Abstract
Background
Small studies have indicated that twinning increases the risk of oral cleft.
Methods
We used data from a Danish national population-based cohort study to investigate whether twinning was associated with isolated oral cleft, and to estimate the twin probandwise concordance rate and heritability. Twins (207 affected/130,710) and singletons (7766 affected/4,798,526) born from 1936 through 2004 in Denmark were ascertained by linkage among the Danish Facial Cleft Database, the Danish Twin Registry and the Civil Registration System. We computed oral cleft prevalence and prevalence proportion ratio for twins versus singletons, stratified for three sub-phenotypes. Probandwise concordance rates and heritability for twins were estimated for two phenotypes—cleft lip with or without cleft palate (CL/P) and cleft palate (CP).
Results
The prevalence of oral cleft was 15.8 per 10,000 twins and 16.6 per 10,000 singletons (prevalence proportion ratio = 0.95; 95% confidence interval = 0.83 – 1.1). This prevalence was similar for monozygotic and dizygotic twins. The probandwise concordance rate was higher for CL/P for monozygotic twins than for dizygotic twins (50 % vs. 8%, respectively). A similar contrast was present for CP. Recurrence risk for both types of clefts was greater in dizygotic twins than in non-twin siblings. Heritability estimates were above 90% for both CL/P and CP.
Conclusion
No excess risk of oral cleft could be demonstrated for twins compared with singletons. The concordance rates and heritability estimates for both types of clefts show a strong genetic component.
Oral clefts, including cleft lip (CL), cleft lip with cleft palate (CLP) and cleft palate only (CP), are among the most common congenital malformations. The three sub-phenotypes have overlapping but distinct etiologies.1 Non-syndromic oral clefts are complex traits with strong familial aggregation and a substantial genetic component.2-5 Known genetic variants seem to explain about 25% of isolated oral clefts and smoking (the only common environmental factor with an established harmful effect) explain approximately 5%.6,7
Several studies have compared the oral cleft occurrence in twins and singletons. Most studies have been limited by small sample size, ascertainment bias, inclusion of syndromic forms of oral cleft, and a lack of zygosity information.8-18 So far, the data have not provided compelling evidence for an association of oral cleft with twinning in general, or with monozygotic twinning in particular.4,5,19-22
The relative contributions of genetic and environmental factors to oral clefts has been estimated with classical twin studies on a small Danish twin population born from 1970 through 1990.4,5 The probandwise concordance rate for monozygotic twins was about 60% and for dizygotic twins between 0 to 10% with corresponding heritability estimates of approximately 70%. However, the estimates were hampered by the small sample size,23 especially for CP. It has not been possible either in the Danish population or in other populations to determine whether dizygotic twins have an excess risk of oral cleft compared with ordinary siblings, as might be expected if intrauterine environmental factors influence this risk.
The aim of the present study was to compare the oral cleft occurrence among twins and singletons in a large, population-based dataset, and to provide estimates of heritability and probandwise concordance rates for monozygotic and dizygotic twins.
Methods
Study population
Persons with an oral cleft were identified through the Danish Facial Cleft Database23,24 from the 1936-2004 birth cohorts and linked to the Danish Twin Registry.25 The linkage between the population-based registries was enabled by the unique personal identification number assigned by the Civil Registration System. Everyone residing in Denmark in 1968 and all subsequent live births have been assigned a personal identification number.
The Danish Facial Cleft Database comprises babies with clefts born from 1936 to 2005 and contains 9146 persons with a valid personal identification number. Both the registration and the treatment of those with oral cleft have been centralized in Denmark since the 1930s. Since the vast majority of persons born in 1936 or later were still alive in 1968, this provides a nearly complete ascertainment for the cohorts under study. Clefts discovered later in a child's life were also registered.24,26 Capture-recapture methods have indicated a 99% ascertainment for the sub-phenotype isolated cleft lips with or without cleft palate (CL/P) in the period 1983 to 1987.26 For phenotypes other than CL, CLP, and CP, ascertainment was low; hence the microforms (bifid uvula, defects in the orbicularis oris muscle, etc.) of oral cleft were excluded from the study. The syndromic forms of oral cleft and oral cleft cases with other major anomalies were also excluded because these are such heterogeneous groups, including both dominantly inherited syndromes (such as Van der Woude and velo-cardio-facial syndrome) and the non-heritable sequence Pierre Robin. Minor malformations such as polydactyly or hip dislocation were included. All cases refer to isolated oral cleft unless otherwise specified. The Danish Facial Cleft Database has previously been described in greater detail with regard to both ascertainment and anomalies.23,24
The Danish Twin Registry comprises more than 80,000 twin pairs born in Denmark since 1870. The twins have been ascertained independently of any disease. The ascertainment of live-born twins from 1930 to 1968 was about 80%. Since the establishment of the Civil Registration System, the ascertainment has been considered complete for live-born twins, and since 1973 for all twins.25 Zygosity determination of same-sex pairs has been made through four standard questions about physical resemblance—a method with less than 5% misclassification for the birth cohorts 1900-1982.27 Zygosity determination of twins with oral cleft was made using the same method. Here also the misclassification was estimated to be less than 5%.28 About 75% of the twins in the register have an assigned zygosity. Information on zygosity is accessible only through the Danish Twin Registry.
