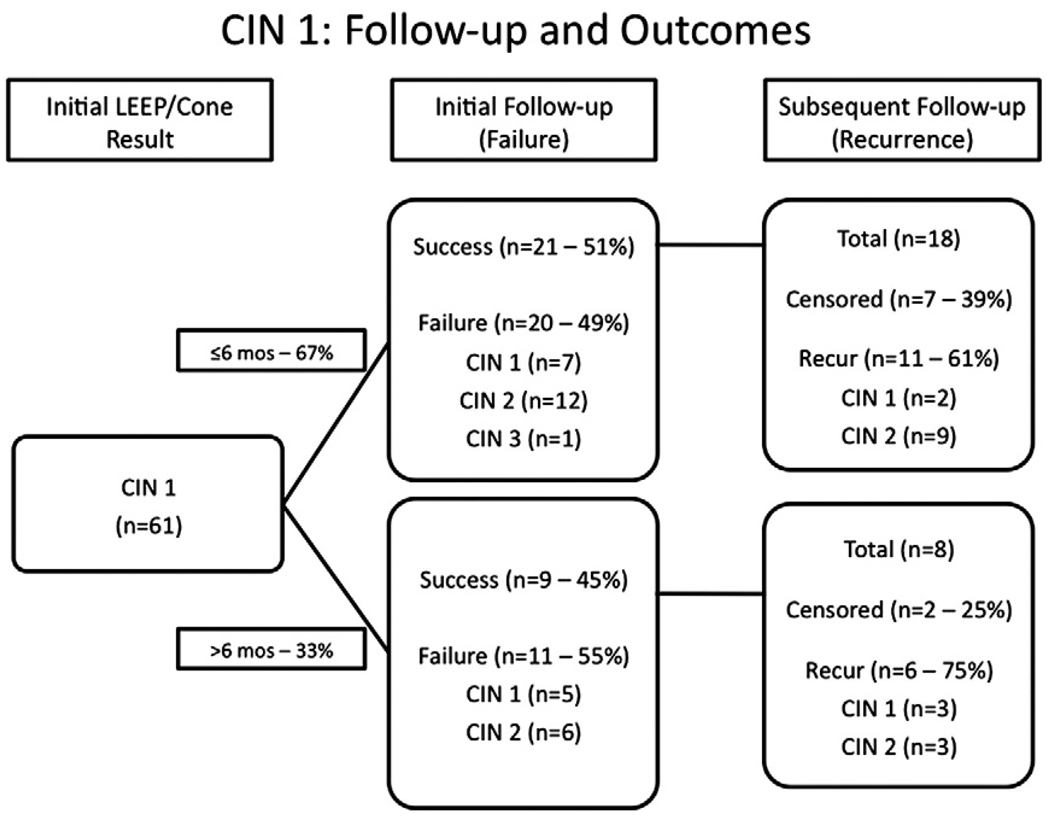
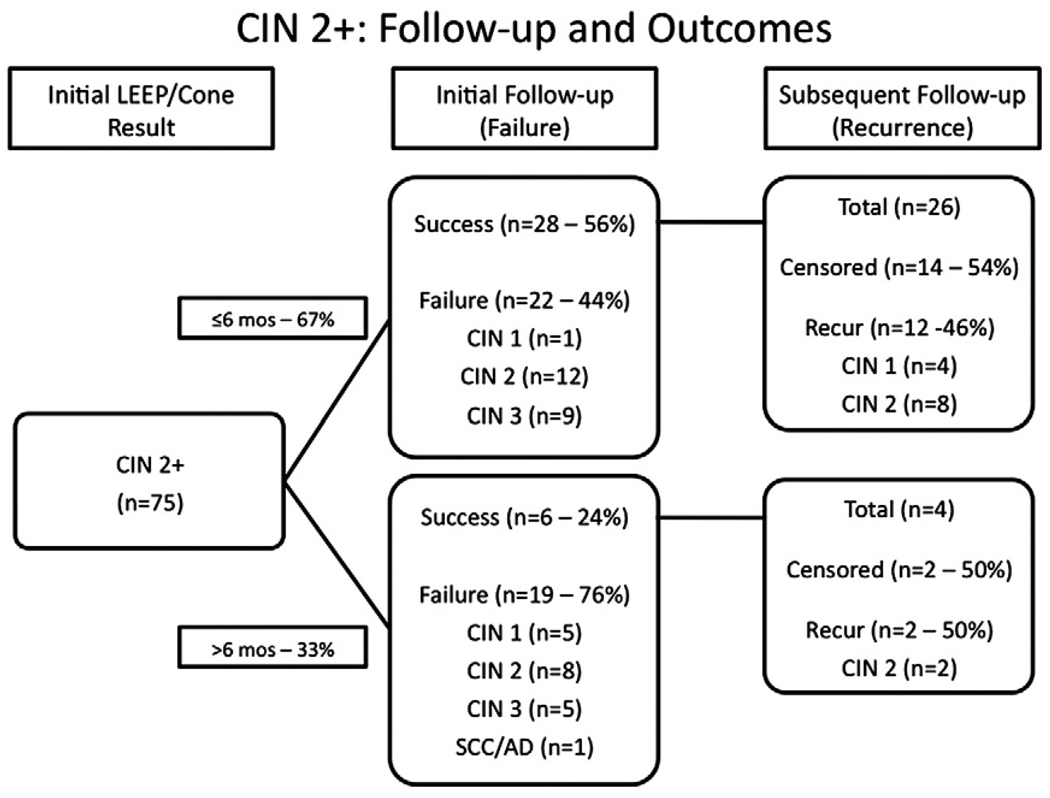
Treatment failure occurred in 31 (51%) of 61 CIN 1 (see Fig. 1) and 41 (55%) of 75 CIN 2+ (see Fig. 2). As might be expected, women with >6 months until their initial follow-up appeared to have a higher rate of treatment failure (55% of CIN 1 and 76% of CIN 2+) compared with those assessed in ≤6 months (49% and 56%, respectively). Time to first follow-up, therefore, was a variable incorporated into our multivariate models (see Table 2).
Fig. 1.
Time to failure and recurrence among 61 HIV-infected women treated for CIN 1 who came for initial follow-up before (top group) or after (bottom group) 6 months.
Fig. 2.
Time to failure and recurrence 75 HIV-infected women treated for CIN 2+ who came for initial follow-up before (top group) or after (bottom group) 6 months.
Table 2.
Univariate and multivariate analyses of failure after extirpative procedure.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Characteristics | RR | 95% CI | p | RR | 95% CI | p |
| Age at procedure (years) | ||||||
| <30 | 1.05 | (0.70–1.57) | 0.81 | 0.94 | (0.60–1.47) | 0.79 |
| 30–45 | 1.17 | (0.72–1.89) | 0.53 | 1.01 | (0.73–1.40) | 0.94 |
| >45 | 1.00 | – | – | 1.00 | – | – |
| Ethnicity | ||||||
| African American | 1.78 | (1.11–2.87) | 0.02 | 1.47 | (0.93–2.34) | 0.10 |
| Caucasian | 1.94 | (1.07–3.51) | 0.03 | 1.63 | (0.94–2.85) | 0.08 |
| Hispanic | 1.00 | – | – | 1.00 | – | – |
| Other | 1.73 | (0.97–3.08) | 0.06 | 1.50 | (0.79–2.82) | 0.21 |
| Current smoker | 0.75 | (0.54–1.05) | 0.09 | |||
| Intravenous drug use | 1.09 | (0.71–1.66) | 0.70 | |||
| Parity (multiparous vs. nulliparous) | 1.09 | (0.39–3.01) | 0.87 | |||
| Ever had an STI | 1.16 | (0.83–1.63) | 0.37 | |||
| Current hormone contraceptive use | 0.82 | (0.35–1.95) | 0.66 | |||
