Abstract
Epidermal growth factor (EGF)-like factors [amphiregulin (AREG), betacellulin, and epiregulin] are induced by LH and activate the EGF receptor (ERBB1)/ERK1/2 pathway in granulosa cells and cumulus cells of preovulatory follicles to impact ovulation. However, the expression and roles of other ERBB family members and their ligands have not been explored in detail. Herein, we document that two transcripts of the neuregulin (Nrg1) gene are expressed in granulosa cells, and that the type III Nrg1 is induced during ovulation in an ERK1/2 and C/EBPβ-dependent manner. Western blotting shows that intact (75 kDa) and secreted (45 kDa) forms of neuregulin 1 (NRG1) are present in the ovary. NRG1 likely binds to ERBB3/ERBB2 complexes that are expressed in granulosa cells and cumulus cells. In cultured granulosa cells, NRG1 selectively stimulates the phosphorylation of AKT/PKB compared to ERK1/2. However, when granulosa cells were cultured with NRG1 and AREG, the phosphorylation of ERK1/2 was markedly enhanced as compared with that by AREG alone. Cotreatment with NRG1 and AREG also increased progesterone production. When cumulus–oocyte complexes (COCs) were cultured with both NRG1 and AREG, the matured oocytes exhibited significantly higher developmental competence as compared with that of oocytes cultured with AREG alone. Collectively, these results document that the expression of type III NRG1 is induced in granulosa cells during ovulation and that NRG1 enhances AREG-induced ERK1/2 phosphorylation in both granulosa cells and cumulus cells. The NRG1 pathway has two roles: one is to enhance AREG-induced progesterone production in granulosa cells, and the other is to regulate oocyte maturation by a cumulus cell-dependent mechanism.
Neuregulin 1 expressed in granulosa cells acts on granulosa cells and cumulus cells during ovulation process, which induces luteinization and regulates oocyte meiotic progression.
FSH acts on granulosa cells in follicles at the secondary and small antral stages to direct and ensure the development of preovulatory follicles where LH receptors (LHCGR) are expressed on granulosa cells and theca cells (1). The surge of LH then acts to induce luteinization, cumulus cell–oocyte complex (COC) expansion, oocyte maturation, and follicle rupture (1,2). During this dramatic developmental progression, the follicular endocrine environment is changed. FSH increases estradiol 17β (E2) production by induction of the Cyp19a1 gene (aromatase), whereas LH decreases Cyp19a1 expression but markedly induces genes (Cyp11a1, Star) regulating progesterone biosynthesis (1). E2 enhances FSH-mediated granulosa cell proliferation and differentiation (Lhcgr induction), whereas progesterone is essential for ovulation (3,4,5,6,7).
Recent genetic and molecular approaches have determined that FSH and LH activate multiple and specific intracellular signaling cascades (8,9,10). Our recent studies indicate that the ERK1/2 (also known as MAPK3/1) are essential mediators by which LH dictates the dramatic changes in follicular cell fate during ovulation and luteinization (11). The PI3K/PKB(AKT)/(FOXO) pathway is related to the survival of granulosa cells not only in preovulatory follicles but also in luteinized granulosa cells (12,13,14,15). Small guanine nucleotide exchange factors of the rat sarcoma virus oncogene homolog (RAS) super family are activated during ovulation, and infection of granulosa cells with adenoviral vectors encoding a dominant active form of KRAS induces the phosphorylation of both AKT/PKB and ERK1/2 in cultured mouse granulosa cells (16,17). It has been well established that growth factors, such as epidermal growth factor (EGF), activate RAS family members via the receptor tyrosine kinase-adaptor protein-dependent mechanisms (18).
The EGF receptor (EGFR, ERBB1) is one member of the EGF receptor super family that is expressed in granulosa cells, and based on specific receptor tyrosine kinase inhibitors, is known to impact oocyte maturation and granulosa cell differentiation in LH-stimulated preovulatory follicle cultures (19,20). Specifically, the EGF-like factors Amphiregulin (Areg), Betacellulin (Btc), and Epiregulin (Ereg) are transiently expressed after LH stimulation in granulosa cells and act by binding to ERBB1 expressed on both granulosa cells and cumulus cells (19,21,22). Additionally, mutant mice null for Areg and homozygous for Egfrwa2 (Areg−/− Egfrwa2/wa2) exhibited significantly reduced phosphorylation of ERBB1 in cumulus cells, impaired COC expansion and oocyte meiotic arrest at the germinal vesicle (GV) stage (20). In these mutant mice, the number of ovulated COCs were dramatically decreased (20), suggesting that the EGF-like factor–ERBB1 pathway plays an essential role in ovulation. Moreover, Downs and Chen (23) showed that the meiotic resumption of oocytes was significantly suppressed when COCs were cultured with FSH in the presence of neutralizing antibodies to ERBB1. On the other hand, when preovulatory follicles were cultured with EREG alone, the expression of ERK1/2 target genes, such as Tnfaip6, was significantly lower in both granulosa cells and cumulus cells as compared with those in follicles cultured with LH (24). Additionally, although the expression of the EGF-like factors was transiently increased after hCG stimulation in vivo, sustained activity of ERBB1 and ERK1/2 was required for the induction of cumulus expansion and oocyte meiotic resumption (25). From these reports, we hypothesized that LH not only induces the expression of known EGF-like factors but also regulates other growth factor receptor system(s) for successful ovulation, COC expansion, and the resumption of meiosis.
To analyze this hypothesis, we have focused on additional members of the ERBB family (ERBB2, ERBB3, and ERBB4) because the receptors, except for ERBB1, have not been examined in detail in granulosa cells or cumulus cells. Our results show that each ERBB family member exhibits different temporal expression and phosphorylation patterns in granulosa cells with both ERBB2 and ERBB3 exhibiting increased phosphorylation. ERBB2 has no ligand binding site but forms a potent signaling complex when dimerized with another ERBB receptor (26). ERBB3 has a ligand binding site but lacks receptor tyrosine (Tyr) kinase activity, and therefore must heterodimerize with another ERBB receptor, mainly ERBB2 to form an active receptor complex (27,28,29). Furthermore, we identified ovarian neuregulin 1 (NRG1) types I and III that are potential ligands for the ERBB3 receptor, analyzed the promoter regions of the Nrg1 gene that are expressed in granulosa cells, and determined the interactions of NRG1 and amphiregulin (AREG) in regulating granulosa cell and cumulus cell functions in culture.
