Abstract
During male development, the testes move from a high intraabdominal position and descend into the scrotum. The gubernaculum, an inguinoscrotal ligament connecting the testis to the lower abdomen, is believed to play a critical role in this process. The first stage of testicular descent is controlled by insulin like3 hormone (INSL3), produced in testicular Leydig cells. Deletion of Insl3 or its receptor, Rxfp2, in mice causes cryptorchidism. We produced Cre/loxP regulated shRNA transgenic mice targeting RXFP2 expression. We have shown that the transgene was able to reduce Rxfp2 gene expression and thus behaved as a hypomorphic allele of Rxfp2. Variable degrees of uni- and bilateral cryptorchidism was detected in males with the activated shRNA transgene on an Rxfp2+/− background. Conditional suppression of Rxfp2 in the gubernaculum led to cryptorchidism. Gene expression analysis of a mutant cremasteric sac using Illumina microarrays indicated abnormal expression of a significant number of genes in Wnt/β-catenin and Notch pathways. We have demonstrated profound changes in the expression pattern of β-catenin, Notch1, desmin, and androgen receptor (AR), in Rxfp2−/− male embryos, indicating the role of INSL3 in proliferation, differentiation, and survival of specific cellular components of the gubernaculum. We have shown that INSL3/RXFP2 signaling is essential for myogenic differentiation and maintenance of AR-positive cells in the gubernaculum. Males with the deletion of β-catenin or Notch1 in the gubernacular ligament demonstrated abnormal development. Our data indicates that β-catenin and Notch pathways are potential targets of INSL3 signaling during gubernacular development.
Expression data and mouse transgenic experiments reveal an involvement of Notch1 and β-catenin in INSL3 signaling in gubernaculum.
The majority of Boreoeutherian land mammals have a sexually dimorphic position of the gonads. The male gonads are located in the scrotum, outside of the abdominal cavity. While different hypotheses exist to explain the evolutionary origin and functional significance of such a dimorphism, experimental data demonstrate that an external testis position is essential to provide a proper temperature environment for efficient spermatogenesis and sperm production (1,2). The externalization of testes occurs through a developmental process called testicular descent and in humans is usually finalized by birth. Cryptorchidism, the absence of one or both testicles from the scrotum, is in most cases caused by abnormal testis position. With an estimated frequency of 2–4% in full-term newborn boys, cryptorchidism is one of the most frequent congenital abnormalities in humans. Approximately half of the cases presenting this defect are spontaneously resolved within the first year. However, if left untreated, cryptorchidism can lead to different degrees of infertility and increase the risk of testicular cancer (2,3,4,5).
During embryonic development, both male and female gonads are differentiated from the genital ridges. After completion of sex determination, both gonads remain in a high pararenal position, attached to the body walls by a mesenterial ligamentous complex believed to be derived from the mesonephric mesenchyme (2,6). At this stage, two major ligaments connect the gonads to the abdominal wall. The cranial mesonephric ligament develops from the mesentery inserted between the border of the mesonephros and gonad, near the hydatid region, connecting it with the posterior abdominal wall (6). The caudal genitoinguinal ligament or the gubernaculum Hunteri, connects differentiating genital ducts and later the cauda epididymis with the lower abdominal wall at the future position of the inner inguinal ring. The development and reorganization of these two ligaments, differentiation of the epididymis, the growth and orientation of the gonads and reproductive tracts, and intraabdominal pressure direct the movement of the testis to the scrotum. The two-stage model of testicular descent proposed by Hutson et al. (2) distinguishes the transabdominal phase, characterized by the descent of the testis into the lower abdominal position, and the inguinoscrotal phase, during which the testis moves through the inguinal canal and into the scrotum. During the transabdominal phase, the gubernacular cord and bulb are formed, followed by the differentiation of muscle layers around the bulb. Further differentiation of the caudal part of the gubernaculum involves the enlargement of the bulb (swelling reaction), caused by active cell proliferation and deposition of hyaluronic acid. In humans, the first stage of testicular descent occurs between 10–15 weeks of gestation; in mice, it begins at embryonic d 14.5 (E14.5) with the testes moving to the inguinal region at birth (2). The gubernaculum then undergoes an inversion to become the tunica of the sac-like processus vaginalis peritonei. The components of the fetal gubernaculum further develop to become the wall of a cremasteric sac, with the testes gliding along the formed inguinal canal and into the scrotum (7).
Mice deficient for the insulin-like 3 (INSL3) peptide have high intraabdominal chryptorchidism (8,9). INSL3 is produced in testicular Leydig cells, beginning at E13.5, thus, coinciding with testis descent (10,11). INSL3 signals through the G protein–coupled receptor, Relaxin family peptide receptor 2 (RXFP2); genetic ablation of the Rxfp2 gene causes the same cryptorchid phenotype as in Insl3−/− males (12,13). Previously, we have established that RXFP2 is the only receptor for INSL3 in vivo (14). RXFP2 is highly expressed in the embryonic gubernaculum and cremaster muscles (15,16). In Insl3−/− or Rxfp2−/− males, the gubernacula do not differentiate and their remnants can be detected as thin, thread-like structures lacking the muscle components (17). Transgenic overexpression of INSL3 in females causes gubernaculum differentiation and movement of the ovaries into a low abdominal position (18,19), indicating that INSL3/RXFP2 signaling alone is sufficient for the first phase of testicular descent. The androgen receptor (AR)–deficient males exhibit a low abdominal gonad position, demonstrating that androgen signaling is not required in the first stages of testis descent (5). It is not clear, however, what the effects of the INSL3 hormone are on the differentiation and/or proliferation of different cellular components of the gubernaculum in vivo, as well as what the local cellular pathways activated by INSL3/RXFP2 are.
To answer these questions, we produced a transgenic mouse with Cre/loxP regulated expression of shRNA targeting Rxfp2 that allowed us to partially reduce the expression of the INSL3 receptor in vivo. We used these mice to knock-down Rxfp2 in the gubernaculum, which resulted in cryptorchidism. Total gene expression analysis of the mutant cremasteric sac identified a number of genes with altered expression. Of those, numerous genes were involved in the Wnt/β-catenin and Notch signaling pathways. We then demonstrated that the expression pattern of some of these genes is severely distorted in Rxfp2−/− embryos during transabdominal testicular descent. We found that the deletion of β-catenin and Notch1 in a mouse gubernaculum compromised its differentiation, suggesting that these two genes are involved in mediating INSL3 signaling.
Results
Suppression of Rxfp2 expression using Tg(shRNA) transgene
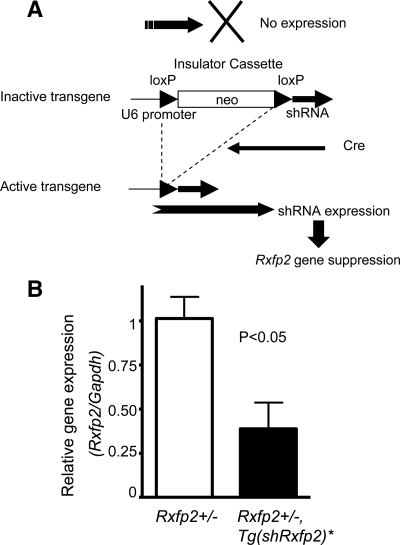
The transabdominal stage of testicular descent occurs during E14.5–15.5 (20). Complete arrest of the mutant gubernacular ligament differentiation in Insl3- or Rxfp2-deficient males makes it difficult to perform a comprehensive analysis of INSL3 signaling. Therefore, we decided to produce a new mutant with only partially reduced expression of Rxfp2. We produced a novel mouse line with an shRNA transgene targeting the Rxfp2 gene (Tg(shRxfp2)). To modulate the expression of shRNA, we used a previously described vector containing a strong U6 promoter separated from the shRNA sequence by a floxed neomycin-resistant cassette (21). Such an insulator prevents the expression of shRNA in a nonrecombined allele. In the presence of Cre recombinase, the insulator cassette is removed, providing a continuous expression of the shRNA transgene (Fig. 1A). First, we selected the most efficient siRNA. The siRNAs were designed to different regions of a cDNA sequence and selected against a mouse genomic sequence to be specific for Rxfp2. We then tested siRNAs using cotransfection with a psiCheck2 system (see Supplemental Fig. 1, published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). The best construct was used to design the shRNA transgene. Three independent mutant mouse lines containing the transgene were established and used in subsequent experiments. In most cases, they behaved similarly, and thus the data was combined.
