Abstract
The glycoprotein hormone receptor hinge region is the least conserved component and the most variable in size; the TSH receptor (TSHR) being the longest (152 amino acids; residues 261–412). The TSHR is also unique among the glycoprotein hormone receptor in undergoing in vivo intramolecular cleavage into disulfide-linked A- and B-subunits with removal of an intervening ‘C-peptide’ region. Experimentally, hinge region amino acids 317–366 (50 residues) can be deleted without alteration in receptor function. However, in vivo, more than 50 amino acids are deleted during TSHR intramolecular cleavage; furthermore, the boundaries of this deleted region are ragged and poorly defined. Studies to determine the extent to which hinge region deletions can be tolerated without affecting receptor function (‘minimal hinge’) are lacking. Using as a template the functionally normal TSHR with residues 317–366 deleted, progressive downstream extension of deletions revealed residue 371 to be the limit compatible with normal TSH binding and coupling with cAMP signal transduction. Based on the foregoing downstream limit, upstream deletion from residue 307 (307–371 deletion) was also tolerated without functional alteration, as was deletion of residues 303–366. Addressing a related issue regarding the functional role of the TSHR hinge region, we observed that downstream hinge residues 377–384 contribute to coupling ligand binding with cAMP signal transduction. In summary, we report the first evaluation of TSHR function in relation to proteolytic posttranslational hinge region modifications. Deletion of TSHR hinge amino acids 303–366 (64 residues) or 307–371 (65 residues) are the maximum hinge region deletions compatible with normal TSHR function.
Deletion of TSHR hinge residues 303–366 or 307–371 are the maximum deletions compatible with normal TSHR function.
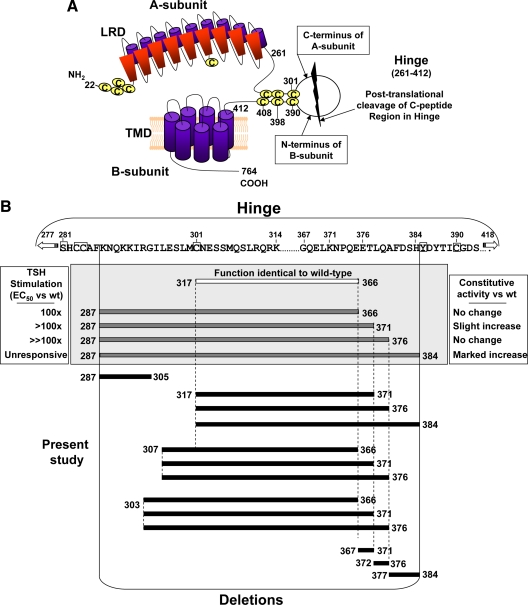
The glycoprotein hormone receptors (GPHR) are a small subgroup of G protein-coupled receptors with large (350–400 amino acid residue) extracellular domains (reviewed in Ref. 1). These ectodomains comprise a ligand binding N-terminal leucine-rich repeat domain (LRD) of approximately 260 amino acid residues (2,3) linked by a hinge region to a membrane-spanning domain (∼350 amino acid residues; Fig. 1A) that mediates signal transduction by interaction with G proteins. The hinge region is the least conserved GPHR component and the most variable in size. As its name implies (and perhaps because it is the only GPHR component whose structure remains unknown), the hinge region is widely considered to be a simple linker, or bridge, between the ligand binding and signaling components and has received little investigative attention relative to the LRD. Nevertheless, early studies on the TSH receptor (TSHR) indicated that the hinge region also contributed to both ligand binding and signal transduction (for example, see Refs. 4,5,6). A strong case for such properties has also been made for the gonadotropin receptors (discussed in Ref. 7). Indeed, based on this evidence, Moyle suggested signaling specificity domain as a more appropriate term for the GPHR hinge region. However, because the latter term is not yet in conventional use, we retain the term hinge in the present report. More recent studies have supported the importance of the TSHR hinge region in TSH binding and receptor activation (8,9,10,11; also reviewed in Refs. 1,12).