To assess the number of twins obtained through the Danish Twin Registry, summary data were extracted from the Statistics Denmark,29 where data on many aspects of life for all residents of Denmark have been consistently collected for administrative purposes. Since the establishment of the Civil Registration System, it has been possible to track persons by use of their personal identification number; before 1968 the data have been aggregated. Of the 130,710 twins and 4,798,526 singletons born from 1936 to 2004, 207 twins and 7966 singletons were born with an isolated oral cleft.
Statistics
We estimated prevalence and prevalence proportion ratio (PPR) of oral clefts for twins versus singletons, stratified by sex and the sub-phenotypes CL, CLP, and CP, for the 1936 to 2004 cohorts.29 For the 1968 to 2004 cohorts, further stratification was made for zygosity using data from the Danish Twin Registry. The proportion of monozygotic to dizygotic twins in the oral cleft twin population was compared with the total twin population. The distribution of monozygotic, dizygotic same-sex, dizygotic opposite-sex, and unknown zygosity was compared among the CL, CLP, and CP cases. All binary comparisons were made using Fisher's exact test or, when possible, Poisson regression, in order to take into account the correlated nature of the twins. This was done by use of the cluster function in Stata 10.1 (StataCorp, USA), which was also used to test for interaction between sex or zygosity with oral cleft.
The relative contribution of genes and environment to oral cleft etiology was estimated for CL/P and CP by the probandwise concordance rates, the tetrachoric correlation (corresponding to the intraclass correlation for a continuous outcome), and calculations of heritability. Twin pairs born from 1936 to 2004 were included. The basic assumption is that the intrauterine environment of monozygotic and dizygotic twins is similar, and therefore any differences in their concordance rates must be attributable to genetic differences (monozygotic twins share 100% of their genes and dizygotic twins share 50% of the parental genetic pool). The probandwise concordance rate is an estimator of the probability that one twin has an oral cleft, given that the co-twin is affected. The probandwise concordance rate denotes two times the number of concordant affected pairs divided by two times the number of concordant affected pairs plus the number of discordant pairs. The oral cleft recurrence risk for full siblings (non-twins) is the number of affected siblings divided by the total number of siblings. Because the probandwise concordance rate provides estimates of risk for the individual rather than for the pair, it can be directly compared with the recurrence risk for ordinary siblings, who are genetically equivalent to dizygotic co-twins.30 This comparison offers the possibility of isolating the effect of the environment: the number of shared genes is similar for both dizygotic twins and full siblings while twins share a single intrauterine environment but siblings don't. A change in the intrauterine environment between pregnancies could be caused by an intentional change in the mother's risk behavior after having a child with oral cleft, or by changes in environmental factors unrelated to the pregnancy outcome. The probandwise concordance rate for monozygotic and dizygotic twins and the recurrence risk for siblings were compared using exact statistical methods. All probandwise-concordance-rate comparisons were verified by the use of bootstrapping, assuming that the prevalence of oral cleft for monozygotic and dizygotic twins was the same.
Tetrachoric correlations for monozygotic twins and for dizygotic twins (same and opposite sex) were compared under the assumption of the multifactorial threshold model (liability threshold model), which is thought to best describe the etiology of oral clefts.31 A higher correlation for monozygotic twins compared with dizygotic twins indicates that genetic factors contribute to the phenotypic variation. The magnitude of the genetic contribution can be computed using heritability estimates that are independent of the prevalence of the malformation studied. For the tetrachoric correlations and the heritability estimates, both same-sex and opposite-sex twin pairs were included, but thresholds were not adjusted for effects of sex. The total variance (V) could be decomposed as V = A + D + C + E where A refers to the additive genetic effects, D refers to the dominant genetic effect (intraloci interaction), C refers to shared environmental effects, and E refers to the unique environmental effect. Univariate genetic models32 were fitted to contingency tables using maximum likelihood estimation with Mx statistical modelling.33 First, a saturated model was fitted and thereafter the following models: ACE, ADE, AE, CE and E. The best-fitting model was chosen by the Akaike Information Criterion (AIC) (χ2-2·df), thereby taking into account both the goodness-of-fit and the simplicity of the model. The 95% confidence intervals (CIs) were calculated for the standardized parameter estimates (heritability) of the best-fitting model.