Figure 1.
Cre/loxP-mediated shRNA targeting of Rxfp2. A, The transgene contains a floxed neo-cassette (insulator cassette) separating the U6 promoter from the shRNA coding sequence. In the presence of Cre recombinase (second transgene), the insulator cassette is removed, providing expression of shRNA and suppression of the target gene (Rxfp2). B, Suppression of Rxfp2 gene expression measured by qRT-PCR in cremasteric sac RNA isolated from four control (Rxfp2+/−) males and four males with activated shRNA transgene (Tg(shRxfp2)*, Rxfp2+/−). The results are expressed as mean ± sem. The difference is statistically significant (P < 0.05).
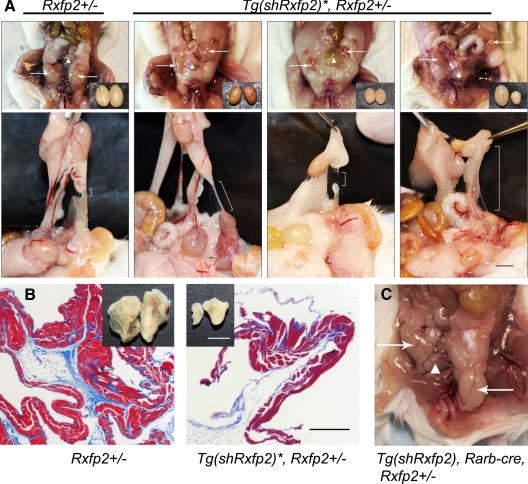
First, we crossed Tg(shRxfp2) transgenics with Hprt-cre mice to produce mice with the activated shRNA transgene in all tissues. Hprt-cre has been previously reported to be a ubiquitously expressed transgene (22). In our experiments, PCR analysis of ear DNA in Tg(shRxfp2), Hprt-cre mice revealed the presence of nonrecombined copies of the Tg(shRxfp2) transgene, possibly due to a mosaic nature of Cre expression. Analysis of the male gonadal phenotype did not show any testicular maldescent. Hence, the activation of the transgene and subsequent down-regulation of Rxfp2 expression was not enough to produce a mutant phenotype. We then crossed Tg(shRxfp2)/+, Hprt-cre/+ males with Rxfp2−/− females to make animals with one functional allele of the Rxfp2 gene and an activated copy of the Tg(shRxfp2) transgene, Tg(shRxfp2)*. About 40% of such males had a variable degree of testicular maldescent at 1 month of age (six of 16 mice) and at 3–4 months (16 of 38 mice) (Fig. 2), demonstrating that by 30 d, the position of the testes in mice is fully established. The mutants demonstrated both bi- and unilateral cryptorchidism, with the testes located at different positions between the caudal pole of the kidney and inguinal ring, located laterally to the bladder. None of the cryptorchid males had gonads in a high intraabdominal position as compared with males with a complete deletion of INSL3 or RXFP2. At 1 month of age, the average size of the testes in Tg(shRxfp2)*, Rxfp2+/− males was slightly smaller than that of the wild-type control (99.8±4.6 mg vs. 116.9±3.5 mg, P < 0.007). The size of the testes was visibly smaller for the gonads located at a higher position (Fig. 2A).
Figure 2.
Cryptorchidism in mice with the activated Tg(shRxfp2) transgene. A, Undescended testes in the Tg(shRxfp2)*, Rxfp2+/− males with the activated shRNA transgene. The position of the testes is indicated by the arrows relative to the bladder (arrowhead). The littermate Rxfp2+/− males had a wild-type phenotype (left). All males are 3 months old. Note the decreased size of the cryptorchid testes (insets). The brackets on the lower panel show the short gonadal ligaments of a wild-type male and the thin elongated gonadal ligaments in cryptorchid animals. Scale bar, 5 mm. B, The size of the cremasteric sac isolated from phenotypically wild-type (Rxfp2+/−) males and the males with the activated shRNA transgene (Tg(shRxfp2)*, Rxfp2+/−). The lower panel shows reduced muscle (red) and collagen (blue) content in a cryptorchid cremasteric sac after Masson’s trichrome staining. Scale bar, 200 μm. C, The undescended right testis of a male with a conditional activation of the Tg(shRxfp2) transgene in the gubernaculum (Tg(shRxfp2), Rarb-cre, Rxfp2+/−). Position of the testes is indicated with arrows; the bladder is marked by an arrowhead.
As shown in Fig. 2 cryptorchid males have a poorly developed cremasteric sac with a long thread-like gonadal ligament connected to the lower cauda epididymis. In wild-type males, the testicles were movable through the inguinal ring; in mutant animals, the cryptorchid testis did not pass into the scrotum. The gonadal ligament, the derivative of the embryonic gubernacular cord, remained stretched, several times longer than the rudimentary gonadal ligament in wild-type male siblings (Fig. 2A). At the histological level, the mutant cremasteric sac was noticeably less developed, containing fewer striated muscle and connective tissue sheaths with increased extracellularity and decreased amounts of collagen (Fig. 2B).
The expression of the Rxfp2 gene in the cremasteric sac was reduced by 61% in mutant Tg(shRxfp2)*, Rxfp2+/− compared with the Rxfp2+/− males as revealed by qRT-PCR (Fig. 1B). No other visible abnormalities were detected in these mice, although the total weight of the mice was slightly lower than that of the controls. Thus, the shRNA transgene behaved as a hypomorphic allele, decreasing but not completely ablating INSL3 signaling. The progeny resulting from the intercrossing of Tg(shRxfp2)/+, Hprt-cre animals yielded several males with a cryptorchid phenotype. It is possible that such mice had two copies of the activated shRNA transgene, which was sufficient to knock down RXFP2 expression and cause abnormal gubernacular development.
Targeted reduction of Rxfp2 expression in gubernacular ligament leads to cryptorchidism
We have previously shown that the strongest Rxfp2 expression in developing embryos during testicular descent was found in the gubernaculum, suggesting that this ligament may be crucial for proper movement of the testis. The ability to conditionally activate the expression of shRNA in our transgenic construct and thus reduce the expression of the INSL3 receptor in specific tissues, allowed us to test this assumption. The Cre transgene driven by retinoic acid receptor beta (Rarb-cre) was shown to be expressed in the mesonephric mesenchyme and in its derivatives, including the gubernacular anlage (23). While not uniquely specific for gubernaculum or different gubernacular cells, this construct allowed us to significantly restrict shRNA suppression of the Rxfp2 gene. As determined by the expression of the reporter gene in the Rarb-cre/ ROSA26 mice, the transgene was expressed in gubernacular striated muscle cells, stromal fibroblasts, and in epithelial cells (Supplemental Fig. 2). We produced males with a Tg(shRxfp2), Rarb-cre, Rxfp2+/− genotype. One of six males containing all three mutations manifested cryptorchidism (Fig. 2C); the wild-type phenotype was detected in littermate males with other combinations of mutant alleles/transgenes. The cryptorchid testis was located on the right side, ventral lateral to the bladder position and connected to the body wall by a peritoneal mesentery fold. The gonadal ligament on the cryptorchid side was much longer than that of the left side. The PCR analysis of genomic DNA isolated from the cryptorchid gubernaculum showed an excision of the neo-cassette in the shRNA transgene. No recombination was detected in two analyzed noncryptorchid gubernacula with the same genotype. The qRT-PCR analysis of Rxfp2 expression in cryptorchid in noncryptorchid Tg(shRxfp2), Rarb-cre, Rxfp2+/−, and in control Tg(shRxfp2), Rxfp2+/− cremasteric sac showed that the Rxfp2 expression was reduced only in the cryptorchid gubernaculum (Supplemental Fig. 3), suggesting to some extent a mosaic expression of the Rarb-cre transgene. Thus, the suppression of Rxfp2 in the gubernaculum caused abnormal testis descent, the result consistent with the mutant phenotype in males with a complete deletion of Rxfp2.