Results
Temporal changes in expression of ERBB family members in granulosa cells and cumulus cells
To analyze the expression of ERBB family members at the protein level, ovaries were recovered from 23-day-old immature (im) mice before and 48 h after treatment with equine gonadotropin (eCG) or eCG followed by human chorionic gonadotropin (hCG) for 48 h and processed for Western blotting. As shown in Fig. 1A, ERBB1, ERBB2 and ERBB3 were expressed constitutively in the ovary during follicular development and ovulation. However, the levels of ERBB4 significantly declined after hCG stimulation. ERBB1 is known to be expressed in both cumulus cells and granulosa cells and is activated by LH in preovulatory follicles (19). Because there is less information about ERBB2 and ERBB3 in ovary, in this study we focused on the latter growth factor receptors. The immunoreactive bands for ERBB2 and ERBB3 were detected in both cumulus cells and granulosa cells, but not in oocytes (Fig. 1B). To examine when ERBB2 and ERBB3 were phosphorylated and formed complex, immunoprecipitation analyses were done using both ERBB3- and ERBB2-specific antibodies. As shown in Fig. 1C, the tyrosine phosphorylated bands were detected at 2–16 h post-hCG stimulation in vivo. The complexes of ERBB2 and ERBB3 were also detected in these samples, but not when control IgG was used or immunoprecipitation. Thus, the ERBB2 and ERBB3 complexes were phosphorylated during ovulation process. On the other hand, the phospho-ERBB1 band was strongly detected at 2 h after hCG (Fig. 2D). We also immunoprecipitated the tyrosine phosphorylated proteins in ovary using anti–Phospho-Tyr antibody and then determined the levels of phosphorylated ERBB4. ERBB4 phosphorylation decreased progressively after eCG and hCG stimulation in a manner similar to that of total ERBB4 (Supplemental Figure S1, published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
Figure 1.
The expression and activation of ERBB family members during follicular development and ovulation in eCG and hCG treated mice. A, Protein levels of ERBB family members in the mouse ovary were determined by Western blot analyses. The intensity of the bands was analyzed using a Gel-Pro Analyzer (Media Cybernetics). Values are mean ± SEM of 3 replicates. *, Significant differences were observed by hormone treatment as compared with those in immature ovary (without any priming) (P < 0.05). im, immature mice; eCG, 48 h after eCG priming; hCG, eCG priming followed 48 h later with hCG. B, ERBB2 and ERBB3 expression was analyzed in the oocytes (O), cumulus cells (CC), or granulosa cells (GC) recovered from eCG-treated mice. C, The phosphorylation status and interactions of ERBB2 and ERBB3 were analyzed in ovaries of eCG-hCG treated mice, the protein samples were used for immunoprecipitation (IP) study by anti-ERBB2 IgG, anti-ERBB3 IgG, or normal rabbit IgG, and then the precipitants were used for Western blotting (WB). D, The phosphorylation status of ERBB1 was analyzed in the mouse ovary. Results in each panel are representative of three separate experiments.
Figure 2.
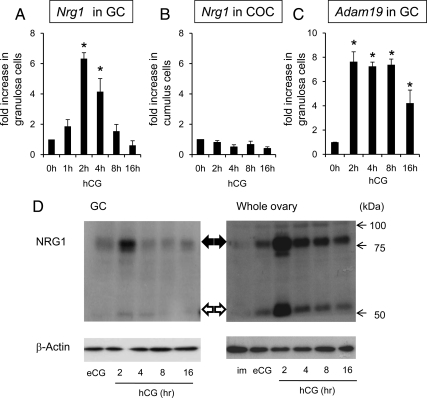
LH/hCG induces the expression of neuregulin 1 (NRG1) in the mouse ovary during ovulation. A, The temporal changes of Nrg1 mRNA expression in granulosa cells collected from ovaries of mice injected with eCG+hCG. For reference, the 0 h value was set as 1, and the data are presented as fold increase. Values are mean ± SEM of three replicates. *, Significant differences were observed as compared with those in granulosa cells recovered from eCG-primed mice (hCG 0 h), *, P < 0.05. B, The expression of Nrg1 mRNA in cumulus cells of COCs collected from ovaries of mice injected with eCG+hCG. For reference, the 0 h value was set as 1, and the data are presented as fold increase. Values are mean ± SEM of three replicates. C, The expression of Adam19 mRNA in granulosa cells. For reference, the 0 h value was set as 1, and the data are presented as fold increase. Values are mean ± SEM of three replicates. *, Significant differences were observed as compared with those in granulosa cells recovered from eCG-primed mice (hCG 0 h) (P < 0.05). D, Western blot analysis of NRG1 protein level in granulosa cells (GC) or whole ovary recovered from mice injected with eCG+hCG. im, immature mice without any injections. Results in each panel are representative of three separate experiments.
The ERBB3 ligand neuregulin1 (NRG1) is expressed in granulosa cells
It is known that ERBB2 lacks a ligand binding domain but has Tyr kinase activity, whereas ERBB3 has impaired kinase activity but binds specific ligands (26). Growth factors of the neuregulin (Nrg) super family are known ligands for ERBB3 in neuronal cells and breast cancer cells (30,31). Therefore, we analyzed the expression of the Nrg1 gene and observed increased Nrg1 expression in granulosa cells after hCG treatment in vivo (Fig. 2A). Nrg1 mRNA levels significantly increased within 2 h and were maintained at 4 h but declined progressively thereafter. By contrast, Ngr1 expression did not increase in cumulus cells before or after hCG stimulation (Fig. 2B). Growth factors undergo proteolytic cleavage at the cell surface enabling their release and activation of their receptors. ADAM19 is an enzyme known to cleave NRG1 in brain (32). In granulosa cells, Adam19 mRNA increased after hCG stimulation suggesting that it might be relevant to NRG1 processing (Fig. 2C). To analyze NRG1 processing, Western blots were done on extracts prepared from isolated granulosa cells and whole ovaries at selected times after hCG stimulation (Fig. 2D). The maximum levels of the ∼75-kDa membrane-bound form of NRG1 were observed at 2 h after hCG stimulation but persisted until 16 h. The ∼45-kDa secreted form of NRG1 was detected more strongly in whole ovary samples than in granulosa cell extracts but also persisted for 2–16 h.
Nrg1 is expressed in a promoter type specific manner in granulosa cells during follicular development and ovulation
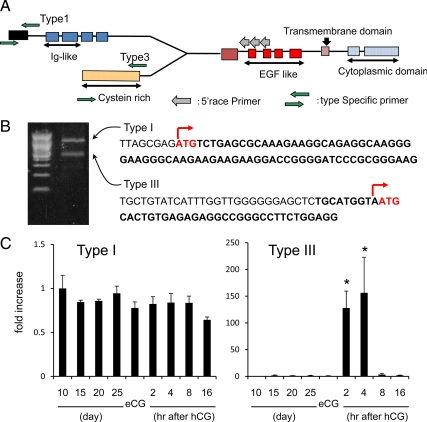
Because the mouse Nrg1 gene has three known transcriptional start sites (Fig. 3A;33), we designed a reverse primer that recognized the common region and performed 5′ rapid amplification of cDNA ends (RACE) analyses to determine which type of Nrg1 mRNA(s) were expressed in granulosa cells. As shown, two PCR products were generated by 5′RACE using mRNA extracted from granulosa cells (Fig. 3B). Sequence data obtained from the amplified products revealed that the types I and type III Nrg1 mRNAs were expressed strongly in granulosa cells 2–4 h after hCG. Using type I and III specific recognition primers, we detected the expression of each mRNA by real-time PCR. Whereas type I was not dramatically changed during follicular development and ovulation, type III mRNA was significantly increased more than 100-fold in granulosa cells after hCG as compared with levels in granulosa cells before hormone treatment (Fig. 3C).
Figure 3.
The expression of type III Nrg1 mRNA is induced selectively by LH/hCG in granulosa cells of mouse ovary during ovulation. A, The structure of mouse Nrg1 gene and the primer sets used to detect the specific types or to perform the 5′RACE analyses. B, The 5′RACE analyses show the expression of two transcripts of the Nrg1 gene in mouse granulosa cells from ovaries treated 4 h after hCG. C, The temporal changes of type I or type III mRNAs in granulosa cells. For reference, the 0 h value was set as 1, and the data are presented as fold increase. Values are mean ±SEM of three replicates. *, Significant differences were observed as compared with that in granulosa cells recovered from eCG-primed mice (hCG 0 h) (P < 0.05).