Figure 1.
A, Schematic representation of the TSH holoreceptor (not to scale). The amino acid limits of the TSHR hinge (residues 261–412) are based on the crystal structure of the TSHR leucine-rich repeat domain (LRD) (3) and the present consensus for insertion of the ectodomain into the plasma membrane (17). Intramolecular cleavage of some TSHR on the cell surface removes a substantial (>50 amino acid residue), but poorly defined portion of the hinge region (‘C peptide region’) leaving an extracellular A-subunit linked by disulfide bonds to a largely transmembrane B-subunit (reviewed in Ref. 25). B, Summary of TSHR hinge deletions examined in the present study. Deletions studied previously, beginning upstream at amino acid residues 317 or 287 are shown within the gray box, with their phenotypic changes indicated in the lateral boxes. The amino acid sequence for part of the 152-residue human TSHR hinge is shown above. Cysteine residues are boxed, as is Y385. The latter is a critical residue for TSH binding (5,8), for which reason residue H384 is the terminus in even the most extreme deletions.
The TSHR hinge region is unique among the GPHR in being the longest and undergoing in vivo intra-molecular cleavage into disulfide-linked A- and B-subunits, thereby removing a ‘C-peptide’ region (13,14,15,16; Fig. 1A). Compared with the LH receptor, the TSHR hinge has 50 additional amino acids, corresponding approximately (homology in the region is poor) to TSHR residues 317–366. Alignment of GPHR suggests that the TSHR hinge begins at amino acid residue 269 (17), however the TSHR LRD crystal structure, which includes all 10 leucine-rich repeats, terminates at residue 260 (3). In the present report we apply the latter value, estimating the TSHR hinge to be 152 amino acid residues in length (TSHR residues 261–412). However, analysis of TSHR protein from thyroid tissue and transfected eukaryotic cells reveals a ragged, poorly defined deletion pattern, with numerous putative cleavage sites in the hinge region ranging from amino acids ∼287–316 upstream (14,15,18) to residues 366–388 downstream (14). There is no amino acid motif whose alteration can prevent TSHR cleavage (19,20), and the enzyme responsible for cleavage, possibly an ADAM (14), is membrane-associated and appears to function in a molecular ruler manner independent of substrate amino acid sequence (21).
Experimentally, TSHR hinge amino acids 317–366 (50 residues) can be deleted without altering receptor function (22,23). However, as mentioned above, the TSHR cleavage region observed in vivo approaches 100 residues in length (approximately residues 287–388). There is strong evidence that these extreme upstream and downstream limits of TSHR hinge region cleavage are nonphysiological TSHR purification artifacts (15,18) incompatible with normal receptor function. In particular, in vitro deletion mutations initiated at amino acid residue 287 and terminating at residue 384 progressively reduce TSH binding and ligand-induced cAMP signal transduction (10; Fig. 1B). Unexpectedly, downstream extension of the hinge deletions to residues 371 and 384 also increased ligand-independent constitutive activity (10). Therefore, there is presently no information as to what portion of the TSHR hinge can be deleted with retention of normal, presumably physiological, receptor function. Consequently, the goal of the present study was to explore the limits to which TSHR hinge deletions can be tolerated without significant loss of TSH binding and TSH-mediated signal transduction; in other words to delineate the approximate boundaries of a physiologically normal hinge region. Such information would provide novel insight into the correlation between normal TSHR function and the aforementioned proteolytic posttranslational structural modifications.