Gene expression analysis of RXFP2-deficient cremasteric sac
The INSL3 hormone produced in testicular Leydig cells acts through its RXFP2 receptor, causing gubernacular development. The cellular pathways activated by INSL3 in gubernacular cells remain unknown. To obtain a global view of the genes misexpressed in cryptorchid RXFP2-deficient gubernacula, we performed a genome-wide expression analysis. We used heterozygous Rxfp2+/− cryptorchid males with an activated Tg(shRxfp2)* transgene and compared them to phenotypically wild-type Rxfp2+/− males. The animals were produced in crosses of Hprt-cre, Tg(shRxfp2) males and Rxfp2−/− females. The cremasteric sac was isolated from three one-month-old cryptorchid and three control males derived from the same litters. As shown above (Fig. 1B) we detected a reduced expression of Rxfp2 in the RNA samples isolated from mutant tissues. The analysis of global gene expression was performed on Illumina’s Mouse WG-6 v2.0 Expression BeadChip.
Of all the misexpressed genes, there were a total of 131 genes for which a more than twofold difference (P < 0.01) in the expression level was detected, including Rxfp2. The full list of misexpressed genes can be found in Supplemental Table 1; the representative genes are shown in Table 1. Functional annotation of the top genes differentially expressed in wild-type vs. cryptorchid tissues using Ingenuity IPA 8.5 Pathway Analysis software identified Wnt/β-catenin signaling (P value for enrichment = 8.3 × 10−5) and Notch signaling (P = 2.4 × 10−2) pathways as the top functional categories. Among others, Cdh5, Lrp1, Lrp5, Fzd7, Sox6, Sox7, Sox17, and Sox18 from the Wnt/β-catenin pathway and Notch1 and Notch4 from the Notch pathway were significantly down-regulated in the mutant cremasteric sac.
Table 1.
Representative genes down-regulated in Tg(shRxfp2) mouse gubernaculum
| Symbol | Fold change (mutant/control) | P value |
|---|---|---|
| Amhr2 | 0.46 | 0.003 |
| Cdh5 | 0.49 | 0.008 |
| Dhh | 0.43 | 0.002 |
| Fzd7 | 0.71 | <0.001 |
| Hr | 0.67 | 0.001 |
| Inhbb | 0.46 | 0.001 |
| Lrp1 | 0.44 | 0.002 |
| Lrp5 | 0.34 | 0.005 |
| Notch1 | 0.37 | 0.003 |
| Notch4 | 0.44 | <0.001 |
| Rxfp2 | 0.32 | 0.001 |
| Sox7 | 0.45 | <0.001 |
| Sox17 | 0.27 | <0.001 |
| Sox18 | 0.33 | <0.001 |
Remarkably, the analysis of the genes with reduced expression in the mutant cremasteric sac also included a number of genes previously shown to participate in sex determination, testis development, or testicular descent, indicating a broad regulatory role of INSL3 signaling (Table 1).
INSL3 /RXFP2 signaling controls myogenic differentiation in gubernacular ligaments
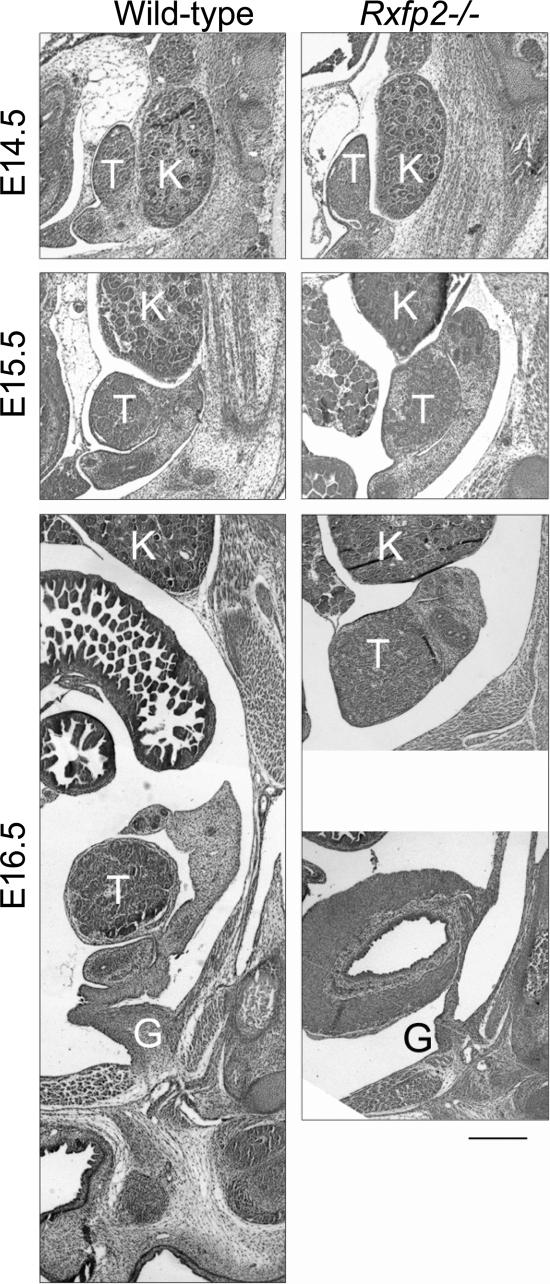
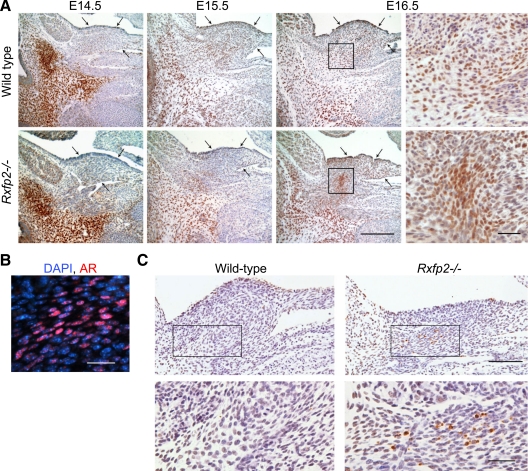
To define the critical stage of INSL3/RXFP2 action during development, we analyzed testis descent in wild-type and Rxfp2−/− male embryos. At E14.5, the cell-rich part of the gubernaculum was connected to the genital duct at the caudal side of the abdominal wall in the future inner inguinal ring and scrotum position (Fig. 3). At E15.5 the testis began to descend; during this time, the testes were still located near the caudal pole of the kidney. By E16.5, the testis moved caudally with the gubernacular cord, containing mesenchymal cells and a larger gubernacular bulb embedded into the abdominal wall. There was an increased differentiation of myogenic cells in the periphery of the gubernacular bulb. The gubernaculum contained two layers of internal oblique muscle cells positive for desmin and continuous with the adjacent ventral abdominal wall (Fig. 4A).
Figure 3.
Testicular maldescent in mice with the deletion of the Rxfp2 gene (Rxfp2−/−). Both mutant and wild-type testes (T) move from a lateral position relative to the kidney (K) on E14.5 to a more caudal location on E15.5. By E16.5, the wild-type testis is located in a low abdominal position, connected through the anlage of the cauda epididymis via a well-developed gubernaculum (G). The mutant testis remains in a high intraabdominal position next to the kidney. Note the poorly developed gubernaculum. Staining with H&E. Scale bar, 200 μm.
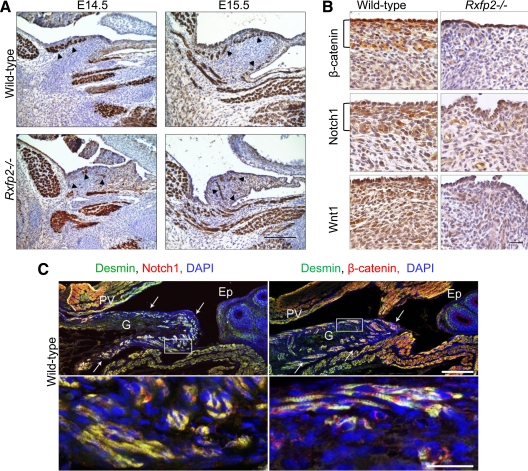
Figure 4.