The type III Nrg1 promoter is regulated in granulosa cells
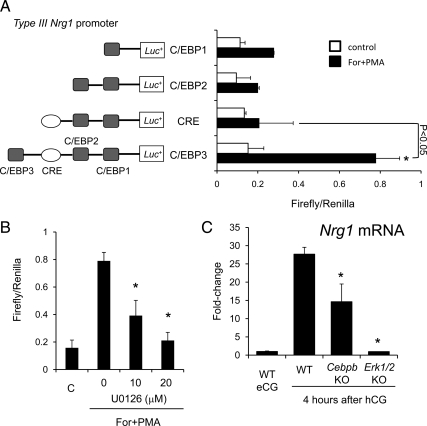
The type III Nrg1 promoter region has three predicted CCAAT enhancer binding protein (C/EBPα/β) binding sites and one CRE site. To analyze the functional activity of these regions within the type III proximal promoter of the Nrg1 gene that confers responsiveness to Forskolin+phorbol 12-myristate 13-acetate (PMA) (mimics LH stimulation for optimal induction of gene expressions in cultured granulosa cells;22), granulosa cells from eCG-primed mice were transfected with different Nrg1 type III promoter-luciferase deletion constructs and treated with the agonists (Fig. 4A). Forskolin+PMA significantly induced the activity of the type III-luciferase construct containing three C/EBP sites and the CRE site (∼4-fold; P < 0.05), whereas limited activity was observed when the most distal C/EBP site was deleted, indicating that activation in granulosa cells may be dependent on C/EBPα/β. Because C/EBPα/β are activated by ERK1/2 in granulosa cells during the ovulation process (11), the effect of the ERK1/2 pathway inhibitor, U0126, on the promoter activity was examined. Strikingly, U0126 significantly suppressed the promoter activity in a dose-dependent manner (Fig. 4B). Additionally, the expression level of type III Nrg1 mRNA in granulosa cells isolated from granulosa cell-specific Erk1/2 double knockout mice and the Cebpb knockout mice was reduced 95% and 50%, respectively, compared with that in WT mice after hCG (Fig. 4C).
Figure 4.
The type III Nrg1 promoter is activated and the type III Nrg1 mRNA is expressed in mouse granulosa cells via ERK1/2-C/EBP pathway. A, The type III Nrg1 promoter is activated in mouse granulosa cells. Mouse granulosa cells were transiently transfected with type III Nrg1 promoter-luciferase constructs and stimulated with or without forskolin+PMA (For+PMA) for 4 h. *, For+PMA treatment significantly increased promoter activities as compared with that nontreated granulosa cells (P < 0.05). The deletion of C/EBP significantly decreased the promoter activity when the granulosa cells were cultured with For+PMA (P < 0.05). Firefly luciferase activities were normalized by Renilla luciferase activities. Values are mean ± SEM of three replicates. B, Activation of the type III Nrg1 promoter by ERK1/2. Mouse granulosa cells were transiently transfected with type III Nrg1 promoter-luciferase constructs (C/EBP 3 type) and then treated with 10 or 20 μm of U0126 (U01) in the presence of For+PMA. Values are mean ± SEM of three replicates. C, The expression of Nrg1 mRNA in mouse granulosa cells recovered from wild-type mice (WT), granulosa cell–specific Erk1/2 knockout mice (Erk1/2KO), or Cebpb knockout mice (CebpbKO), WT eCG; granulosa cells were recovered from eCG-primed wild-type mice ovary. *, Significant differences were observed at 4 h after hCG injection as compared with that in granulosa cells recovered from WT mice (P < 0.05). Values are mean ± SEM of three replicates.
NRG1 activates PKB/AKT and coordinately enhances AREG activation of ERK1/2 in cultured granulosa cells
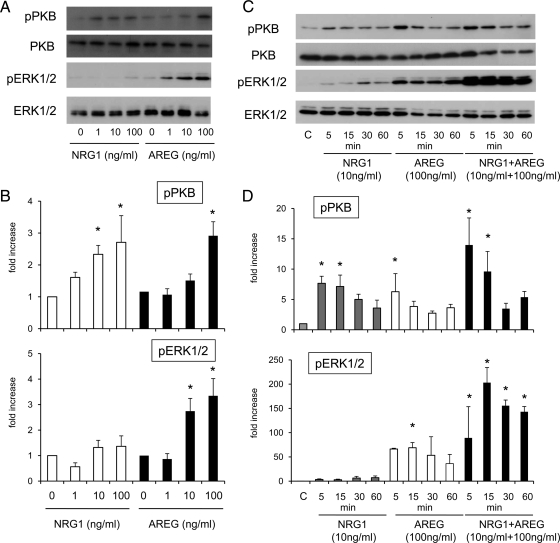
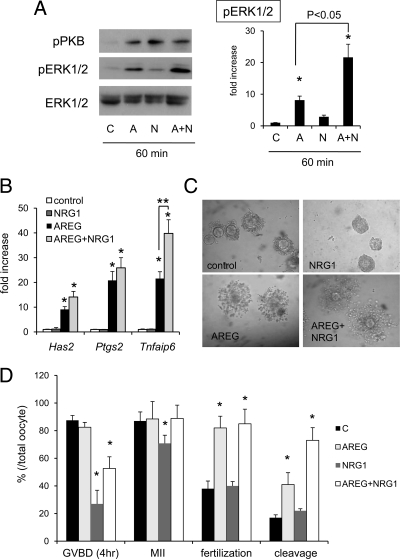
To determine the cellular signaling pathways activated by NRG1 compared with AREG, the dose-dependent effects of NRG1 and AREG alone or in combination on the phosphorylation status of ERK1/2 and PKB/AKT were analyzed. When granulosa cells were cultured with 1 to 100 ng/ml of NRG1 alone for 5 min, the phosphorylation of PKB/AKT was increased significantly in a dose-dependent manner as compared with that in control (Fig. 5, A and B). However, these same doses of NRG1 did not significantly induce the phosphorylation of ERK1/2 (Fig. 5, A and B). When the temporal effects of NRG1-induced PKB/AKT phosphorylation were analyzed, NRG1 (10 ng/ml) maintained PKB/AKT phosphorylation up to 15 min, whereas ERK1/2 phosphorylation was not significantly induced even at 60 min (Fig. 5, C and D). By contrast, AREG alone (10 or 100 ng/ml) rapidly and significantly induced the phosphorylation of ERK1/2 within 5 min (Fig. 5, A and B). However, AREG-induced phosphorylation of PKB/AKT was only detected at the 100 ng/ml dose (Fig. 5, A and B). Although the PKB/AKT phosphorylation was not changed by costimulation with NRG1 and AREG, the combinatorial treatment dramatically enhanced ERK1/2 phosphorylation as compared with that by AREG alone at 15 min (P < 0.05), and the induction was maintained up to 60 min (Fig. 5, C and D).
Figure 5.