| Missed appointments | ||||||
| Rarely (0–3) | 1.00 | – | – | |||
| Frequently (3+) | 1.23 | (0.86–1.75) | 0.26 | |||
| Current HAART use | 0.98 | (0.71–1.35) | 0.89 | |||
| Time to first follow-up | ||||||
| ≤6 months | 1.00 | – | – | 1.00 | – | – |
| >6 months | 1.44 | (1.07–1.96) | 0.02 | 1.13 | (0.82–1.54) | 0.46 |
| Result of LEEP/cone specimen | ||||||
| <CIN 2 | 1.00 | – | – | |||
| ≥CIN 2 | 1.08 | (0.78–1.48) | 0.66 | |||
| Procedure type | ||||||
| LEEP | 1.39 | (0.94–2.05) | 0.10 | 1.76 | (1.17–2.64) | 0.007 |
| Conization | 1.00 | – | – | 1.00 | – | – |
| Margin status (endo- and ectocervical) | ||||||
| Both positive | 1.98 | (1.23–3.19) | 0.01 | 1.23 | (0.77–1.91) | 0.35 |
| Either positive | 1.73 | (1.11–2.69) | 0.02 | 0.99 | (0.66–1.48) | 0.97 |
| Both negative | 1.00 | – | – | 1.00 | – | – |
| CD4 count at time of procedure (±8 weeks) | ||||||
| ≤200 cells/µL | 3.55 | (1.44–8.74) | 0.006 | 2.93 | (1.06–8.11) | 0.04 |
| 201–500 cells/µL | 2.86 | (1.16–7.07) | 0.02 | 2.23 | (0.79–6.29) | 0.13 |
| >500 cells/µL | 1.00 | – | – | 1.00 | – | – |
| Viral load at time of procedure (±8 weeks) | ||||||
| Undetectable | 1.00 | – | – | |||
| Detectable –20,000 copies/mL | 1.12 | (0.76–1.65) | 0.56 | |||
| >20,000 copies/mL | 1.17 | (0.79–1.72) | 0.44 | |||
All significant p-values (p<0.05) were italicized.
CIN treatment failure occurred in 27 (71%) of 38 women with CD4 ≤200 cells/µL, 32 (57%) of 56 with CD4 201–500, and 4 (20%) of 20 with >500 cells/µL. Most of the lesions detected at treatment failure were high grade (>70%), regardless of the grade of lesion at the time of treatment. In multivariate models, CD4 ≤200 cells/µL (relative risk [RR]=2.93; 95% confidence intervals [CI]: 1.06–8.11) was associated with significantly increased risk of CIN treatment failure compared with CD4 >500 cells/µL, and the risk increased