Abnormal myogenesis in Rxfp2−/− gubernaculum. A, Desmin expression in the wild-type and Rxfp2−/− gubernaculum (arrowheads) revealed by immunohistochemistry. Two layers of muscle cells in the rim of the gubernacular bulb in a wild-type gubernaculum are visible at E15.5. The mutant gubernaculum contains a sharply reduced number of disorganized desmin-positive cells. Scale bar, 100 μm. B, Reduced expression of Notch1, β-catenin, and Wnt1 in the Rxfp2−/− gubernaculum revealed by immunohictochemistry. Note the intense staining for Notch1 and β-catenin in the rim of the gubernacular bulb in wild-type males (brackets). Scale bar, 20 μm. C, Coexpression (yellow) of desmin (green) and β-catenin (red) or Notch1(red) in gubernacular myoblasts revealed by confocal microscopy. Arrows indicate gubernaculum borders. Nuclei are stained with DAPI (blue). Scale bars, 50 μm (top) and 20 μm (bottom). G, gubernaculum; Ep, epididymis; PV, processus vaginalis.
The analysis of Rxfp2−/− mutants revealed that the testes remained in the E15.5 position throughout embryonic development. The gubernacular development appears to be arrested; it appeared as a loose mesenchymal cord (Fig. 4A). At E14.5 there was a clear difference in the position and organization of myoblast cells, which were located inside the cord, not forming layers and reduced in numbers. Thus, even before transabdominal descent, there were structural differences between mutant and wild-type gubernacula, the former lacking muscle enforcement.
Specification of myoblasts in mammals is regulated by a number of signals, which among others include members of the Wnt family (24). As indicated above, in the cryptorchid cremasteric sac of animals with a reduced expression of Rxfp2, a significant number of genes in Wnt/β-catenin and Notch pathways were misregulated. We have analyzed the expression of Notch1, Wnt1, and β-catenin, a key mediator of canonical Wnt signaling, in wild-type and Rxfp2−/− embryos at E15.5, before testis descent. In wild-type males, there was a clear band of Notch1 and β-catenin positive cells in the outer rim of the gubernacular bulb (Fig. 4B). The overall expression of both proteins was reduced in the mutant gubernaculum; the pattern of expression was uniform. Immunohistological analysis of Wnt1 expression also showed decreased expression in the mutant gubernacular bulb (Fig. 4B). The colocalization experiment indicated that differentiating myoblast cells positive for desmin were also stained with β-catenin and Notch1 (Fig. 4C). Strong staining for β-catenin was also detected in peritoneal surface epithelial cells covering the gubernaculum. There was high proliferation activity of epithelial cells, in both wild-type and Rxfp2-deficient gubernacula, as revealed by proliferating cell nuclear antigen staining (data not shown). The wild-type gubernaculum also showed proliferating cells in the outer rim of the gubernacular bulb, the site of desmin-positive myoblasts. In contrast, only few proliferating cells were detected in this region of the mutant gubernaculum.
Ablation of INSL3/RXFP2 signaling causes reduction of AR-positive cells in gubernaculum
Subsequently, we analyzed the effect of RXFP2 ablation on the pattern of AR expression in a developing gubernaculum. In wild-type males, the mesenchymal core of the gubernacular cord is populated by an increased number of AR-positive cells, beginning at E14.5 through E16.5 (Fig. 5A). These cells form a dense core, first at the base of the bulb and then inside this structure in parallel with the swelling reaction, increasing the size of the gubernaculum. In contrast, in Rxfp2−/− animals, the number of AR-positive cells located inside the gubernacular bulb did not increase. It appears that AR-positive cells remained at the base of the mutant bulb and do not enter into the gubernaculum. The swelling reaction was not observed in mutants. In both wild-type and mutant gubernacula, there was nuclear localization of AR (Fig. 5, A and B). We analyzed the cell proliferation and apoptosis inside the gubernacular ligament using PCNA and terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) immunochemistry. No significant differences were observed in the number of proliferating cells between the wild-type and mutant embryos (data not shown). We did detect a high level of apoptosis in the base of the gubernacular bulb in mutant males (Fig. 5C). Consequently, the absence of INSL3/RXFP2 appeared to be responsible for the reduction of AR-positive cells migration inside the gubernaculum, increased apoptosis and failure to yield a gubernacular swelling reaction.
Figure 5.
Regression of the gubernacular ligament in Rxfp2−/− males. A, Immunohistochemistry using anti-AR antibodies to detect AR-positive cells in developing gubernacular ligaments of wild-type and Rxfp2−/− male embryos. Increase of AR-positive cells (brown staining) is suppressed in a Rfxp2−/− gubernacular bulb (arrows). Scale bar, 100 μm. Right images, AR-positive cells inside a wild-type gubernacular bulb (top) and at the base of a mutant gubernaculum (bottom). Scale bar, 20 μm. B, Nuclear localization of the AR in the gubernacular bulb cells revealed by fluorescent immunohistochemistry. AR, red; nuclei are stained with DAPI (blue). Scale bar, 20 μm. C, Cell apoptosis in an E15.5 Rfxp2−/− gubernacular bulb detected by TUNEL assay. Scale bars, 50 μm (top), 20 μm (bottom).
Ablation of β-catenin leads to abnormal myogenesis in gubernacular ligament
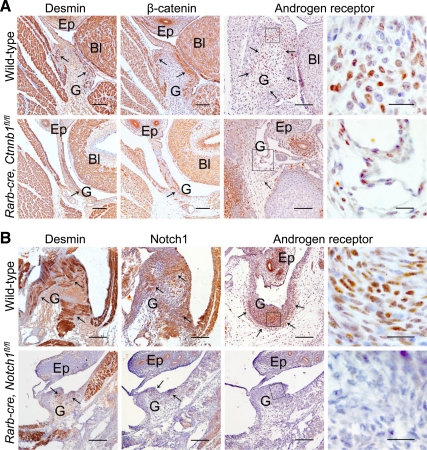
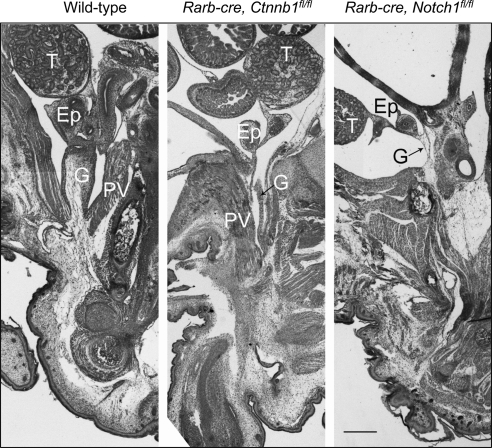
To analyze the effect of β-catenin ablation on gubernacular development, we used a conditional targeting approach. Mice with a floxed allele of β-catenin (Ctnnb1floxed) (25) were intercrossed with Rarb-cre transgenics and then backcrossed to Ctnnb1floxed/Ctnnb1floxed homozygotes. We did not find any Ctnnb1floxed/Ctnnb1floxed Rarb-cre animals among the 3-week-old progeny, with all other genotypes present at the expected ratio. Further analysis indicated that these animals die within the first several days after birth. Analysis of the gubernaculum in mutant males at E17.5 showed that it was dramatically underdeveloped (Fig. 6A). Although the testes were located in the lower abdominal position, the gubernaculum cord was significantly elongated, whereas the gubernacular bulb was completely absent. In the wild-type gubernaculum, β-catenin staining was very prominent in epithelial peritoneal cells and in layers of muscle cells, particularly in the gubernacular bulb (Fig. 6A). In mutants, no β-catenin staining was present in the gubernacular epithelium and a significantly lower number of cells were positive inside the gubernaculum cord, indicating an ablation of the β-catenin gene expression in the cells due to Rarb-cre mediated recombination. Desmin staining demonstrated the absence of striated muscles in the base of the gubernaculum, and only a few unorganized myoblasts within the gubernacular cord. Reduced cellularity at the base of gubernaculum was clearly visible, resulting in a reduced number of AR-positive cells (Fig. 6A). Significantly reduced staining for Notch1 was detected in the mutant gubernacula (data not shown). In newborn males, processus vaginalis, the outpouching of the peritoneum, was visible in both controls and mutants, although the latter had a splinter-like appearance with a narrow inguinal ring (Fig. 7).