The relative effects of AREG and/or NRG1 on ERK1/2 and AKT/PKB pathway activation in mouse granulosa cells. A and B, Dose-dependent effects of AREG or NRG1 on the phosphorylation of ERK1/2 and AKT/PKB in granulosa cells cultured for 5 min. The intensity of the bands was analyzed using a Gel-Pro Analyzer (Media Cybernetics). Values are mean ± SEM of three replicates. *, Significant differences were observed by the addition of NRG1 or AREG as compared with those in control (without any ligand) (P < 0.05). C and D, Time-dependent changes of the phosphorylation of ERK1/2 and AKT/PKB in granulosa cells cultured with NRG1, AREG, or NRG1+AREG. The intensity of the bands was analyzed using a Gel-Pro Analyzer (Media Cybernetics). Values are mean ± SEM of three replicates. *, Significant differences were observed by the addition of NRG1, AREG, or NRG1+AREG as compared with those in control (without any ligand) (P < 0.05).
The functional roles of NRG1 in AREG/LH-induced granulosa cell luteinization
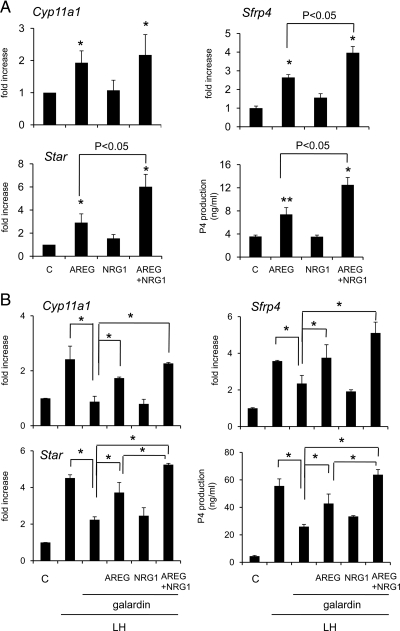
Because progesterone production is a measure of granulosa cell luteinization, conditioned media samples were collected from eCG-primed granulosa cells cultured with AREG and/or NRG1 for 8 h and then the progesterone levels were analyzed. As shown, progesterone levels in cultured eCG-primed granulosa cells were significantly increased by AREG alone but not by NRG1 (Fig. 6A). Addition of NRG1 to the AREG-containing medium enhanced the progesterone production (P < 0.05) compared with that by AREG alone (Fig. 6A). Because the expression of the Star and Cyp11a1 genes are critical for regulating progesterone biosynthesis (34), and Sfrp4 expression is a marker of granulosa cell luteinization (35), the expression levels of these mRNAs were analyzed at 4-hr culture. The results show that Star, Cyp11a1, and Sfrp4 expression, like progesterone production, was increased by AREG but not by NRG1 and that addition of NRG1 to AREG containing medium significantly increased the mRNA levels of Star and Sfrp4 (Fig. 6A).
Figure 6.
The functional roles of NRG1 in AREG/LH-induced granulosa cell luteinization. A, Progesterone production and the expression of genes involved in luteinization (Cyp11a1, Sfrp4, Star) in granulosa cells cultured with 100 ng/ml of AREG, 10 ng/ml of NRG1, or AREG+NRG1 for 4 h (RNA sample) or 8 h (progesterone assay). For reference, the control (C) value was set as 1, and the data are presented as fold increase. Values are mean ± SEM of three replicates. *, Significant differences were observed as compared with that in control (without any agonist) (P < 0.05). B, The relationship between LH-induced luteinization and the EGF-like factors, granulosa cells were cultured with LH and the broad metalloprotease inhibitor galardin (10 μm) to suppress the release of the EGF domain from EGF like factors. Additional effects of AREG (100 ng/ml), NRG1 (10 ng/ml), or both on progesterone production and the expression of genes involved in luteinization (Cyp11a1, Sfrp4, Star) in granulosa cells were analyzed. For reference, the control (C) value was set as 1, and the data are presented as fold increase. Values are mean ± SEM of three replicates. *, Significant differences were observed between the treatment groups (P < 0.05).
To determine the relationship between LH-induced luteinization and the EGF-like factors, mouse granulosa cells were cultured with LH and the broad metalloprotease inhibitor galardin (10 μm;36) to suppress the release of the EGF domain from EGF-like factors. LH-induced progesterone production and the expression of genes regulating relevant steroidogenic enzymes were significantly suppressed by the treatment with galardin (Fig. 6B). The addition of AREG alone but not NRG1 alone overcame the inhibitory effects of galardin, whereas NRG1 enhanced the effect of AREG. Thus, we conclude that both AREG and NRG1 play an important role in LH-induced luteinization of granulosa cells (Fig. 6B).
The effects of NRG1 on cumulus expansion, oocyte maturation of in vitro cultured COCs, and early embryo development
In cultured COCs, either 10 ng/ml of NRG1 or 100ng/ml of AREG induced PKB/AKT phosphorylation, whereas ERK1/2 phosphorylation was only induced by AREG (Fig. 7A). Cotreatment with AREG and NRG1 significantly enhanced ERK1/2 phosphorylation in a manner similar to that observed in granulosa cells (Fig. 7A). The expression of genes (Has2, Ptgs2, Tnfaip6) involved in cumulus expansion was significantly increased by AREG alone but not by NRG1 alone at 4-hr culture (Fig. 7B). However, costimulation of COCs with both growth factors enhanced expression of Tnfaip6 mRNA as compared with that in cumulus cells of COCs cultured with AREG alone (Fig. 7B).
Figure 7.
The role of NRG1 in cumulus cell functions and oocyte maturation in cultured COCs. A, The phosphorylation of ERK1/2 in cumulus cells of COCs cultured with 100 ng/ml of AREG (A), 10 ng/ml of NRG1 (N), or AREG+NRG1 (AN). Values are mean ± SEM of three replicates. *, Significant differences were observed by the addition of NRG1, AREG, or NRG1+AREG as compared with those in control (without any ligand) (P < 0.05). The additional NRG1 to AREG-containing medium significantly increased the phosphorylation level of ERK1/2 (P < 0.05). B, The expression of genes involved in cumulus expansion in cumulus cells of COCs, For reference, the control value was set as 1, and the data are presented as fold increase. Values are mean ± SEM of three replicates. *, Significant differences were observed as compared with that in control (without any agonist) (P < 0.05). **, The addition of NRG1 to AREG-containing medium significantly increased the expression of Tnfaip6 in cumulus cells of COCs as compared with that in COCs cultured with AREG alone (P < 0.05). C, The morphology of COCs cultured with 100 ng/ml of AREG, 10 ng/ml of NRG1, or AREG+NRG1 for 16 h. D, Oocyte nuclear and cytoplasmic maturation when COCs were cultured with 100 ng/ml of AREG, 10 ng/ml of NRG1, or AREG+NRG1. GVBD (4 h), the rate of oocytes exhibiting germinal vesicle breakdown (GVBD) at 4-hr culture, MII, the rate of oocytes reached to the metaphase II stage at 16-hr culture, fertilization, the rate of oocytes in which pronuclear formation was observed, cleavage, the rate of cleaved embryo that has at least two blastomeres. *, The percentage data were subjected to arcsine transformation before analysis. Significant differences were observed as compared with that in control (without any agonist) (P < 0.05).