The Intercooled Stata 10.1 version (StataCorp, College station, TX, USA) was used for all computations except for the tetrachoric correlations and heritability estimates, for which Mx (freeware from www.vcu.edu/mx/) was used.
Results
Prevalence
Table 1 shows the number of twins and singletons by oral cleft phenotype from the Danish 1936 to 2004 cohorts, together with cleft prevalence. The prevalence of clefts overall was similar for twins and singletons (15.8 and 16.6 per 10,000, respectively; prevalence proportion ratio (PPR) = 0.95 [95% confidence interval (CI) = 0.83 – 1.10). The sex distribution was similar for twins and singletons (P = 0.13). Twins were much less likely to have CP than singletons (0.63 [0.53 – 0.76]). When stratifying into two time periods with a cut-point in 1968 (corresponding to the establishment of the Civil Registration System), the overall oral cleft prevalence was lowest for twins born before 1968 (0.73 [0.73 – 0.93]). This difference was due to fewer CPs among the twins. From 1968 to 2004, the oral cleft prevalence for twins and singletons was similar (1.15 [0.95 – 1.38]), although twins had a higher prevalence of CLP than singletons (1.43 [1.09 – 1.90])(Table 2). There was no interaction of sex or zygosity with oral cleft. The Danish Twin Registry identified 110,556 of the 130,710 twins (85%) registered in Statistics Denmark in the complete time period, but the ascertainment was nearly complete (99%) from 1968 to 2004 (Table 1). For both twins and singletons, there was the expected male preponderance for CL and CLP and the female preponderance for CP, with these differences more pronounced among twins.
TABLE 1.
Number and prevalence of twins and singletons (N=4,929,236) with isolated Oral Cleft, stratified for phenotype and sex; Denmark 1936-2004.
| Twins | Singletons | ||||||
|---|---|---|---|---|---|---|---|
| Cohort | Phenotype/Source | Total No. | % Male | Prevalence per 10,000a (95% CI) |
Total No. | % Male | Prevalence per 10,000a (95% CI) |
| 1936 – 2004 | |||||||
| Total Oral Clefts | 207 | 66 | 15.8(13.8-18.1) | 7,966 | 60 | 16.6 (16.2-17.0) | |
| Cleft lip | 72 | 75 | 5.5 (4.3-6.9) | 2,495 | 64 | 5.2 (5.0-5.4) | |
| Cleft lip and palate | 93 | 74 | 7.1 (5.7-8.7) | 3,039 | 70 | 6.3 (6.1-6.6) | |
| Cleft palate | 42 | 33 | 3.2 (2.3-4.3) | 2,432 | 45 | 5.1 (4.9-5.3) | |
| Total Danish Population | |||||||
| Danish Twin Registry | 110,556 | 52.3 | - | - | |||
| Statistics Denmark | 130,710 | 51.2 | 4,798,526 | 51.4 | |||
|
| |||||||
| 1936 – 1967 | |||||||
| Total Oral Clefts | 71 | 70 | 10.5 (8.2-13.2) | 3,559 | 61 | 14.3 (13.9-14.8) | |
| Cleft lip | 32 | 78 | 4.7 (3.2-6.7) | 1,131 | 65 | 4.6 (4.3-4.8) | |
| Cleft lip and palate | 30 | 70 | 4.4 (3.0-6.3) | 1,401 | 71 | 5.6 (5.4-6.0) | |
| Cleft palate | 9 | 44 | 1.3 (0.6-2.5) | 1,027 | 42 | 4.1 (3.9-4.4) | |
| Total Danish Population | |||||||
| Danish Twin Registry | 48,142 | 53.6 | - | - | |||
| Statistics Denmark | 67,746 | 51.1 | 2,482,528 | 51.4 | |||
| 1968 – 2004 | |||||||
| Total Oral Clefts | 136 | 64 | 21.6 (18.1-25.6) | 4,407 | 60 | 19.0 (18.5-19.6) | |
| Cleft lip | 40 | 73 | 6.4 (4.5-8.7) | 1,364 | 63 | 5.9 (5.6-6.2) | |
| Cleft lip and palate | 63 | 76 | 10.0 (7.7-12.8) | 1,638 | 69 | 7.1 (6.7-7.4) | |
| Cleft palate | 33 | 30 | 5.2 (3.6-7.4) | 1,405 | 47 | 6.1 (5.8-6.4) | |
| Total Danish Population | |||||||
| Danish Twin Registry | 62,414 | 51.2 | - | - | |||
| Statistics Denmark | 62,964 | 51.2 | 2,315,998 | 51.4 | |||
Based on all livebirths using Statistics Denmark Data as the reference
TABLE 2.