monotonically with diminishing CD4 count (ptrend<0.001). HAART use, however, was not significantly associated with risk of treatment failure, even in univariate analysis. The only other significant variable was the use of LEEP versus conization (RR=1.76; 95% CI: 1.17–2.64). Positive tissue margins following treatment and race/ethnicity were significant risk factors in univariate but not in multivariate models.
Recurrence after initial treatment success
Of the 56 women who initially had treatment success, 31 (55%) experienced recurrence of CIN (see Figs. 1 and 2). Eight patients had no further follow-up after their initial postprocedure follow-up. Among the 26 CIN 1 and 30 CIN 2+ patients with an initial normal clinical evaluation and appropriate follow-up, 65% and 47% recurred, respectively. In both groups, recurrence was high grade (CIN 2+) in 22 (71%) of 31 patients. Even among women with clear margins (data not shown), there was an overall higher recurrence rate among the CIN 2+ population.
The risk of recurrence was significantly associated with host immune status as measured by HIV RNA level. Specifically, the hazard ratio (HR) for recurrence among women with HIV RNA level >20,000 copies/mL (hazard ratio [HR]=6.32; 95% CI: 2.22–17.94; Table 3) was higher compared with those who had undetectable levels. However, there was no monotonic trend to these results, nor was CD4 T-cell count or use of HAART associated with risk of recurrence. Significant risk factors included the presence of positive tissue margins involving both the endocervical and ectocervical tissue (HR=6.12; 95% CI: 1.90–19.73) and the use of LEEP versus conization (HR=3.38; 95% CI: 1.55–7.39). Women who underwent a LEEP had a median recurrence time of 33 months, whereas women who underwent a conization had a median time of recurrence of 81 months.