When COCs were isolated and selected under 4 mm of hypoxanthine and then moved to 1 mm hypoxanthine-containing medium, oocytes resumed meiosis and progressed to the meiotic metaphase II stage spontaneously. Specifically, at 4 h of culture, the number of oocytes exhibiting germinal vesicle breakdown (GVBD) was more than 80% in control. This rate was not significantly different when COCs were cultured with AREG alone (Fig. 8D). However, the addition of NRG1 significantly suppressed spontaneous meiotic resumption of oocytes (Fig. 7D). Suppression of GVBD was also observed in oocytes of COCs cultured with AREG and NRG1 (Fig. 7D). After 16 h of culture, the number of oocytes that had entered the MII stage of meiosis was slightly but significantly lower in NRG1-treated group as compared with that in control, whereas oocytes in COCs cultured with AREG+NRG1 were in MII (Fig. 7D), suggesting that NRG1 did not suppress but delayed the progression of oocytes to the MII stage. When spontaneous meiotic resumption was suppressed by 4 mm of hypoxanthine, AREG significantly increased GVBD at 6 h and progression to the MII stage at 16 h (Supplemental Figure S2). The addition of NRG1 to AREG-containing medium suppressed GVBD at 6 h but did not prevent oocyte progression to the MII stage at 16 h (Supplemental Figure S2), suggesting that AREG accelerated meiotic progression but NRG1 delayed GVBD and MII progression during in vitro culture of COCs.
When COCs were matured under 1 mm of hypoxanthine and analyzed for competence in in vitro fertilization assays (without stripping cumulus cells), the rate of pronuclear formation was lower in control and NRG1 groups as compared with that in AREG alone or AREG and NRG1 treatment groups (Fig. 7D). However, the percent of oocytes undergoing cleavage was significantly higher in AREG+NRG1 group as compared with those in AREG alone (Fig. 7D).
Discussion
For successful ovulation and fertility, COC expansion and oocyte maturation must occur in a timely manner. Because cumulus cells express low levels of the LH receptor, factors secreted from granulosa cells are required to mediate LH action to cumulus cells (37). Park et al. (19) showed that the EGF like factors, AREG, BTC, and EREG are induced in granulosa cells by LH and act on cumulus cells to induce cumulus expansion and oocyte maturation. Specifically, these EGF-like factors bind to ERBB1 in granulosa cells and cumulus cells, leading to ERBB1 phosphorylation in vivo and in vitro and to the subsequent activation of downstream signaling cascades including activation of RAS and ERK1/2 that are critical for COC expansion, oocyte maturation, ovulation, and luteinization (11,17). In this study, we show other ERBB family members, ERBB2 and ERBB3, form complexes that are phosphorylated by hCG stimulation in vivo, suggesting that specific endogenous ligands are induced to activate ERBB2 and/or ERBB3 during ovulation.
Because ERBB2 lacks a ligand binding domain but has Tyr kinase activity, whereas ERBB3 has impaired kinase activity but binds specific ligands (26), we focused on identifying ERBB3 ligands that are expressed in granulosa cells of ovulating follicles. Of the known members of the ERBB3 ligand family that have been reported in mammary gland and neuronal cells (26), we detected strong induction of Nrg1 mRNA in granulosa cells but not in cumulus cells within 2–4 h after hCG injection. The murine Nrg1 gene has three promoter and transcriptional start sites (33), and type I and type III are expressed in granulosa cells during ovulation. Expression of type III Nrg1 was dramatically increased in granulosa cells by hCG, whereas that of type I was not changed. Type III NRG1 has a trans-membrane domain and an EGF-like domain between the trans-membrane domain and a cysteine-rich domain (38). The cysteine rich domain also functions as a trans-membrane domain (39,40), indicating that the type III NRGI protein is a loop-like structure with the EGF domain located on the outside of cells. Western blot analyses showed that at least two immunoreactive bands (about 75 kDa and 45 kDa) were detected in whole ovarian extracts after hCG stimulation. However, the small-molecular-weight band was less intense in granulosa cells. Because the full-length type III NRG1 is more than 110 kDa (41,42), the present results suggest that the 75-kDa band is a membrane bound form that has already been cleaved by ADAM19 to a straight structure that has one trans-membrane site (32) and that subsequent cleavage by an unknown enzyme releases the ∼45-kDa secreted form of NRG1. This form is presumed to include the EGF domain released by an enzyme that recognizes the N-terminal region of type III NRG1, because in this study we used an antibody that recognizes the extracellular N-terminal domain of NRG1. Wang et al. (43) reported that the candidate site for the second cleavage reaction is between Gly117 and Leu118. Gly-Leu-Gly amino acid sequence within C-terminal region of type III NRG1 is predicted to be recognized by ADAMTS4, MMP2 and MMP12 (MEROPS the Peptidase Database, http://merops.sanger.ac.uk). In granulosa cells, ADAMTS4 and MMP2 are transiently increased after hCG stimulation (44,45), whereas expression of MMP12 is undetectable during the ovulation process (44). Although further analyses are required to determine what controls the formation of secreted type III NRG1 during the ovulation process, ADAMTS4 and MMP2 are likely candidates for this processing. Because type III NRG1 is only expressed in granulosa cells but its receptor is expressed in both granulosa cells and cumulus cells, it is likely that NRG1 participates not only in an autocrine manner but also in a paracrine manner during ovulation.
The type III Nrg1 promoter region has three putative C/EBP binding sites and a CRE site (Fig. 3). Based on the transfection of specific promoter-reporter constructs in granulosa cells, the most distal C/EBP binding site appears to play a critical role in the increase of promoter activity after Forskolin+PMA (hCG) stimulation. Because we have shown recently that the ERK1/2–C/EBPβ pathway is essential for inducing cell fate decisions in granulosa cells and cumulus cells of preovulatory follicles (11), it is clear that Nrg1 expression is also dependent on this pathway. Specifically, when granulosa cells or COCs were cocultured with NRG1 and AREG, the phosphorylation of ERK1/2 was enhanced whereas NRG alone did not induce ERK1/2 phosphorylation. Furthermore, NRG1 and AREG acted synergistically to enhance the production of progesterone by granulosa cells, the expression of selected genes in cumulus cells, and the developmental competence of oocytes in cultured COCs. Therefore, NRG1 appears to enhance AREG activation of ERK1/2 phosphorylation during ovulation. The extended activation of ERK1/2 phosphorylation by these two growth factor pathways appears to be required to induce the differentiation of both granulosa cells and cumulus cells and oocyte maturation with a high developmental competence.
When NRG1 was included in the in vitro maturation medium of (COCs), spontaneous meiotic resumption of oocytes and their progression to the MII stage was delayed. Moreover, the induction of meiosis initiated by AREG was delayed by NRG1 in the presence of 4 mm of hypoxanthine. AREG induces oocyte meiosis primarily by activating ERK1/2 in cumulus cells (46). Activated phospho-ERK1/2 phosphorylates gap junction proteins, leading to gap junction closure, thereby blocking the transfer of small molecules, including cyclic GMP (cGMP) that inhibits phosphodiesterase type III (PDEIII) in the oocyte (47,48,49). Active PDEIII degrades cAMP allowing meiosis to resume (50). Although NRG1 can enhance and extend AREG-mediated phosphorylation of ERK1/2, NRG1 also activates the PI3-kinase-PKB/AKT pathway and by this pathway may mediate the delay in the resumption of meiosis and progression to the MII stage in oocytes, confirming studies in porcine oocytes (51). However, the molecular mechanisms by which NRG1 delays GVBD in oocytes remain unclear. We are currently trying to identify the genes selectively expressed in cumulus cells by NRG1 using microarray analysis.