Results
Deletions in the TSHR hinge region downstream of residue 317
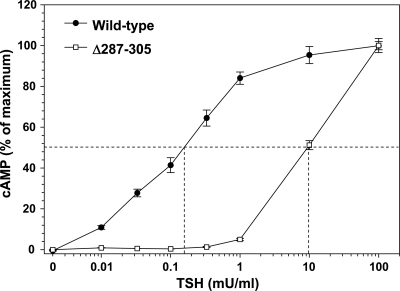
In previous deletion mutagenesis studies on the TSHR hinge region, we initiated TSHR deletions at upstream residue 287, terminating at downstream residues 371, 376, and 384 (10). The rationale for the choice of upstream residue 287 was that a TSHR that we had generated with hinge residues 287–305 deleted retained a vigorous cAMP response to a high TSH concentration (100 mU/ml). However, when we reevaluated TSHR Δ287–305 by performing a full TSH dose-response curve, despite a robust response to 100 mU/ml TSH, the sensitivity of this receptor was at least two orders of magnitude less than for the wild-type TSHR (Fig. 2). The TSH concentration required for half-maximal stimulation (EC50) could not be estimated because no plateau was attained, but was at least 10 mU/ml, approximately 100-fold higher than for the wild-type TSHR. TSH binding was detectable but too low for kinetic analysis. For this reason, and because TSHR Δ317–366 was functionally identical to the wild-type TSHR (21,22), in the present study we generated hinge deletions beginning at upstream residue 317 and terminating at downstream residues, namely 371, 376, and 384 (TSHR Δ317–371, Δ317–376, and Δ317–384; Fig. 1B). The latter residues correspond approximately to three B-subunit N termini identified by direct amino acid sequencing in TSHR purified from thyroid tissue (14). Although the most C-terminal of the potential hinge region cleavage sites identified in structural studies was at residue 388 (14), for our functional studies we did not delete beyond TSHR residue 384 because hinge residue Y385 is clearly established as an important residue for TSH binding and function (5,8).
Figure 2.
TSH stimulation of cAMP generation by a TSHR with amino acid residues 287–305 deleted (TSHR Δ287–305). Stably transfected CHO cell lines expressing the wild-type TSHR and TSHR Δ287–305 were incubated for 2 h at 37 C with the indicated concentrations of TSH (see Materials and Methods). cAMP values are expressed as a percent of maximum levels attained for each TSHR, an underestimate for TSHR Δ287–305 because cAMP levels did not plateau. The dashed lines indicate the effective TSH concentrations required for half-maximal stimulation (an underestimate for TSHR D287–305). In absolute terms, TSHR Δ287–305 cAMP levels increased to 72% and 82% of wild-type TSHR values. Each point represents the mean ± range of cAMP determinations in duplicate wells of cells. These data are representative of two experiments.
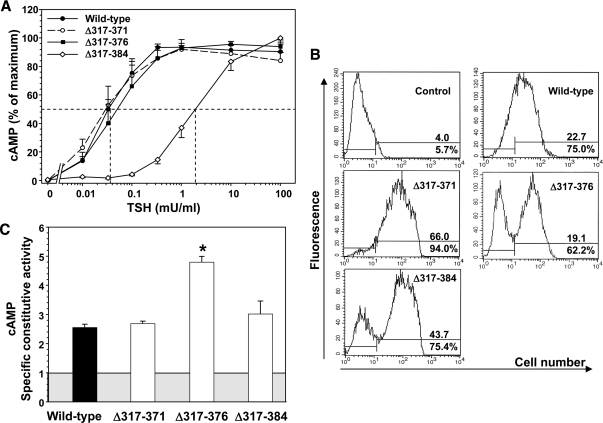
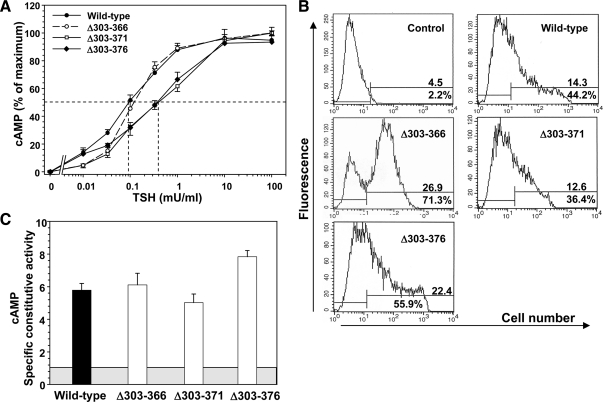
TSHR Δ317–371 and Δ317–376 responded to TSH with EC50s similar to that of the wild-type TSHR (Fig. 3A and Table 1). However, the most downstream deletion (TSHR Δ317–384) responded far less well to TSH stimulation, with an EC50 38-fold greater than for the wild-type TSHR. Reduced sensitivity to TSH stimulation could be caused by a decrease in TSH binding affinity to the TSHR or functional receptor insensitivity despite normal TSH binding affinity. The latter alternative would indicate an uncoupling between ligand binding and receptor activation. Therefore, we also examined 125I-TSH binding to TSHR Δ317-384 and, in subsequent experiments, to other TSHR whose sensitivity to TSH stimulation was reduced. The Kd for TSH binding to TSHR Δ317-384 was not significantly different from that of the wild-type TSHR (Table 1). Flow cytometry using a monoclonal antibody to the N-terminal portion of the ectodomain revealed that TSHR Δ317–384 trafficked normally to the cell surface, and the level of expression per transfected cells was not diminished relative to the wild-type TSHR (Fig. 3B).