Figure 6.
Abnormal gubernaculum development in males with the conditional inactivation of β-catenin and Notch1 genes. A, Gubernaculum development in males with the conditional inactivation of the β-catenin gene at E17.5. Note the well-developed layers of muscle cells (desmin-positive cells, arrows) in the wild-type gubernaculum and the absence of muscle layers in the rim of the gubernacular bulb (arrows) of the mutant male. The decrease of β-catenin expression in the Rarb-cre, Ctnnb1fl/fl gubernaculum indicates an efficient Cre-induced deletion of the gene. Note the poorly developed mutant gubernacular bulb. The two columns on the left show immunohistochemistry using an anti-AR antibody. Mutant animals show hypocellularity at the gubernacular bulb base (arrows) with a low number of AR-positive cells. B, Gubernaculum development in males with the conditional inactivation of the Notch1 gene at P01. Note the dramatic reduction in the gubernacular bulb size and differentiation, with the decreased immunohistochemical staining for desmin, Notch1, and AR. The arrows indicate the positively stained areas. A higher resolution image of the boxed area of the AR staining is located on the right. Scale bars, 100 μm (first three columns), 20 μm (right column). Bl, bladder; Ep, epididymis; G, gubernaculum.
Figure 7.
Abnormal processus vaginalis formation in mice with conditional inactivation of β-catenin or Notch1 gene at P01. The formation of a well-developed processus vaginalis (PV) occurs through the inversion of the gubernaculum (G) in wild-type males (left). The narrow processus vaginalis with an abnormal gubernaculum in Rarb-cre, Ctnnb1fl/fl mice and the complete absence of PV in Rarb-cre, Notch1fl/fl males. T, testis; Bl, bladder; Ep, epididymis. Scale bar, 100 μm.
Targeted deletion of Notch1 caused abnormal gubernaculum development and arrest of processus vaginalis
Using the conditional floxed allele of Noch1 (Notch1floxed) (26) and Rarb-cre, we produced animals with a deletion of Notch1 in the gubernaculum. Some variations in the degree of gubernacular development were detected between the left and right gubernacula, as well as the gubernacula from different mutant animals. At birth, the mutant gubernacula were severely underdeveloped, with dramatic hypocellularity both in the thread-like gubernacular cord and undeveloped bulb (Figs. 6B and 7). The Notch1 expression was barely detected (Fig. 6B). Dramatically reduced muscle cell development was seen in the gubernaculum. As in Rxfp2-deficient males, the Notch1-knockouts did not show processus vaginalis (Fig. 7), indicating the importance of Notch1 signaling in the development of this structure. The deletion of Notch1 dramatically affected the number of AR-positive cells within the mutant gubernacular bulb (Fig. 6B).
Discussion
Mouse gene targeting experiments have led to the identification of INSL3 and its receptor RXFP2, as the major players in the differentiation of gubernacular ligaments and the transabdominal phase of testicular descent. The completion of this phase in AR-deficient mutants and gubernaculum development in female mice with transgenic overexpression of INSL3 indicated that this hormone was not only required, but sufficient for the first step of testis descent (2,20). Until now, no characterization of the effects of INSL3/RXFP2 ablation on differentiation of specific gubernacular cells or analysis of local cell signaling pathways activated by INSL3 in vivo was performed. Such analysis was in part complicated due to the dramatic effect of INSL3/RXFP2 ablation on the gubernaculum development, preventing in-depth comparisons between mutant and wild-type tissues. To overcome this problem, we created a novel mouse mutant with an shRNA transgene that caused suppression but not complete ablation of Rxfp2 expression. Mice with the activated shRNA demonstrated different degrees of cryptorchidism along with a defective gubernaculum. Expression analysis of a mutant cremasteric sac using whole-genome expression arrays identified Wnt/β-catenin and Notch cell signaling pathways as possible INSL3 targets. Their role in gubernaculum development was further confirmed by the analysis of gene expression in Rxfp2-deficient mutants and analysis of gubernacular development in the β-catenin and Notch1-deficient mice.
The recently developed shRNA transgenic approach allowed us to attenuate, but not to eliminate, the expression of the INSL3 receptor (21). As a result, shRNA transgenic mice did develop a gubernaculum followed by a cremasteric sac. We estimated that the activated Tg(shRxfp2) transgene partly suppressed target gene expression and thus behaved as a hypomorphic allele of the Rxfp2 gene. Cryptorchidism was present in 40% of transgenic males and only on an Rxfp2+/− background, suggesting a gene dose-dependence. An additional variation in target gene suppression may be attributable to the variability in the neo-cassette excision and the activation of different transgene copy numbers, recombination between loxP sites of different transgenes, and other Cre/loxP induced rearrangements. The majority of the cryptorchid animals had testis located in a low abdominal position, suggesting that the partial inactivation of RXFP2 was not sufficient for the interruption of transabdominal testis descent. The delay in testis descent at birth was previously reported in Insl3+/− heterozygous male mice (8), however no such effect was noted in Rxfp2+/− animals (12,13). In humans, the heterozygosity for a nonfunctional T222P RXFP2 allele with a point mutation in an extracellular domain was strongly associated in some European populations with a variable degree of testis maldescent (12,27). Our data showed that the reduction of INSL3 signaling affects myogenic differentiation, maintenance, and proliferation of AR-positive cells in the gubernacular bulb and thus might interfere with the second phase of testicular descent when the inversion of the gubernacular bulb results in formation of the cremasteric sac (7). It is also possible that the inguinoscrotal descent requires INSL3 stimulation. The inhibition of gubernacular differentiation in animals treated during early postnatal period with INSL3 antagonist suggests that this might be the case (28).
Conditional Cre/loxP regulation of transgene activation allowed us to target shRNA expression in gubernacular ligaments. The expression of the Rarb-cre transgene is first detected in the embryonic mesodermic mesenchyme and then in the gubernaculum during embryonic development (23), confirming the previously suggested origin of this ligament (6). We demonstrated that in 12-d-old males, the Rarb-cre–induced recombination was present in peritoneal epithelial cells, gonadal ligament, and cremaster muscles. Cryptorchidism detected in Tg(shRxfp2), Rarb-cre, Rxfp2+/− male suggests that the gubernaculum ligament is a main target of INSL3/RXFP2 signaling during testicular descent. The low penetrance of the mutant phenotype in triple transgenics might be related to the low Cre expression level, resulting in a low recombination frequency and the absence of shRNA suppression as demonstrated in wild-type triple transgenics. Importantly, no cryptorchidism was detected in any other males with the combinations of two mutations or in any heterozygote Rxfp2/+ males from the breeding colony. It should be noted that the Rarb-cre expression pattern is not restricted to the gubernaculum and appears to some extent to be mosaic. It is possible therefore that the Rxfp2 inactivation in other organs, such as the epididymis, might affect testis descent. The more specific Cre-expressors will be required to delineate the contribution of different gubernacular cellular components in INSL3/RXFP2 signaling.
It is important to note that the Tg(shRxfp2) transgene caused a very specific developmental phenotype; we did not find any additional abnormalities in these mice. It was shown previously that the shRNAs or siRNAs may induce an interferon response in transfected cells or in transgenic animals (29,30,31). In our experiments, the RNA expression analysis performed on mutant Tg(shRxfp2) transgenic gubernacula did not reveal any up-regulation of the genes involved into interferon pathway. We conclude, therefore, that the effect of shRNA expression was specific to RXFP2 signaling.