The NRG1-mediated extension of the time during which oocytes progress to the MII resulted in oocytes that acquired a high degree of developmental competence, suggesting that critical biochemical changes in the oocyte must follow a precise temporal pattern. For example, during the meiotic maturation of oocytes, cytoplasmic maturation occurs during which protein synthesis and the localization of cytoplasmic organelles, such as cortical granules, mitochondria change dramatically (52,53), and these changes may regulate developmental competence. Specific inhibition of meiotic resumption by dibutyryl cAMP, a CDK1 inhibitor, or IBMX also enhances the cytoplasmic maturation of bovine and porcine oocytes once the inhibitors are removed from the COC cultures (54,55,56). Moreover, oocytes matured without gonadotropins exhibit low developmental competence after in vitro fertilization (57). Thus, we propose that LH induction of AREG and NRG1 leads to a balance that is important for the in vitro maturation of oocytes with high developmental competence. AREG acts an the accelerator, and NRG1 acts as a brake regulating temporal changes in the oocyte required for proper meiotic resumption and competence.
Our previous study reported that the PI3-kinase–PKB/AKT pathway is involved in the survival of cumulus cells during in vitro maturation of oocytes in porcine COCs (51). Because NRG1 has been implicated in cell survival in other tissues (58), we determined whether it might serve a survival function in granulosa cells. Indeed, when the immature granulosa cells were cultured with NRG1, the rate of apoptotic positive cells detected by TUNEL method was significantly lower than that in control cells (Supplemental Figure S3). Thus, it is possible that the expression of NRG1 may be involved in granulosa cell survival as well as oocyte meiotic arrest by stimulating the PI3-kinase–PKB/AKT pathway.
In conclusion, the present study shows that NRG1 is expressed in granulosa cells and may act as a ligand for ERBB3 present in both granulosa cells and cumulus cells but not in the oocyte. The dramatic increase in the expression of NRG1 that is associated with LH induction of ovulation and that is dependent on the ERK1/2-C/EBP pathway appears to be part of a mechanism to enhance luteinization of granulosa cells and regulate proper oocyte maturation and developmental competence. Thus, NRG1 type III is a novel factor that is expressed in ovarian granulosa cells after the LH surge and may impact luteinization and oocyte maturation.
Materials and Methods
Materials
Equine and human chorionic gonadotropins (eCG and hCG) were purchased from Asuka Seiyaku (Tokyo, Japan). Forskolin and PMA were purchased from Calbiochem (San Diago, CA); DMEM:F12 medium, penicillin-streptomycin from Invitrogen (Carlsbad, CA); fetal bovine serum (FBS) from Life Technologies Inc. (Grand Island, NY); oligonucleotide poly-(dT) from Invitrogen, and AMV reverse transcriptase, Taq polymerase from Promega (Madison, WI, USA). Routine chemicals and reagents were obtained from Nakarai Chemical Co (Osaka, Japan), or Sigma Chemical Co. (Sigma; St. Louis, MO).
Animals
Immature female C57BL/6 mice were obtained from Clea Japan (Tokyo, Japan). On d 23 of age, female mice were injected ip with 4 IU of eCG to stimulate follicular growth followed 48 h later with 5 IU hCG to stimulate ovulation and luteinization. Granulosa cell-specific Erk1/2 knockout mice (11) and granulosa cell specific Cebpb knockout mice (11) were used in selected experiments. Animals were housed under a 16-h light/8-h dark schedule in the Experiment Animal Center at Hiroshima University or the Center for Comparative Medicine at Baylor College of Medicine and provided with food and water ad libitum. Animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as approved by the Animal Care and Use Committee at Baylor College of Medicine or at Hiroshima University.
Granulosa cell culture
Granulosa cells were harvested by needle puncture from immature mice at d 23–25 of age or after the treatment with eCG for 48 h as described previously (22). Briefly, 1×106 cells were cultured in 12-well culture plates in 1% (vol/vol) serum-containing medium (DMEM:F12 containing penicillin and streptomycin). After 8 h culture, cells were washed then cultured in fresh serum-free medium containing recombinant mouse amphiregulin (AREG, R&D systems, Minneapolis, MN) or recombinant mouse neuregulin 1 (NRG1, R&D systems) or both and harvested for protein and RNA analysis.
COC isolation and culture
Ovaries of immature mice primed with eCG for 48 h contain multiple preovulatory follicles. COCs were isolated from these follicles by needle puncture and collected by pipette. Nonexpanded COCs were selected and 50 COCs were cultured in separate wells of a 96-well Falcon plate (Becton Dickinson, Franklin Lakes, NJ) in 150 μl of defined medium (22) containing 1% FBS with AREG, NRG1, or AREG+NRG1. After 4 h, total RNA or protein was extracted from the COCs (see below). The COCs cultured for 16 h were used for in vitro fertilization analyses.
In Vitro Fertilization
In vitro fertilization was analyzed as described previously (59). COCs that were matured in vitro as described above for 16 h and placed in 150 μl of human tubal fluid (HTF) medium. Spermatozoa were collected from the cauda epididymis of 4 month old ICR mice into 500 μl of HTF medium. After 60 min, the spermatozoa were introduced into the fertilization medium at a final concentration of 1000 spermatozoa/μl. Twelve hr after insemination, the oocytes were washed thoroughly five times, and then examined for formation of pronuclei under a phase-contrast microscopy. The gametes were further cultured for an additional day in the developing medium (KSOM+AA, Millipore, Billerica, MA) to check the cleavage rate.
Real-time RT-PCR analyses
Total RNA was obtained from COCs or granulosa cells using the RNAeasy Mini Kit (Qiagen Sciences, Germantown, MD) according to the manufacturer’s instructions and quantitative real-time PCR analyses were performed as previously (60). Briefly, total RNA was reverse transcribed using 500 ng poly-dT (Invitrogen) and 0.25 U avian myeloblastosis virus-reverse transcriptase (Promega) at 42 C for 75 min and 95 C for 5 min. cDNA and primers were added to 15 μl total reaction volume of the Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). PCR reactions were then performed using the iCycler thermocycler (Bio-Rad, Hercules, CA). Conditions were set to the following parameters: 10 min at 95 C followed by 45 cycles each of 15 sec at 95 C and 1 min at 62 or 64 C. Specific primers pairs were selected and analyzed as indicated in Supplemental Table S1.