Comparison of twins and singletons according to oral cleft phenotype, sex and zygosity; Denmark 1968-2004.
| Cohort | Phenotype/Source | Total No. | % Male | Twins vs. Singletons Prevalence Ratio (95% CI)a |
|---|---|---|---|---|
| All Oral Cleft Twins | ||||
| Total Oral Cleft | 136 | 64 | 1.15 (0.95-1.38) | |
| Cleft lip | 40 | 73 | 1.09 (0.77-1.53) | |
| Cleft lip and palate | 63 | 76 | 1.43 (1.09-1.9) | |
| Cleft palate | 33 | 30 | 0.87 (0.61-1.26) | |
| Monozygotic Twins | ||||
| Total Oral Cleft | 22 | 59 | 1.29 (0.77-2.17) | |
| Cleft lip | 9 | 56 | 1.71 (0.83-3.52) | |
| Cleft lip and palate | 9 | 78 | 1.42 (0.65-3.12) | |
| Cleft palate | 4 | 25 | 0.74 (0.22-2.45) | |
| Dizygotic Twins | ||||
| Total Oral Cleft | 76 | 61 | 1.14 (0.90-1.45) | |
| Cleft lip | 17 | 65 | 0.82 (0.50-1.37) | |
| Cleft lip and palate | 39 | 77 | 1.57 (1.13-2.20) | |
| Cleft palate | 20 | 25 | 0.94 (0.59-1.49) | |
Controlled for cluster
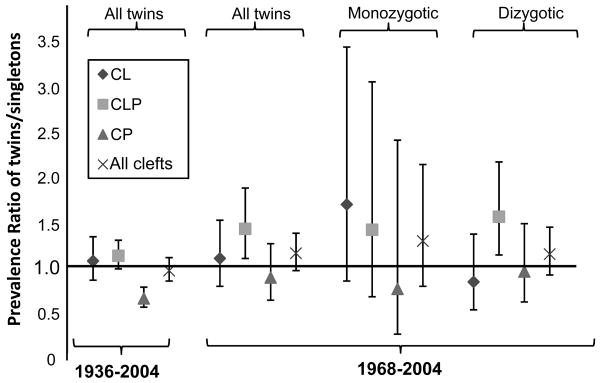
The PPR for oral cleft in twins compared with singletons is provided in Table 2, stratified by phenotype and zygosity for the 1968 to 2004 cohorts. The key prevalence ratios from Tables 1 and 2 are summarized in Figure 1. Similar prevalences were found for monozygotic and dizygotic twins and for singletons for all of the oral cleft phenotypes, with the exception of a higher, CL prevalence for monozygotic twins compared with dizygotic twins (PPR = 1.71 [0.83 – 3.52] and 0.82 [0.50 – 1.37] respectively). A similar pattern was seen for twins with unknown zygosity (oral cleft PPR = 1.08 [0.79 – 1.49]).
FIGURE 1.
Prevalence Proportion Ratio for Oral Cleft, Twins vs. Singletons. For 1936-2004, 130,710 twins/4,798,526 singletons; for 1968-2004, 62,414 twins/2,315,998 singletons.
Similar proportions of monozygotic to dizygotic twins were found for cleft twins and the total twin population – 1:3.9 and 1:3.5, respectively (P = 0.62). Likewise, the proportions of monozygotic, dizygotic same-sex, dizygotic opposite-sex, and unknown-zygosity twins were similar for CL, CLP or CP persons (P = 0.26).
Probandwise concordance rates, tetrachoric correlations, and heritability
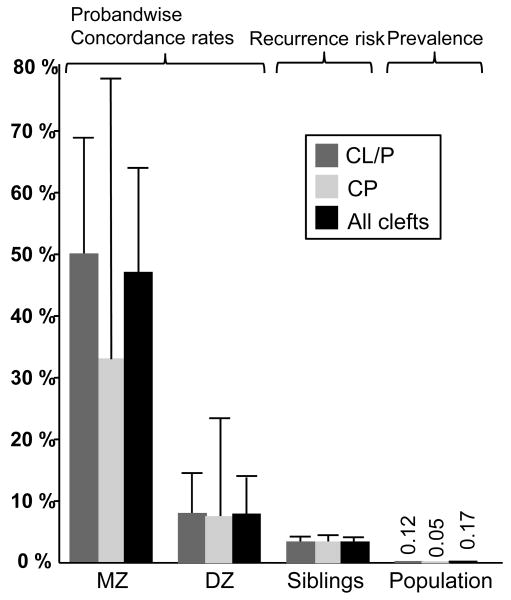
The probandwise concordance rates for monozygotic twins, all dizygotic twins, the subset of same-sex dizygotic twins, and twins with unknown zygosity were stratified by CL/P and CP. These ratios are provided in Table 3, along with the recurrence risk for ordinary siblings. The probandwise concordance rate for CL/P was 50% among monozygotic twins compared with 8% among dizygotic twins. For CP, the probandwise concordance rate was 33% among monozygotic twins compared with 7% among dizygotic twins. No pairs had the combination of CL/P in one twin and CP in the other. Estimates of oral cleft recurrence risk for siblings were derived from the Danish 1952-2005 cohorts.31 The probandwise concordance rate for dizygotic twins was greater than the recurrence risk for ordinary siblings for both phenotypes. When stratified by sex and the three cleft sub-phenotypes, the confidence intervals were wide, but the patterns were consistent: the probandwise concordance rates for monozygotic twins ranged from 33% to 67%, for dizygotic twins from 6% to 12%, and for unknown zygosity twins from 13% to 33%. Figure 2 shows probandwise concordance rates for monozygotic and dizygotic twins, the recurrence risk for ordinary siblings and the population prevalence.