Table 3.
Univariate and multivariate analyses of recurrence after extirpative procedure.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Characteristics | HR | 95% CI | p | HR | 95% CI | p |
| Age at procedure (years) | ||||||
| <30 | 1.39 | (0.46–4.18) | 0.56 | 0.28 | (0.10–1.29) | 0.07 |
| 30–45 | 1.33 | (0.52–3.37) | 0.55 | 1.84 | (0.36–1.98) | 0.77 |
| >45 | 1.00 | – | – | 1.00 | – | – |
| Ethnicity | ||||||
| Black | 0.63 | (0.23–1.71) | 0.34 | |||
| Caucasian | 0.70 | (0.14–3.62) | 0.66 | |||
| Hispanic | – | – | – | |||
| Other | 1.00 | (0.28–3.53) | 1.00 | |||
| Current smoker | 0.49 | (0.22–1.11) | 0.09 | |||
| Intravenous drug use | 0.49 | (0.10–2.29) | 0.34 | |||
| Parity (multiparous vs. nulliparous) | 1.04 | (0.09–11.70) | 0.97 | |||
| Ever had an STI | 1.74 | (0.80–3.77) | 0.16 | |||
| Current hormone contraceptive use | 2.74 | (0.41–18.30) | 0.28 | |||
| Missed appointments | ||||||
| Rarely (0–3) | 1.00 | – | – | |||
| Frequently (3+) | 1.49 | (0.78–2.86) | 0.21 | |||
| Current HAART use | 0.84 | (0.38–1.84) | 0.67 | |||
| Time to first follow-up | ||||||
| ≤6 months | 1.00 | – | – | |||
| >6 months | 0.81 | (0.36–1.81) | 0.61 | |||
| Procedure type | ||||||
| LEEP | 3.26 | (1.52–7.00) | 0.005 | 3.38 | (1.55–7.39) | 0.02 |
| Conization | 1.00 | – | – | 1.00 | – | – |
| Margin status (endo-and ectocervical) | ||||||
| Both positive | 3.85 | (1.22–12.27) | 0.03 | 6.12 | (1.90–19.73) | 0.03 |
| Either positive | 2.07 | (0.94–4.60) | 0.09 | 2.58 | (0.16–6.16) | 0.12 |
| Both negative | 1.00 | – | – | 1.00 | – | – |
| CD4 count at time of procedure (±8 weeks) | ||||||
| ≤200 cells/µL | 1.47 | (0.44–4.95) | 0.53 | |||
| 201–500 cells/µL | 0.74 | (0.30–1.86) | 0.52 | |||
| >500 cells/µL | 1.00 | – | – | |||
| Viral load at time of procedure (±8 weeks) | ||||||
| Undetectable | – | – | – | –– | – | |
| Detectable –20,000 copies/mL | 0.56 | (0.21–1.50) | 0.28 | 0.62 | (0.26–1.48) | 0.43 |
| >20,000 copies/mL | 1.89 | (0.79–4.52) | 0.18 | 6.32 | (2.22–17.94) | 0.01 |
All significant p-values (p<0.05) were italicized.