The analysis of genes misregulated in a mutant cremasteric sac indicated a number of candidates with well-known effects on testis development and testicular descent. Mutation of the desert hedgehog (Dhh) leads to abnormal testis development and cryptorchidism (32). Males deficient for the anti-Mullerian hormone receptor 2 (Amhr2) develop as internal pseudohermaphrodites, possessing a complete male reproductive tract as well as a uterus and oviducts (33). A number of SOX genes and other important developmental regulators were also affected in mutants. The question arises then: which cell signaling pathway mediates the INSL3 effects in the gubernacular ligament during development? The analysis of microarray data revealed that a number of genes in two major cell signaling pathways, Wnt/β-catenin and Notch, were primarily affected. Indeed, we have shown that the expression pattern of Wnt1, β-catenin, and Notch1 genes were dramatically altered in a Rxfp2-deficient gubernaculum during the first transabdominal phase of testicular descent.
Recently, an importance of other members of the Wnt signaling pathway in gubernacular differentiation was reported (34). As noted above, a deletion of two secreted frizzled-related proteins (Sfrp1 and Sfrp2), the antagonists of Wnt signaling, caused widespread abnormalities of gonad and gonadal tracts morphology and failed gubernacular differentiation. Similar phenotypes were also described in mice deficient for noncanonical Wnt signaling molecules Wnt5a, wingless-related MMTV integration site 5A, or Vangl2, vang-like 2 (34). No changes in INSL3 or RXFP2 gene expression were detected in these three mutants, suggesting that the INSL3/RXFP2 may in fact be upstream of Wnt signaling. The question then arises regarding the relative significance of canonical or noncanonical Wnt pathways in gubernaculum differentiation. It should be noted that in the presence of LRP5/FZ4, Wnt5A is capable to induce a canonical pathway (35) and the complex interactions between canonical and noncanonical Wnt signaling might further obscure cellular mechanisms of action. The available data does not allow delineating the precise mechanism or possible interaction of different molecules of this signaling machinery; however, taken together, our shRNA transgenic array data, embryonic expression data and the analysis of β-catenin-deficient mice along with the published reports imply an importance of various Wnt molecules in the developing gubernaculum.
The use of Rarb-cre allowed us to overcome the early embryonic lethality in Ctnnb1 and Notch1-deficient mice and to analyze the effect of conditional, albeit not fully specific, gubernacular ablation of these two genes. Interestingly, a knock-out of Notch1 had more dramatic consequences for the development of the gubernacular ligament. Poor muscle differentiation, swelling reaction, and processus vaginalis in the gubernaculum was observed in mutants; the phenotype strikingly resembling Rxfp2−/− gubernaculum development (17).
Surprisingly, despite all deficiencies in the gubernaculum differentiation, the testes in Ctnnb1 or Notch1 mutants were located in the lower abdominal position. Two explanations can be put forward. First, it is possible that the Rarb-cre transgene had a mosaic or a low level expression in the gubernacular cord, the part of the gubernaculum that may have a crucial role in transabdominal testis descent. Indeed β-catenin–positive cells, as well as some muscle cells were detected in the Ctnnb1floxed/Ctnnb1floxed Rarb-cre gubernaculum cord at d E17.5, suggesting the absence of Cre expression in this structure. Second, it is also probable that a combination of Notch and Wnt/β-catenin pathways may be required for testis descent. We suggest that INSL3 might regulate both signaling pathways, contributing to proper gubernaculum development.
The crosstalk between Wnt/β-catenin and Notch signaling in myogenic differentiation is well-established (36). We have demonstrated that in the developing gubernaculum, β-catenin is colocalized with desmin, one of the earliest markers for muscle cells in embryogenesis. Analysis of normal gubernacular development showed the formation of two layers of internal oblique muscles positive for desmin, β-catenin, and Notch1, continuous with the adjacent ventral abdominal wall. Those were subsequently differentiated into cremaster muscles (7). Strong expression of RXFP2 in cremaster muscles (16) suggests that those cells might be the targets of Wnt and Notch signaling. Deletion of either Rxfp2, β-catenin, or Notch1 led to a dramatic suppression of myogenic differentiation in the gubernacular bulb, resulting in partial (β-catenin) or complete (Rxfp2, Notch1) failure of the cremasteric sac formation and the development of processus vaginalis (17) (Figs. 7 and 8). However, the proposed scheme of INSL3/RXFP2 action needs further confirmation. The experiments with more specific genetic targeting of the critical genes in different cellular components of the developing gubernaculum, as well as the gene rescue experiments with constitutively active forms of the RXFP2 downstream targets, might shed light on the mechanism of INSL3 signaling.
Figure 8.
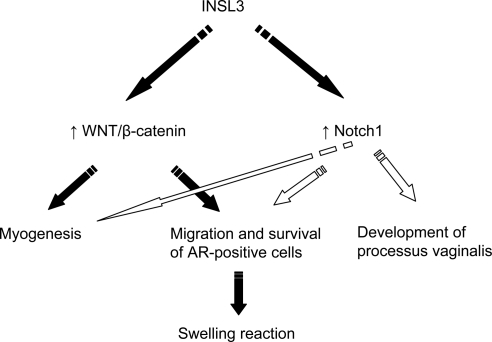
Proposed INSL3 signaling cascade for gubernacular development. The INSL3 hormone activates Wnt/β-catenin and Notch1 signaling through its receptor RXFP2. Both Wnt/β-catenin and Notch1 signaling are required for myogenic differentiation of the gubernaculum, migration, and survival of AR-positive cells in the base of the gubernacular bulb and swelling reaction. Notch1 signaling plays a major role in INSL3 induction of processus vaginalis.
The second inguinoscrotal phase of testicular descent is believed to be mediated mainly by androgens. Previously, we have shown that the treatment of neonatal gubernacular cells in vitro with INSL3 did not stimulate AR production, suggesting that the functional link between the two hormones might be indirect (37). In our present study, we have showed that in Rxfp2−/− mutants, there was a significant reduction of AR-positive cells in gubernaculum. The reduction coincided with an increased cellular apoptosis. Antiapoptotic effects of INSL3 were described in testis germ cells treated with GnRH antagonist in vitro and in vivo (38), as well as in cancer cells expressing RXFP2 (39,40). These results are also consistent with the antiapoptotic effects of Wnt and Notch signaling on other embryonic cells. Our data suggest that the role of INSL3 in gubernacular differentiation is cell-specific: on one side, INSL3 supports myogenic differentiation of cremaster muscles; on the other side, it promotes migration, proliferation, and survival of AR-positive cells inside the gubernacular bulb (Fig. 8). We hypothesize that the latter cells mediate testosterone stimuli and are primarily responsible for the gubernacular swelling reaction and rearrangement of the extracellular matrix during the second androgen-dependent stage of testicular descent (Kaftanovskaya, Feng, Agoulnik, unpublished data). Significantly, both published data and the results of our work strongly indicate the significance of cell–cell interaction between different components of the developing gubernaculum, and possible effect of endocrine and exocrine stimuli from other regions of developing urogenital tract.
In summary, our data indicate the diverse effects of INSL3/RXFP2 signaling during gubernacular development. The most significant effect of decreasing INSL3 signaling was associated with the misregulation of genes in the Wnt/β-catenin and Notch cell signaling pathways. Deletion of β-catenin and Notch1 suppressed gubernacular development, suggesting that these genes might mediate INSL3 effects on a cellular level. Further analysis of INSL3/RXFP2 signaling will be important in understanding the precise mechanisms of testicular descent and the etiology of cryptorchidism.
Materials and Methods
siRNA cell transfection experiments
Four siRNA constructs targeting mouse Rxfp2 transcripts were synthesized in MWG-Biotech AG (Huntsville, AL). For evaluation of siRNA efficiency, we used psiCheck2 reporter system (Promega, Madison, WI). Mouse Rxfp2 cDNA 2.3-kb fragment containing full open reading frame of the gene was amplified from the mouse brain RNA by RT-PCR and subcloned into PmeI sites of psiCheck2. HEK293T cells were obtained from the American Type Culture Collection (Rockville, MD) and maintained in DMEM (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cells were plated in a 96-well plate at 8000 cells per well. After cotransfection with 30 ng psiCheck2 Vector and 50 pmol of siRNA, Dual-Glo Luciferase Assay System (Promega) was used to measure Firefly and Renilla luciferase activity. The samples were analyzed in triplicates and the experiments were repeated twice.