TABLE 3.
Probandwise concordance rates for twins and recurrence risk for siblings for isolated Oral Cleft, Denmark 1936-2004 (n=185 twin pairs/7,654 sib pairs)
| Dizygotic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype | Monozygotic | All | Same Sex | Unknown Zygosity | Siblings | |||||
| Concordance | No. | CPr, % (95% CI)a |
No. | CPr, % (95% CI)a |
No. | CPr, % (95% CI)a |
No. | CPr, % (95% CI)a |
No. | Recurrence riskb, % (95% CI)a |
| Cleft lip with or without cleft palate | ||||||||||
| Concordant pairs | 8 | 50 (32-68) | 4 | 7.9 (3.5-15) | 2 | 7.7 (2.1-19) | 5 | 31 (16-50) | 86 | 3.2 (2.7-3.7) |
| Discordant pairs | 16 | 93 | 48 | 22 | 5224 | |||||
| Cleft palate | ||||||||||
| Concordant pairs | 1 | 33 (4.3-78) | 1 | 7.4 (0.9-24) | 0 | 0 | 0 | 0 | 36 | 3.0 (2.3-3.8) |
| Discordant pairs | 4 | 25 | 17 | 6 | 2308 | |||||
| Total Oral Clefts | ||||||||||
| Concordant pairs | 9 | 47 (31-64) | 5 | 7.8 (3.8-14) | 2 | 5.6 (1.6-14) | 5 | 26 (13-43) | 122 | 3.1 (2.8-3.5) |
| Discordant pairs | 20 | 118 | 67 | 28 | 7532 | |||||
CPr indicates Probandwise Concordance Rate; Exact methods for 95% confidence intervals
Recurrence risk from 1952 to 2005
FIGURE 2.
Probandwise Concordance Rates for Monozygotic (MZ) and Dizygotic (DZ) Twins, Recurrence Risk for Singleton Siblings, and Background Population Prevalence for Cleft lip with or without Cleft Palate and Cleft Palate alone.
The tetrachoric correlation was higher for monozygotic than for dizygotic twins for both CL/P and CP. The AE model was the best fitting model for both CL/P and CP (with lowest AIC). Heritability estimates (a2) were very similar for CL/P and CP (91% and 90% respectively), and the unique environmental factor (e2) was small (9% and 10%, respectively) eTable, (http://links.lww.com). These estimates did not take infant sex into account due to small sample size. When restricting to same-sex dizygotic twins, the results did not change considerably, and when we included the syndromic forms of oral cleft in the analyses, the estimates of heritability increased slightly (results not shown).
Discussion
We found very little evidence of excess risk of oral cleft for twins compared with singletons. More specifically, we could not demonstrate an excess risk of oral cleft among monozygotic twins compared with singletons. While the concordance rate was highest for monozygotic twins, the concordance was highest among dizygotic twins than among ordinary siblings.
Strength and weaknesses
This large data set comes from a nearly complete nationwide registry of isolated oral clefts collected over 69 years. Even so, clefts are relatively rare, and power issues continue to be a limiting factor. Over the period observed, the average frequency of twin births was 1.3%. With an oral cleft prevalence of 0.17% for the same period, the probability of co-occurrence in twins was one in 45,000 individuals. Nonetheless, our results are more reliable than previous estimates based on the Danish 1970-1990 cohorts (207 twins vs. the previous 65).4,5 Subgroup analyses required multiple comparisons, which increases the possibility of chance findings. Caution should be taken when interpreting differences in subgroup analyses when only small and imprecise differences in oral cleft prevalence between twins and singletons were found.