Discussion
Failure and recurrence following excisional procedures in our retrospective cohort of HIV-infected women with CIN were very high and were particularly high in patients with poorly controlled HIV, women who had a LEEP instead of conization, and women with positive tissue margins following treatment. The rates of treatment failure were similar to those reported in other captured patient cohort studies, but our rates of recurrence was higher [7,8,11,13,15,16,21]. Moreover, most lesions diagnosed in our patient population that were detected at treatment failure or recurrence were high-grade CIN. This is in contrast to findings of a recent observational cohort study, which found most treatment failures and recurrences were low grade [8]. The reason for these differences is unknown. However, a difference in the characteristics of the unselected subjects in the current study and those of the volunteer subjects in that prior observational cohort (e.g., a higher rate of concurrent STI) [8] is one possible explanation. In any event, the high rates of CIN 2+ at the time of treatment failure and recurrence we observed are concerning and provide a rationale for careful and frequent follow-up of HIV-infected women after treatment of CIN. This frequency should also likely increase, or continue to remain frequent with worsening HIV status. Also, even CIN 1 is relevant in the setting of HIV since this requires regular, frequent follow-up. Until better therapies are available, clinicians who treat cervical dysplasia in HIV-infected women should avoid raising expectations of cure and instead focus on the achievable goal of cancer prevention.
The other major finding was the superiority of cervical conization to LEEP in treatment of CIN in our population. This included lower risk of treatment failure as well as a lower risk of recurrence over time. It is possible the lower risk is due to an often smaller resection in a LEEP when compared to a cone, but the size of the biopsy was not available for this analysis. Previous studies have shown that the length and weight, as well as the general size of specimen, are often less in a LEEP than in a conization [22,23]. In our population, negative endocervical and ectocervical margins were found in 60% of conizations versus 31% of LEEPs, suggesting less of the lesion was completely removed in a LEEP than in a conization. In women who underwent a LEEP, but had positive endocervical and ectocervical margins, only 32% of the positive margins were positive for CIN 2+. Most of these positive margins were found to be koilocytosis, low-grade dysplasia, or CIN 1, as is typical for an general HPV effect in these often persistently HPV-infected women. Fewer short-term side effects and office availability without the need for an operating room have prompted surgeons to choose a LEEP over a conization; however, women may ultimately have to return for re-treatment. In prior studies, researchers found no significant differences between excisional treatments [24,25], and in a population of HIV-infected women, researchers also found no difference between treatment type [13]. A recent study found similar results as ours where loop excision was inferior to conization; however, the investigators saw no difference in margin positivity between the two treatment types [21]. Because of the potential importance of these results, further study of LEEP versus conization in this patient population is warranted.
Given that type of procedure is provider-directed, bias was potentially introduced. However, generally cones have been chosen or were preferred to a LEEP for lesions that were larger, to ensure complete excision or due to margin concerns. Thus, our analysis should not have been affected by provider preference and further supports that LEEPs are an inefficient excisional procedure in the setting of HIV. Since LEEPs often fail and/or recur even when there are smaller lesions, or persistent CIN 1. Additional bias could have been introduced with the number of women who were lost to follow-up; however, we feel that given the similarity in diagnosis and histopathology between those women and the subjects remaining in the cohort, the chance of these patients affecting the overall analysis is slight. Along with loss to follow-up, another limitation of our study is that the time between follow-up visits varied for each woman, as is typical in observational cohorts. The interval analysis, however, allowed us to take these follow-up variations into account.
It is worth noting that the relationship of CD4 T-cell count with treatment failure was stronger than its relationship with CIN recurrence. This helps point to an important limitation of the current study. Both the CD4 and HIV RNA levels were based on the values obtained at time of procedure. Therefore, these values may be less representative of each patient's immune status over increasing time of follow-up. When longer follow-up periods are involved, this aspect is more likely to affect our analysis of recurrence. These same concerns probably affected our analysis of HAART use. Further, since the HAART use data found in patients' records was based on self-report, our analysis of HAART was also subject to misclassification. Recently, reported data regarding adherent HAART use suggest that HAART has a beneficial effect on control of both HPV and cervical dysplasia [26].
Despite these weaknesses, our study has an important strength that cannot be addressed in carefully followed voluntary prospective cohorts where subjects usually have more time with providers, more support staff, and are often reimbursed for added time and travel expenses. Our study may provide results that are more representative of outcomes in the real-world situation that clinicians face with HIV-infected women with dysplasia. This includes barriers to regular follow-up, variation in patient compliance, including treatment for sexually transmitted infections, as well as other high-risk behaviors that may be less prevalent in subjects who remain in long-term cohort studies.