Mice
The mice with the targeted ablation of the Rxfp2 gene were described previously (12). Rarb-cre (Tg(Rarb-cre)1Bhr) transgenic mice (23) were generously provided by Dr. Richard Behringer (University of Texas MD Anderson Cancer Center, Houston, TX). Mice with β-catenin floxed allele (Ctnnb1tm2Kem) (25), Notch1-floxed allele (Notch1tm2Rko) (26), ROSA R26R-LacZ reporter (Gtrosa26tm1Sor) (41), and Hprt-cre (Hprttm1(cre)Mnn) (22) were obtained from the Jackson Laboratory (Bar Harbor, ME). Genotyping protocols are described on the Jackson Laboratory website. The mice were maintained under standard conditions at Baylor College of Medicine (BCM) and Florida International University (FIU) animal facilities. The BCM and FIU Institutional Committees on Animal Care have approved all animal experiments described in this article; all experiments were conducted in accordance with the accepted standards of humane animal care.
shRNA transgenic mouse strain
The shRNA construct was designed based on the pBS/U6-ploxPneo vector as described (21). The complimentary oligonucleotides containing sense and antisense sequences (uppercase letters) corresponding to the most efficient siRNA#3 with hairpin sequence (lowercase letters) in between: GGAATAAAGTACATAACGACttcctgtcaGTCGTTATGTACTTTATTCCctttttg and aattcaaaaagGGAATAAAGTACATAACGACtgacaggaaGTCGTTATGTACTTTATTCC were annealed and cloned into EcoRI and blunted ApaI sites of the vector. The construct was verified by sequencing. After releasing the plasmid backbone via restriction digests with AflIII, KpnI, and PvuI, the transgene was purified by agarose gel electrophoresis. The transgene microinjection into fertilized FVB/N eggs was performed at the Transgenic Core in Baylor College of Medicine. Mice with Tg(shRxfp2) were selected by PCR from DNA isolated from ear pieces with primers specific for the transgene: U6F, 5′-CCCTTGGAGAAAAGCCTTG-3′ and T3. Three independent transgenic lines were established by backcrossing to inbred FVB/N mice (Tg(shRxfp2)3–5Aia; Tg(shRxfp2)3Aia; Tg(shRxfp2)5Aia). The U6no-loxPscreenF primer, 5′-CGCACAGACTTGTGGGAGAA-3′, and U6no-loxPscreenR primer, 5′-CACAATTACTTTACAGTTAG-3′, located in the U6 promoter outside the floxed neo-cassette were used to detect the recombinant (activated) transgene. The reverse primer PPGK, 5′-AGAGGCCACTTGTGTAGCGC-3′, from the PGK promoter of the neo-cassette was used in combination with the U6no-loxPscreenF primer to detect the non-recombined transgene.
Galactosidase staining
Rarb-cre mice were crossed with R26R to generate Rarb-cre, R26R mice. The embryos and adult organs were stained for β-galactosidase according to the previously described protocol (42). After staining, the tissues were fixed, dehydrated, embedded in paraffin, and sectioned. Tissue sections were counterstained with eosin.
Histology and immunohistochemistry
The animal organs or embryos were collected and fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned using standard protocols. For embryo collection, time-pregnant females were used. The 12 AM of the day when the vaginal plug was detected was counted as E0.5. All embryos were genotyped using tail DNA. The immunohistochemistry was performed using the following antibodies: AR (1:800, sc-816, Santa Cruz Biotechnology, Santa Cruz, CA,); β-catenin, Notch1, Wnt1 (1:1200, ab6302; 1:200, ab27526; 1:300, ab15251, respectively, Abcam, Cambridge, MA); Desmin (1:1000, D1033, Sigma-Aldrich Corp., St. Louis, MO). For negative control normal rabbit IgG or mouse IgG (Vector Laboratories, Burlingame, CA) were used at appropriate primary antibody dilutions. Detection was performed using a Vectastain ABC (avidin–biotin–peroxidase) kit (Vector Laboratories) as recommended. The color was developed with diaminobenzidine (DAB) as chromogen. Samples were counterstained with Harris Hematoxylin. Histological analysis was performed on sections stained with hematoxylin and eosin (H&E), using standard protocols. Collagen and muscle fibers of the cremasteric sac were stained using a Trichrome stain (Masson) kit (Sigma). A TUNEL assay was performed using an ApopTaq Plus peroxidase in situ Apoptosis detection kit (Millipore, Billerica, MA). Stained slides were examined with a Carl Zeiss Axio A1 Microscope, and images were captured by an AxioCam MRc5 CCD camera. For β-catenin, Notch1, AR, and Desmin immunofluorescence, an Alexa Fluor 555 goat anti-rabbit and Alexa Fluor 488 goat anti-mouse (Invitrogen, Carlsbad, CA), respectively, were used at a dilution of 1:500 for 1 h. Nuclei were counterstained with DAPI. Confocal images were captured using Zeiss LSM 510 laser scanning microscope. Images were edited with Adobe Photoshop CS.
RNA isolation and cDNA synthesis
Total RNA was isolated from cells and mouse tissues using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. cDNA was synthesized using an oligo(dT) primer and RETROscript kit (Ambion, Austin, TX).
Real-time quantitative RT-PCR
Real-time quantitative RT-PCR (QRT-PCR) was performed according to a Q-PCR SybrGreen real time protocol on an Eppendorf Mastercycler ep realplex instrument (Westbury, NY). Primers for all genes were designed from different exons; the primer sequences are available upon request. Gapdh expression was used for normalization of SybrGreen data. The relative fold change in mRNA level was calculated by the comparative Ct (2−ΔΔCt) method.
Expression microarray analysis
Gene expression profiles were analyzed using the Illumina Mouse WG-6 v2.0 Expression BeadChip platform (Illumina, San Diego, CA). Each profile represented RNA taken from three cryptorchid and three wild-type cremasteric sac samples isolated from animals derived from the same litters. The array experiments were performed in the Microarray Core Laboratory at the University of Texas Health Science Center in Houston, using standard Illumina protocols. Data was analyzed using BeadStudio software (Illumina). Ingenuity IPA 8.5 Pathway Analysis software (Ingenuity Systems, Mountain View, CA) was used to identify pathways enriched by the genes differentially expressed in wild-type vs. cryptorchid gubernacula.
Statistical analysis
Student t test and ANOVA were used to assess significance of differences among the different groups. Differences were expressed as mean ± sem with P values of < 0.05 considered as statistically significant. All analyses were performed using the GraphPad Software package (GraphPad Software, La Jolla, CA).
Supplementary Material
Acknowledgments
We thank Dr. Chuxia Deng (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland) for providing shRNA plasmids; Dr. Richard Behringer (University of Texas MD Anderson Cancer Center, Houston, Texas) for Rarb-cre mice; Dr. Franco DeMayo and Baylor College of Medicine Transgenic Core for the help with transgenic mouse production; the University of Texas Health Science Center at Houston Microarray Core Laboratory for help with microarray analysis. The authors also thank Dr. Gen Yamada (Kumamoto University, Japan) for helpful discussions and Dr. Zhen Li and Ms. Rhea Pereira for technical assistance.
Footnotes
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health Grant 7R01HD37067 (to A.I.A.).
Disclosure Summary: The authors have nothing to declare.
Abbreviations: AR, Androgen receptor; E14.5, embryonic d 14.5; Dhh, desert hedgehog; INSL3, insulin-like 3; RXFP2, relaxin family peptide receptor 2; Sfrp, secreted frizzled-related protein; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling.