The upward trend over time in the oral cleft prevalence for twins compared with singletons was likely due to a decrease in infant mortality for twins in general, and for oral cleft twins in particular (Figure 1). The oldest cohorts were most prone to this bias because only after 1954 were the midwifes in Denmark obliged to report an oral cleft identified at birth. Before that, children with oral cleft had to survive until the age of 2 months to be evaluated for surgery and thereby be included in the database.26 Persons with CP were most susceptible to this selection bias because the diagnosis is more often made after birth. Persons with the mildest CPs were not in need of surgery at all. Moreover, CP cases with an associated syndrome or other anomalies may have had an even higher infant mortality because babies with cleft palate were twice as likely to have an associated syndrome or other anomalies as those with CL or CLP.24 For the oral-cleft twins, this problem might have been magnified because at least one-third of twins are born preterm, with an accompanying higher infant mortality. It seems less plausible that the smaller number of CPs was due to a difference in diagnosis of CP or oral cleft for twins relative to singletons. Survival bias is even stronger for twins in the oldest cohorts, who had to survive until the age of 6 to be included in the Registry.25 For the 1968 cohorts and onwards, 99% of all live-born twins had been ascertained.
Because we excluded syndromic forms and oral clefts with other major anomalies, our results describe isolated oral clefts only. Major anomalies and syndromes should also have been excluded among all other twins and singletons, but those data were not available. More anomalies and syndromes could be expected to be found among twins compared with singletons in general,18 thereby reducing the twin prevalence more than the singleton prevalence and working against any increased risk of oral cleft in twins.
The zygosity determination in the Danish Twin Registry has a high degree of validity.27 However; the use of questionnaires regarding physical resemblance might not be the best method in a study of facial malformation. From studies on two subsets of our Danish oral cleft twin population from 1941-196928 and 1970-19904,5 it was evident that the method resulted in less than 5% of monozygotic twins being misclassified as dizygotic twins. Both studies used blood, serum, and enzyme determinants to verify the information obtained from the questionnaire. In our study, the difference between the oral cleft probandwise concordance rate for all dizygotic twins relative to the dizygotic same-sex twins could indicate such bias. For the CL/Ps, however, no such difference could be found, and because those with CL/P would be the most prone to misclassification due to facial asymmetry, any information bias thus introduced was likely to be minimal. This assumption was supported by the similar proportions of monozygotic to dizygotic twins among the cleft twin population and the total twin population, as well as by the similar proportions of monozygotic and dizygotic twins among the CL, CLP, or CP sub-groups.
Comparison with previously published studies
The oral cleft occurrence among twins and singletons has also been studied in populations other than the Danish.4,5,28 Some previous studies were too small to interpret.9,12 Others were large but limited by ascertainment bias,14,34 inclusion of syndromic forms of oral cleft,5,18,19,34 or lack of stratification by zygosity or type of cleft.10,11,17 The majority of studies found no difference in the oral cleft prevalence for twins relative to singletons. These studies were based on a total number of twins more than twice the size of the twin sample used in the studies suggesting a difference.4,5,11,13,15,17,19-22,28,35 A number of the previous large studies also provided pairwise or probandwise concordance rates.4,5,19,22,28,35,36 Only one recent study estimated heritability (among CL/P cases, for males, a2 = 0.73 [standard error = 0.42] and for females, a2 = 0.66 [0.39]).4
Most of these challenges were overcome in our study, and our results are in accordance with the majority of previous literature. (The decreased risk for twins found by Shields et al.28 on a subset of the Danish populations was likely due to survival bias.) Moreover, our study added further support for the two phenotypes CL/P and CP being different entities, as no pairs in which one twin had CL/P and the other CP have been observed in Denmark in 69 years.
The best-fitted model in the variance-component analysis was the AE model. This suggests that the variance in oral clefts is due to additive genetic factors (A) and unique (non-shared) environment (E). Our demonstration of a more than four-fold increased probandwise concordance rate for monozygotic twins relative to dizygotic twins supports the possibility that several loci affect oral cleft risk.2,37 The less-than-100% phenotypic concordance may indicate that environmental factors could be of importance, in that the genomic sequence alone does not explain disease occurrence. However, monozygotic twin discordance could also result form genetic, cytogenetic or epigenetic anomalies in the affected twin, and not the other.38-40 An environmental component is supported by the higher risk among dizygotic twins than among singleton siblings. The difference between twin concordance and sibling concordance could have been overestimated due to non-paternity (i.e. if some of the presumed full siblings were in fact half-siblings), but because the non-paternity rate is likely to be low, the effect would be small.
Supplementary Material
Acknowledgments
Financial support: University of Southern Denmark, Danish Graduate School in Public Health and NIH (Grant numbers R01 DE-11948 and DE-08559).