Overall, the current study reports provocative results suggesting that in HIV-infected women who are not volunteers in long-term observational or clinical cohorts: treatment of CIN often fails; even after initial successful treatment of CIN often recurs; and that a substantial fraction of CIN at treatment failure and recurrence may be high grade. Furthermore, cold knife conization may be more effective than a LEEP in treating CIN in HIV-infected women. Larger, more comprehensive retrospective cohort studies of high-risk HIV-infected women are, therefore, greatly needed.
Acknowledgments
Funding
This work was supported by an American Medical Association (AMA) Foundation Seed Grant (D. Daniel, MH Einstein). This work was also supported in part by the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center funded by the National Institutes of Health (NIH AI-51519).
Footnotes
Conflicts of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Strickler HD. Does HIV/AIDS have a biological impact on the risk of human papillomavirus-related cancers? J Natl Cancer Inst. 2009;101:1103–1105. doi: 10.1093/jnci/djp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hocke C, Leroy V, Morlat P, Rivel J, Duluc MC, Boulogne N, et al. Cervical dysplasia and human immunodeficiency virus infection in women: prevalence and associated factors. Groupe d'Epidemiologie Clinique du SIDA en Aquitaine (GESCA) Eur J Obstet Gynecol Reprod Biol. 1998;81:69–76. doi: 10.1016/s0301-2115(98)00150-x. [DOI] [PubMed] [Google Scholar]
- 4.Robinson WR, Hamilton CA, Michaels SH, Kissinger P. Effect of excisional therapy and highly active antiretroviral therapy on cervical intraepithelial neoplasia in women infected with human immunodeficiency virus. Am J Obstet Gynecol. 2001;184:538–543. doi: 10.1067/mob.2001.111103. [DOI] [PubMed] [Google Scholar]
- 5.Boardman LA, Peipert JF, Hogan JW, Cooper AS. Positive cone biopsy specimen margins in women infected with the human immunodeficiency virus. Am J Obstet Gynecol. 1999;181:1395–1399. doi: 10.1016/s0002-9378(99)70382-0. [DOI] [PubMed] [Google Scholar]
- 6.Strickler HD, Burk RD, Fazzari M, Anastos K, Minkoff H, Massad LS, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 7.Wright TC, Jr, Koulos J, Schnoll F, Swanbeck J, Ellerbrock TV, Chiasson MA, et al. Cervical intraepithelial neoplasia in women infected with the human immunodeficiency virus: outcome after loop electrosurgical excision. Gynecol Oncol. 1994;55:253–258. doi: 10.1006/gyno.1994.1286. [DOI] [PubMed] [Google Scholar]
- 8.Massad LS, Fazzari MJ, Anastos K, Klein RS, Minkoff H, Jamieson DJ, et al. Outcomes after treatment of cervical intraepithelial neoplasia among women with HIV. J Low Genit Tract Dis. 2007;11:90–97. doi: 10.1097/01.lgt.0000245038.06977.a7. [DOI] [PubMed] [Google Scholar]
- 9.Holcomb K, Matthews RP, Chapman JE, Abulafia O, Lee YC, Borges A, et al. The efficacy of cervical conization in the treatment of cervical intraepithelial neoplasia in HIV-positive women. Gynecol Oncol. 1999;74:428–431. doi: 10.1006/gyno.1999.5479. [DOI] [PubMed] [Google Scholar]
- 10.Tate DR, Anderson RJ. Recrudescence of cervical dysplasia among women who are infected with the human immunodeficiency virus: a case-control analysis. Am J Obstet Gynecol. 2002;186:880–882. doi: 10.1067/mob.2002.123607. [DOI] [PubMed] [Google Scholar]