Western blot analyses
Protein samples from granulosa cells or cumulus cells were prepared by homogenization in whole-cell extract buffer and then diluted by same volume of 2× SDS sample buffer. Some extracts were used for immunoprecipitation using the Immunoprecipitation kit (Roche Diagnostics GmbH, Mannheim, Germany) and 1:100 dilution of anti ErbB2 antibody (Santa Cruz Biotechnology, Santa, Cruz, CA), 1:100 of anti ErbB3 antibody (Santa Cruz Biotechnology), 1:200 of anti–phosphor-Tyr antibody (BD Biosciences, San Jose, CA) or normal rabbit IgG (Sigma). Extracts (10 μg protein) or precipitates were resolved by SDS polyacrylamide gel (7.5, 10 or 12%) electrophoresis and transferred to PVDF membranes (GE Healthcare, Piscataway, NJ). Membranes were blocked in Tris-buffered saline and Tween 20 [TBST; 10 mm Tris (pH 7.5), 150 mm NaCl and 0.05% Tween 20] containing 5% nonfat Carnation instant milk (Nestle Co., Solon, OH). Blots were incubated with primary antibody (1:2000 dilution of anti-EGFR antibody, Santa Cruz Biotechnology; 1:1000 of anti-ERBB2 antibody, Santa Cruz Biotechnology; 1:1000 of anti-ERBB3 antibody, Santa Cruz Biotechnology; 1:2000 of anti-ERBB4 antibody, Epitomics, CA; 1:2000 of anti-phospho EGFR antibody, Cell Signaling Technology, CA; 1:2000 of anti–phospho-tyrosine antibody, Cell Signaling Technology; 1:2000 of anti–neuregulin 1 antibody, LifeSpan Biosciences, WA; 1:2000 of anti-ERK1/2 antibody, Cell Signaling Technology; 1:2000 of anti–phospho-ERK1/2 antibody, Cell Signaling; 1:1000 of anti-PKB, Cell Signaling; 1:2000 of anti–phospho-PKB antibody, Cell Signaling Technology; 1:10000 of anti–β-actin antibody, Sigma) overnight at 4 C. After washing in TBST, enhanced chemiluminescence (ECL) detection was performed by using the ECL system according the manufacture’s specifications (GE Healthcare) and appropriate exposure of the blots to Fuji X-ray film (Fujifilm, Tokyo, Japan). The intensity of the bands was analyzed using a Gel-Pro Analyzer (Media Cybernetics, MD).
Transient transfection and luciferase reporter assay
Granulosa cells from eCG-primed mice were cultured as described previously (60,61). Transfections of specific promoter-reporter constructs were done 3 h after plating cells using Fugene 6 (Roche Molecular Biochemicals, Indianapolis, IN) according to the manufacturer’s instructions. Cells were transfected with 0.5 μg of the indicated Nrg1 Type III promoter-reporter constructs (shown in Fig. 4) and 10 ng of pRL Renilla luciferase control vector (Promega). After overnight culture, cells were washed in serum-free medium then placed in the same media containing agonists and/or antagonists as indicated. After 4 h of agonist treatment, the luciferase activity was measured using Dual-Glo Luciferase Assay System (Promega). Firefly luciferase activities were normalized by Renilla luciferase activities. Each experiment was performed in triplicate at least three times.
5′RACE analysis
To determine the transcriptional start site of each Nrg1 subtype, 5′ rapid amplification of cDNA ends (RACE) was done using the 5′RACE System (Invitrogen) according to the manufacturer’s instructions. One microgram of total RNA recovered from granulosa cells of ovaries 4 h after hCG stimulation was used for the synthesis of first-strand cDNA using the Nrg1 specific primer shown in Fig. 3A. The homopolymeric tail was added to the 3′-end of the cDNA, and then PCR amplification was done using the nested Nrg1-specific primer that annealed to a site located within the cDNA molecule. After amplification, the products were cloned into TOPO vector (Invitrogen) and used for sequencing analysis. The primers are shown in Supplemental Table S1.
Progesterone assay
Progesterone in the cultured medium was measured by specific AIA 1800 system (TOSOH) as described previously (62).
Statistics
Statistical analyses of all data from three or four replicates for comparison were carried out by one-way ANOVA followed by Duncan’s multiple-range test (Statview; Abacus Concepts, Inc., Berkeley, CA).
Supplementary Material
Acknowledgments
We thank Dr. M. Nishibori and Dr. N. Yanaka, Hiroshima University, for technical advice, Dr. H. Negishi, Women’s clinic Ooizumi Gakuen for supporting to analyze progesterone level. We also thank K. Tabata, M. Shitanaka, M. Enjoji, and K. Nishimatsu, Hiroshima University for technical assistance.
Footnotes
This work was supported by Grant-in-Aid for Scientific Research (No. 21688019, No.21028014, No. 21248032) from the Japan Society for the Promotion of Science (JSPS) (to M.S.), Young Research Fellowship from JSPS (No. 09J04118) (to I.K.), and National Institutes of Health (NIH)-HD-16229 and HD-07495 (Project III, Specialized Cooperative Centers Program in Reproduction and Infertility Research, SCCPIR) (to J.S.R).
Present address for H.-Y.F.: Life Science Institute, Zhejiang University, Hangzhou, 310058, China.
Disclosure Summary: The authors have nothing to declare.
Abbreviations: AREG, Amphiregulin; BTC, betacellulin; C/EBP, CCAAT enhancer binding protein; cGMP, cyclic GMP; COC, cumulus cell–oocyte complex; eCG, equine gonadotropin; ECL, enhanced chemiluminescence; EGF, epidermal growth factor; EGFR, EGF receptor; ERG, epiregulin; FBS, fetal bovine serum; GV, germinal vesicle; GVBD, germinal vesicle breakdown; hCG, human chorionic gonadotropin; im, immature; NRG, neuregulin; PDEIII, phosphodiesterase type III; PMA, phorbol 12-myristate 13-acetate; RACE, rapid amplification of cDNA ends.