Footnotes
SDC Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
Reference List
- 1.Harville EW, Wilcox AJ, Lie RT, Vindenes H, Abyholm F. Cleft lip and palate versus cleft lip only: are they distinct defects? Am J Epidemiol. 2005;162(5):448–453. doi: 10.1093/aje/kwi214. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell LE, Christensen K. Analysis of the recurrence patterns for nonsyndromic cleft lip with or without cleft palate in the families of 3,073 Danish probands. Am J Med Genet. 1996;61(4):371–376. doi: 10.1002/(SICI)1096-8628(19960202)61:4<371::AID-AJMG12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 3.Christensen K, Mitchell LE. Familial recurrence-pattern analysis of nonsyndromic isolated cleft palate--a Danish Registry study. Am J Hum Genet. 1996;58(1):182–190. [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen K, Fogh-Andersen P. Cleft lip (+/- cleft palate) in Danish twins, 1970-1990. Am J Med Genet. 1993;47(6):910–916. doi: 10.1002/ajmg.1320470620. [DOI] [PubMed] [Google Scholar]
- 5.Christensen K, Fogh-Andersen P. Isolated cleft palate in Danish multiple births, 1970-1990. Cleft Palate Craniofac J. 1993;30(5):469–474. doi: 10.1597/1545-1569_1993_030_0469_icpidm_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 6.Little J, Cardy A, Munger RG. Tobacco smoking and oral clefts: a meta-analysis. Bull World Health Organ. 2004;82(3):213–218. [PMC free article] [PubMed] [Google Scholar]
- 7.Vieira AR. Unraveling human cleft lip and palate research. J Dent Res. 2008;87(2):119–125. doi: 10.1177/154405910808700202. [DOI] [PubMed] [Google Scholar]
- 8.Hay S, Wehrung DA. Congenital malformations in twins. Am J Hum Genet. 1970;22(6):662–678. [PMC free article] [PubMed] [Google Scholar]
- 9.Myrianthopoulos NC. Congenital malformations in twins. Acta Genet Med Gemellol (Roma) 1976;25:331–335. doi: 10.1017/s0001566000014380. [DOI] [PubMed] [Google Scholar]
- 10.Layde PM, Erickson JD, Falek A, McCarthy BJ. Congenital malformation in twins. Am J Hum Genet. 1980;32(1):69–78. [PMC free article] [PubMed] [Google Scholar]
- 11.Windham GC, Bjerkedal T. Malformations in twins and their siblings, Norway, 1967-79. Acta Genet Med Gemellol (Roma) 1984;33(1):87–95. doi: 10.1017/s0001566000007558. [DOI] [PubMed] [Google Scholar]
- 12.Little J, Nevin NC. Congenital anomalies in twins in Northern Ireland. I: Anomalies in general and specific anomalies other than neural tube defects and of the cardiovascular system, 1974-1979. Acta Genet Med Gemellol (Roma) 1989;38(1-2):1–16. doi: 10.1017/s0001566000002786. [DOI] [PubMed] [Google Scholar]
- 13.Shaw GM, Croen LA, Curry CJ. Isolated oral cleft malformations: associations with maternal and infant characteristics in a California population. Teratology. 1991;43(3):225–228. doi: 10.1002/tera.1420430306. [DOI] [PubMed] [Google Scholar]
- 14.Menegotto BG, Salzano FM. Epidemiology of oral clefts in a large South American sample. Cleft Palate Craniofac J. 1991;28(4):373–376. doi: 10.1597/1545-1569_1991_028_0373_eoocia_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 15.Doyle PE, Beral V, Botting B, Wale CJ. Congenital malformations in twins in England and Wales. J Epidemiol Community Health. 1991;45(1):43–48. doi: 10.1136/jech.45.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos-Arroyo MA. Birth defects in twins: study in a Spanish population. Acta Genet Med Gemellol (Roma) 1991;40(3-4):337–344. doi: 10.1017/s0001566000003524. [DOI] [PubMed] [Google Scholar]
- 17.Kallen B. Congenital malformations in twins: a population study. Acta Genet Med Gemellol (Roma) 1986;35(3-4):167–178. doi: 10.1017/s0001566000005687. [DOI] [PubMed] [Google Scholar]
- 18.Mastroiacovo P, Castilla EE, Arpino C, et al. Congenital malformations in twins: an international study. Am J Med Genet. 1999;83(2):117–124. doi: 10.1002/(sici)1096-8628(19990312)83:2<117::aid-ajmg7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Hay S, Wehrung DA. Twins with clefts: a descriptive statistical analysis of selected variables. Cleft Palate J. 1971;8:379–386. [PubMed] [Google Scholar]