- 11.Russomano F, Reis A, Camargo MJ, Grinsztejn B, Tristao MA. Recurrence of cervical intraepithelial neoplasia grades 2 or 3 in HIV-infected women treated by large loop excision of the transformation zone (LLETZ) São Paulo Med J. 2008;126:17–22. doi: 10.1590/S1516-31802008000100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah S, Montgomery H, Crow JC, Smith CJ, Moore A, Sabin CA, et al. Cervical intraepithelial neoplasia treatment in Human Immunodeficiency Virus-positive women. J Obstet Gynaecol. 2008;28:327–332. doi: 10.1080/01443610802054964. [DOI] [PubMed] [Google Scholar]
- 13.Heard I, Potard V, Foulot H, Chapron C, Costagliola D, Kazatchkine MD. High rate of recurrence of cervical intraepithelial neoplasia after surgery in HIV-positive women. J Acquir Immune Defic Syndr. 2005;39:412–418. doi: 10.1097/01.qai.0000167157.83098.60. [DOI] [PubMed] [Google Scholar]
- 14.Gilles C, Manigart Y, Konopnicki D, Barlow P, Rozenberg S. Management and outcome of cervical intraepithelial neoplasia lesions: a study of matched cases according to HIV status. Gynecol Oncol. 2005;96:112–118. doi: 10.1016/j.ygyno.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Lehtovirta P, Paavonen J, Heikinheimo O. Risk factors, diagnosis and prognosis of cervical intraepithelial neoplasia among HIV-infected women. Int J STD AIDS. 2008;19:37–41. doi: 10.1258/ijsa.2007.005672. [DOI] [PubMed] [Google Scholar]
- 16.Lima MI, Tafuri A, Araujo AC, de Miranda Lima L, Melo VH. Cervical intraepithelial neoplasia recurrence after conization in HIV-positive and HIV-negative women. Int J Gynaecol Obstet. 2009;104:100–104. doi: 10.1016/j.ijgo.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Maiman M, Fruchter RG, Serur E, Levine PA, Arrastia CD, Sedlis A. Recurrent cervical intraepithelial neoplasia in human immunodeficiency virus-seropositive women. Obstet Gynecol. 1993;82:170–174. [PubMed] [Google Scholar]
- 18.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 19.Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol. 1986;123:174–184. doi: 10.1093/oxfordjournals.aje.a114212. [DOI] [PubMed] [Google Scholar]
- 20.Allison PD SAS Institute. Survival analysis using the SAS system: a practical guide. Cary, NC: SAS Institute; 1995. [Google Scholar]
- 21.Foulot H, Heard I, Potard V, Costagliola D, Chapron C. Surgical management of cervical intraepithelial neoplasia in HIV-infected women. Eur J Obstet Gynecol Reprod Biol. 2008;141:153–157. doi: 10.1016/j.ejogrb.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Fanning J, Padratzik J. Cold knife conization vs. LEEP. Are they the same procedure? J Reprod Med. 2002;47:33–35. [PubMed] [Google Scholar]
- 23.Simmons JR, Anderson L, Hernandez E, Heller PB. Evaluating cervical neoplasia. LEEP as an alternative to cold knife conization. J Reprod Med. 1998;43:1007–1013. [PubMed] [Google Scholar]
- 24.Prendiville W. The treatment of CIN: what are the risks? Cytopathology. 2009;20:145–153. doi: 10.1111/j.1365-2303.2009.00669.x. [DOI] [PubMed] [Google Scholar]
- 25.Mathevet P, Chemali E, Roy M, Dargent D. Long-term outcome of a randomized study comparing three techniques of conization: cold knife, laser, and LEEP. Eur J Obstet Gynecol Reprod Biol. 2003;106:214–218. doi: 10.1016/s0301-2115(02)00245-2. [DOI] [PubMed] [Google Scholar]
- 26.Minkoff H, Ahdieh L, Massad LS, Anastos K, Watts DH, Melnick S, et al. The effect of highly active antiretroviral therapy on cervical cytologic changes associated with oncogenic HPV among HIV-infected women. AIDS. 2001;15:2157–2164. doi: 10.1097/00002030-200111090-00011. [DOI] [PubMed] [Google Scholar]