First Published Online December 8, 2010
References
- Heyns CF, Hutson JM 1995 Historical review of theories on testicular descent. J Urol 153:754–767 [PubMed] [Google Scholar]
- Hutson JM, Hasthorpe S, Heyns CF 1997 Anatomical and functional aspects of testicular descent and cryptorchidism. Endocr Rev 18:259–280 [DOI] [PubMed] [Google Scholar]
- Feng S, Ferlin A, Truong A, Bathgate R, Wade JD, Corbett S, Han S, Tannour-Louet M, Lamb DJ, Foresta C, Agoulnik AI 2009 INSL3/RXFP2 signaling in testicular descent. Ann NY Acad Sci 1160:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonisch T, Fowler PA, Hombach-Klonisch S 2004 Molecular and genetic regulation of testis descent and external genitalia development. Dev Biol 270:1–18 [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Agoulnik AI 2005 INSL3/LGR8 role in testicular descent and cryptorchidism. Reprod Biomed Online 10:49–54 [DOI] [PubMed] [Google Scholar]
- Barteczko KJ, Jacob MI 2000 The testicular descent in human. Origin, development and fate of the gubernaculum Hunteri, processus vaginalis peritonei, and gonadal ligaments. Adv Anat Embryol Cell Biol 156:III-X, 1–98 [PubMed] [Google Scholar]
- van der Schoot P 1996 Towards a rational terminology in the study of the gubernaculum testis: arguments in support of the notion that the cremasteric sac should be considered the gubernaculum in postnatal rats and other mammals. J Anat 189 (Pt 1):97–108 [PMC free article] [PubMed] [Google Scholar]
- Nef S, Parada LF 1999 Cryptorchidism in mice mutant for Insl3. Nat Genet 22:295–299 [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayernia K, Holstein AF, Engel W, Adham IM 1999 Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol Endocrinol 13:681–691 [DOI] [PubMed] [Google Scholar]
- Pusch W, Balvers M, Ivell R 1996 Molecular cloning and expression of the relaxin-like factor from the mouse testis. Endocrinology 137:3009–3013 [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Schöttler P, Engel W, Adham IM 1997 Mouse Leydig insulin-like (Ley I-L) gene: structure and expression during testis and ovary development. Mol Reprod Dev 47:30–38 [DOI] [PubMed] [Google Scholar]
- Gorlov IP, Kamat A, Bogatcheva NV, Jones E, Lamb DJ, Truong A, Bishop CE, McElreavey K, Agoulnik AI 2002 Mutations of the GREAT gene cause cryptorchidism. Hum Mol Genet 11:2309–2318 [DOI] [PubMed] [Google Scholar]
- Overbeek PA, Gorlov IP, Sutherland RW, Houston JB, Harrison WR, Boettger-Tong HL, Bishop CE, Agoulnik AI 2001 A transgenic insertion causing cryptorchidism in mice. Genesis 30:26–35 [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Truong A, Feng S, Engel W, Adham IM, Agoulnik AI 2003 GREAT/LGR8 is the only receptor for insulin-like 3 peptide. Mol Endocrinol 17:2639–2646 [DOI] [PubMed] [Google Scholar]
- Agoulnik AI 2007 Relaxin and related peptides in male reproduction. Adv Exp Med Biol 612:49–64 [DOI] [PubMed] [Google Scholar]
- Feng S, Bogatcheva NV, Truong A, Korchin B, Bishop CE, Klonisch T, Agoulnik IU, Agoulnik AI 2007 Developmental expression and gene regulation of insulin-like 3 receptor RXFP2 in mouse male reproductive organs. Biol Reprod 77:671–680 [DOI] [PubMed] [Google Scholar]
- Tomiyama H, Hutson JM, Truong A, Agoulnik AI 2003 Transabdominal testicular descent is disrupted in mice with deletion of insulinlike factor 3 receptor. J Pediatr Surg 38:1793–1798 [DOI] [PubMed] [Google Scholar]
- Adham IM, Steding G, Thamm T, Büllesbach EE, Schwabe C, Paprotta I, Engel W 2002 The overexpression of the insl3 in female mice causes descent of the ovaries. Mol Endocrinol 16:244–252 [DOI] [PubMed] [Google Scholar]
- Koskimies P, Suvanto M, Nokkala E, Huhtaniemi IT, McLuskey A, Themmen AP, Poutanen M 2003 Female mice carrying a ubiquitin promoter-Insl3 transgene have descended ovaries and inguinal hernias but normal fertility. Mol Cell Endocrinol 206:159–166 [DOI] [PubMed] [Google Scholar]
- Adham IM, Agoulnik AI 2004 Insulin-like 3 signalling in testicular descent. Int J Androl 27:257–265 [DOI] [PubMed] [Google Scholar]
- Shukla V, Coumoul X, Deng CX 2007 RNAi-based conditional gene knockdown in mice using a U6 promoter driven vector. Int J Biol Sci 3:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SH, Silva FJ, Tsark WM, Mann JR 2002 A Cre/loxP-deleter transgenic line in mouse strain 129S1/SvImJ. Genesis 32:199–202 [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR 2005 Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132:2809–2823 [DOI] [PubMed] [Google Scholar]
- Cossu G, Borello U 1999 Wnt signaling and the activation of myogenesis in mammals. EMBO J 18:6867–6872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R 2001 Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128:1253–1264 [DOI] [PubMed] [Google Scholar]
- Yang X, Klein R, Tian X, Cheng HT, Kopan R, Shen J 2004 Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol 269:81–94 [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Ferlin A, Feng S, Truong A, Gianesello L, Foresta C, Agoulnik AI 2007 T222P mutation of the insulin-like 3 hormone receptor LGR8 is associated with testicular maldescent and hinders receptor expression on the cell surface membrane. Am J Physiol Endocrinol Metab 292:E138–E144 [DOI] [PubMed] [Google Scholar]
- Yuan FP, Li X, Lin J, Schwabe C, Büllesbach EE, Rao CV, Lei ZM 2010 The role of RXFP2 in mediating androgen-induced inguinoscrotal testis descent in LH receptor knockout mice. Reproduction 139:759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge AJ, Pebernard S, Ducraux A, Nicoulaz AL, Iggo R 2003 Induction of an interferon response by RNAi vectors in mammalian cells. Nat Genet 34:263–264 [DOI] [PubMed] [Google Scholar]
- Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BR 2003 Activation of the interferon system by short-interfering RNAs. Nat Cell Biol 5:834–839 [DOI] [PubMed] [Google Scholar]
- Pebernard S, Iggo RD 2004 Determinants of interferon-stimulated gene induction by RNAi vectors. Differentiation 72:103–111 [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP 1996 Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol 6:298–304 [DOI] [PubMed] [Google Scholar]
- Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, Behringer RR 1996 Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev 10:2577–2587 [DOI] [PubMed] [Google Scholar]
- Warr N, Siggers P, Bogani D, Brixey R, Pastorelli L, Yates L, Dean CH, Wells S, Satoh W, Shimono A, Greenfield A 2009 Sfrp1 and Sfrp2 are required for normal male sexual development in mice. Dev Biol 326:273–284 [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R 2006 Wnts as ligands: processing, secretion and reception. Oncogene 25:7461–7468 [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA 2008 A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2:50–59 [DOI] [PubMed] [Google Scholar]
- Feng S, Bogatcheva NV, Truong A, Engel W, Adham IM, Agoulnik AI 2006 Over expression of insulin-like 3 does not prevent cryptorchidism in GNRHR or HOXA10 deficient mice. J Urol 176:399–404 [DOI] [PubMed] [Google Scholar]
- Kawamura K, Kumagai J, Sudo S, Chun SY, Pisarska M, Morita H, Toppari J, Fu P, Wade JD, Bathgate RA, Hsueh AJ 2004 Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci USA 101:7323–7328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach-Klonisch S, Bialek J, Radestock Y, Truong A, Agoulnik AI, Fiebig B, Willing C, Weber E, Hoang-Vu C, Klonisch T 2010 INSL3 has tumor-promoting activity in thyroid cancer. Int J Cancer 127:521–531 [DOI] [PubMed] [Google Scholar]
- Klonisch T, Müller-Huesmann H, Riedel M, Kehlen A, Bialek J, Radestock Y, Holzhausen HJ, Steger K, Ludwig M, Weidner W, Hoang-Vu C, Hombach-Klonisch S 2005 INSL3 in the benign hyperplastic and neoplastic human prostate gland. Int J Oncol 27:307–315 [PubMed] [Google Scholar]
- Soriano P 1999 Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71 [DOI] [PubMed] [Google Scholar]
- Kamat AA, Feng S, Bogatcheva NV, Truong A, Bishop CE, Agoulnik AI 2004 Genetic targeting of relaxin and insulin-like factor 3 receptors in mice. Endocrinology 145:4712–4720 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.