- 20.Mitchell LE, Christensen K. Evaluation of family history data for Danish twins with nonsyndromic cleft lip with or without cleft palate. Am J Med Genet. 1997;72(1):120–121. doi: 10.1002/(sici)1096-8628(19971003)72:1<120::aid-ajmg25>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Robert E, Kallen B, Harris J. The epidemiology of orofacial clefts. 1. Some general epidemiological characteristics. J Craniofac Genet Dev Biol. 1996;16(4):234–241. [PubMed] [Google Scholar]
- 22.Nordstrom RE, Laatikainen T, Juvonen TO, Ranta RE. Cleft-twin sets in Finland 1948-1987. Cleft Palate Craniofac J. 1996;33(4):340–347. doi: 10.1597/1545-1569_1996_033_0340_ctsif_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 23.Christensen K. The 20th century Danish facial cleft population--epidemiological and genetic-epidemiological studies. Cleft Palate Craniofac J. 1999;36(2):96–104. doi: 10.1597/1545-1569_1999_036_0096_tcdfcp_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 24.Bille C, Knudsen LB, Christensen K. Changing lifestyles and oral clefts occurrence in Denmark. Cleft Palate Craniofac J. 2005;42(3):255–259. doi: 10.1597/03-139.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skytthe A, Kyvik K, Holm NV, Vaupel JW, Christensen K. The Danish Twin Registry: 127 birth cohorts of twins. Twin Res. 2002;5(5):352–357. doi: 10.1375/136905202320906084. [DOI] [PubMed] [Google Scholar]
- 26.Christensen K, Holm NV, Olsen J, Kock K, Fogh-Andersen P. Selection bias in genetic-epidemiological studies of cleft lip and palate. Am J Hum Genet. 1992;51(3):654–659. [PMC free article] [PubMed] [Google Scholar]
- 27.Christiansen L, Frederiksen H, Schousboe K, et al. Age- and sex-differences in the validity of questionnaire-based zygosity in twins. Twin Res. 2003;6(4):275–278. doi: 10.1375/136905203322296610. [DOI] [PubMed] [Google Scholar]
- 28.Shields ED, Bixler D, Fogh-Andersen P. Facial clefts in Danish twins. Cleft Palate J. 1979;16(1):1–6. [PubMed] [Google Scholar]
- 29.Statistics Denmark. [Accessed November 8, 2009]; http://www.dst.dk.
- 30.McGue M. When assessing twin concordance, use the probandwise not the pairwise rate. Schizophr Bull. 1992;18(2):171–176. doi: 10.1093/schbul/18.2.171. [DOI] [PubMed] [Google Scholar]
- 31.Grosen D, Chevrier C, Skytthe A, et al. A cohort study of recurrence patterns among more than 54,000 relatives of oral cleft cases in Denmark: support for the multifactorial threshold model of inheritance. J Med Genet. 2010;47(3):162–168. doi: 10.1136/jmg.2009.069385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. 1st edition. Dordrecht: Kluwer Academic Publisher; 1992. [Google Scholar]
- 33.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th edition. VCU Box 900126, Richmond, VA 23298: Department of Psychiatry; 2003. [Google Scholar]
- 34.Greene JC, Vermillion JR, Hay S, Gibbens SF, Kerschbaum S. Epidemiologic study of left lip and cleft palate in four states. J Am Dent Assoc. 1964;68:386–404. [PubMed] [Google Scholar]
- 35.Natsume N, Sato F, Hara K, Kawai T, Ogi N. Description of Japanese twins with cleft lip, cleft palate, or both. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):6–8. doi: 10.1016/s1079-2104(00)80004-9. [DOI] [PubMed] [Google Scholar]
- 36.Fogh-Andersen P. Inheritance of Harelip and Cleft Palate. Nyt Nordisk Forlag, Arnold Busck; Oct 7, 1942. [Google Scholar]
- 37.Schliekelman P, Slatkin M. Multiplex relative risk and estimation of the number of loci underlying an inherited disease. Am J Hum Genet. 2002;71(6):1369–1385. doi: 10.1086/344779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mansilla MA, Kimani J, Mitchell LE, et al. Discordant MZ twins with cleft lip and palate: a model for identifying genes in complex traits. Twin Res Hum Genet. 2005;8(1):39–46. doi: 10.1375/1832427053435373. [DOI] [PubMed] [Google Scholar]
- 39.Kimani JW, Shi M, Daack-Hirsch S, et al. X-chromosome inactivation patterns in monozygotic twins and sib pairs discordant for nonsyndromic cleft lip and/or palate. Am J Med Genet A. 2007;143A(24):3267–3272. doi: 10.1002/ajmg.a.32098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimani JW, Yoshiura K, Shi M, et al. Search for Genomic Alterations in Monozygotic Twins Discordant for Cleft Lip and/or Palate. Twin Res Hum Genet. 2009;12(5):462–468. doi: 10.1375/twin.12.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.