First Published Online November 3, 2010
References
- Richards JS 1994 Hormonal control of gene expression in the ovary. Endocr Rev 15:725–751 [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Robker RL, Dajee M, Alliston TN 1998 Molecular mechanisms of ovulation and luteinization. Mol Cell Endocrinol 145:47–54 [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A 190:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robker RL, Richards JS 1998 Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol 12:924–940 [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS 2000 Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA 97:4689–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS 2001 New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol 15:209–218 [DOI] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Park JY, Su YQ 2006 Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol 20:715–723 [DOI] [PubMed] [Google Scholar]
- Dekel N 2009 Master regulators of female fertility. N Engl J Med 361:718–719 [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS 2009 MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS 2000 Follicle-Stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol 14:1283–1300 [DOI] [PubMed] [Google Scholar]
- Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, Woodruff TK, Unterman TG, Lee EJ, Jameson JL, Hunzicker-Dunn M 2005 Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. J Biol Chem 280:9135–9148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Cahill N, Richards JS 2008 Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol 22:2128–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam H, Weck J, Maizels E, Park Y, Lee EJ, Ashcroft M, Hunzicker-Dunn M 2009 Role of the phosphatidylinositol-3-kinase and extracellular regulated kinase pathways in the induction of hypoxia-inducible factor (HIF)-1 activity and the HIF-1 target vascular endothelial growth factor in ovarian granulosa cells in response to follicle-stimulating hormone. Endocrinology 150:915–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS 2008 Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development 135:2127–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Richards JS 2010 Minirevew: physiological and pathological actions of RAS in the ovary. Mol Endocrinol 24:286–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Yao S, Lin YZ 1996 Controlling epidermal growth factor (EGF)-stimulated Ras activation in intact cells by a cell-permeable peptide mimicking phosphorylated EGF receptor. J Biol Chem 271:27456–27461 [DOI] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M 2004 EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M 2007 Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espey LL, Richards JS 2002 Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod 67:1662–1670 [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS 2006 Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 20:1352–1365 [DOI] [PubMed] [Google Scholar]
- Downs SM, Chen J 2008 EGF-like peptides mediate FSH-induced maturation of cumulus cell-enclosed mouse oocytes. Mol Reprod Dev 75:105–114 [DOI] [PubMed] [Google Scholar]
- Motola S, Popliker M, Tsafriri A 2008 Response of follicle cells to ovulatory stimuli within the follicle and in primary culture. Mol Cell Endocrinol 282:26–31 [DOI] [PubMed] [Google Scholar]
- Reizel Y, Elbaz J, Dekel N 2010 Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol Endocrinol 24:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Yarden Y 2006 EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7:505–516 [DOI] [PubMed] [Google Scholar]
- Wallasch C, Weiss FU, Niederfellner G, Jallal B, Issing W, Ullrich A 1995 Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J 14:4267–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin BJ, Sela M, Yarden Y 1996 Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J 15:2452–2467 [PMC free article] [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Shelly M, Glathe S, Ratzkin BJ, Yarden Y 1996 Neu differentiation factor/neuregulin isoforms activate distinct receptor combinations. J Biol Chem 271:19029–19032 [DOI] [PubMed] [Google Scholar]
- Rahmatullah M, Schroering A, Rothblum K, Stahl RC, Urban B, Carey DJ 1998 Synergistic regulation of Schwann cell proliferation by heregulin and forskolin. Mol Cell Biol 18:6245–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita YA, Barff J, Luo Y, Wen D, Brankow D, Hu S, Liu N, Prigent SA, Gullick WJ, Nicolson M 1994 NDF/heregulin stimulates the phosphorylation of Her3/erbB3. FEBS Lett 349:139–143 [DOI] [PubMed] [Google Scholar]
- Shirakabe K, Wakatsuki S, Kurisaki T, Fujisawa-Sehara A 2001 Roles of Meltrin beta /ADAM19 in the processing of neuregulin. J Biol Chem 276:9352–9358 [DOI] [PubMed] [Google Scholar]
- Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE, Birchmeier C 1997 Isoform-specific expression and function of neuregulin. Development 124:3575–3586 [DOI] [PubMed] [Google Scholar]
- Murphy BD 2000 Models of luteinization. Biol Reprod 63:2–11 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Boerboom D, Shimada M, Lo Y, Parlow AF, Luhmann UF, Berger W, Richards JS 2005 Mice null for Frizzled4 (Fzd4−/−) are infertile and exhibit impaired corpora lutea formation and function. Biol Reprod 73:1135–1146 [DOI] [PubMed] [Google Scholar]
- Santiskulvong C, Rozengurt E 2003 Galardin (GM 6001), a broad-spectrum matrix metalloproteinase inhibitor, blocks bombesin- and LPA-induced EGF receptor transactivation and DNA synthesis in rat-1 cells. Exp Cell Res 290:437–446 [DOI] [PubMed] [Google Scholar]
- Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T 1991 Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology 129:3200–3207 [DOI] [PubMed] [Google Scholar]
- Falls DL 2003 Neuregulins: functions, forms, and signaling strategies. Exp Cell Res 284:14–30 [DOI] [PubMed] [Google Scholar]
- Esper RM, Pankonin MS, Loeb JA 2006 Neuregulins: versatile growth and differentiation factors in nervous system development and human disease. Brain Res Rev 51:161–175 [DOI] [PubMed] [Google Scholar]
- Nave KA, Salzer JL 2006 Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol 16:492–500 [DOI] [PubMed] [Google Scholar]
- Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R 2006 Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci 9:1520–1525 [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC 2008 Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci USA 105:5585–5590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Miller SJ, Falls DL 2001 The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J Biol Chem 276:2841–2851 [DOI] [PubMed] [Google Scholar]
- Curry Jr TE, Osteen KG 2003 The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 24:428–465 [DOI] [PubMed] [Google Scholar]
- Richards JS 2005 Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol 234:75–79 [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Kawashima I, Yanai Y, Nishibori M, Richards JS, Shimada M 2007 Hormone-induced expression of tumor necrosis factor alpha-converting enzyme/A disintegrin and metalloprotease-17 impacts porcine cumulus cell oocyte complex expansion and meiotic maturation via ligand activation of the epidermal growth factor receptor. Endocrinology 148:6164–6175 [DOI] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA 2008 Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 135:3229–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA 2009 Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 136:1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari S, Weeks JL 2nd, Hsieh M, Menniti FS, Conti M 2009 Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod 81:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Vaccari S, Nedachi T, Andersen CB, Kovacina KS, Roth RA, Conti M 2006 Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J 25:5716–5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Ito J, Yamashita Y, Okazaki T, Isobe N 2003 Phosphatidylinositol 3-kinase in cumulus cells is responsible for both suppression of spontaneous maturation and induction of gonadotropin-stimulated maturation of porcine oocytes. J Endocrinol 179:25–34 [DOI] [PubMed] [Google Scholar]
- Brevini TA, Cillo F, Antonini S, Gandolfi F 2007 Cytoplasmic remodelling and the acquisition of developmental competence in pig oocytes. Anim Reprod Sci 98:23–38 [DOI] [PubMed] [Google Scholar]
- Ferreira EM, Vireque AA, Adona PR, Meirelles FV, Ferriani RA, Navarro PA 2009 Cytoplasmic maturation of bovine oocytes: structural and biochemical modifications and acquisition of developmental competence. Theriogenology 71:836–848 [DOI] [PubMed] [Google Scholar]
- Funahashi H, Cantley TC, Day BN 1997 Synchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilization. Biol Reprod 57:49–53 [DOI] [PubMed] [Google Scholar]
- Shimada M, Nishibori M, Isobe N, Kawano N, Terada T 2003 Luteinizing hormone receptor formation in cumulus cells surrounding porcine oocytes and its role during meiotic maturation of porcine oocytes. Biol Reprod 68:1142–1149 [DOI] [PubMed] [Google Scholar]
- Lonergan P, Faerge I, Hyttel PM, Boland M, Fair T 2003 Ultrastructural modifications in bovine oocytes maintained in meiotic arrest in vitro using roscovitine or butyrolactone. Mol Reprod Dev 64:369–378 [DOI] [PubMed] [Google Scholar]
- Cross PC, Brinster RL 1970 In vitro development of mouse oocytes. Biol Reprod 3:298–307 [DOI] [PubMed] [Google Scholar]
- Lawlor MA, Alessi DR 2001 PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114:2903–2910 [DOI] [PubMed] [Google Scholar]
- Shimada M, Yanai Y, Okazaki T, Noma N, Kawashima I, Mori T, Richards JS 2008 Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development 135:2001–2011 [DOI] [PubMed] [Google Scholar]
- Shimada M, Yanai Y, Okazaki T, Yamashita Y, Sriraman V, Wilson MC, Richards JS 2007 Synaptosomal-associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol 21:2487–2502 [DOI] [PubMed] [Google Scholar]
- Doyle KM, Russell DL, Sriraman V, Richards JS 2004 Coordinate transcription of the ADAMTS-1 gene by luteinizing hormone and progesterone receptor. Mol Endocrinol 18:2463–2478 [DOI] [PubMed] [Google Scholar]
- Sato C, Shimada M, Mori T, Kumasako Y, Otsu E, Watanabe H, Utsunomiya T 2007 Assessment of human oocyte developmental competence by cumulus cell morphology and circulating hormone profile. Reprod Biomed Online 14:49–56 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.