Figure 3.
Deletions in the TSHR hinge region downstream of residue 317. TSHR with deletion of amino acid residues 317–371, 317–376, and 317–384 (TSHR Δ317–371, Δ317–376, and Δ317–384) were stably expressed in CHO cells (see Materials and Methods). A, TSH stimulation of intracellular cAMP levels in the wild-type TSHR and TSHR Δ317–371, Δ317–376, and Δ317–384 (see Materials and Methods). For each TSHR cAMP values are expressed as a percent of maximum levels attained. Each point represents the mean ± sem. cAMP value from five experiments for the wild-type TSHR, three experiments for TSHR Δ317–371 and TSHR Δ317–376, and four experiments for TSHR Δ317–384 (duplicate determinations in each experiment). The dashed lines indicate the effective TSH concentration required for half-maximal stimulation (EC50). B, Flow cytometry of TSHR-expressing CHO cells used for TSH stimulation (A). The top number in each panel indicates the fluorescence geometric mean, whereas the lower number indicates the percentage of cells (M2) that express the TSHR. Control, wild-type TSHR cells incubated with normal mouse IgG. C, TSHR-specific constitutive activities. The CHO cell lines used in the foregoing experiments were incubated for 2 h at 37 C in medium lacking TSH but supplemented with IBMX followed by assay of intracellular cAMP levels (see Materials and Methods). The specific constitutive activities of the TSHR were determined by adjustment for the variation in receptor expression using flow cytometry with monoclonal antibody CS-17 (see Materials and Methods). The shaded box represents cAMP levels in untransfected CHO cells, and the extension of the bars above this box indicates the contribution from the TSHR to cellular cAMP levels. Each bar indicates the mean ± sem of intracellular cAMP determinations in quadruplicate wells of cells for each TSHR-expressing cell line. The data are representative of three experiments with similar results. * P = 0.001; one-way ANOVA involving multiple comparisons vs. the control, wild-type TSHR (Holm-Sidak method; SigmaPlot).
Table 1.
Summary of functional and TSH binding data for TSHR deletion mutants
| EC50 TSH mU/ml | EC50 ratio mutant/wt | Rmax ratio mutant/wt | Kd nm | Bmax fmoles cAMP/well | |
|---|---|---|---|---|---|
| Wild-type | 0.06 ± 0.003 (10) | 1.0 | 1.0 | 0.79 ± 0.13 | 95.7 ± 19.8 |
| Δ287-305 | >10 (2) | >100 | Unable | Unable | |
| Δ317-371 | 0.05 ± 0.04 (3) | 0.85 ± 0.13 | 0.69 | ||
| Δ317-376 | 0.10 ± 0.07 (3) | 1.77 ± 0.28 | 0.43 | ||
| Δ317-384 | 3.21 ± 1.91 (4) | 38.21 ± 10.8 | 0.70 | 0.82 ± 0.10 | 14.0 ± 0.05 |
| Δ307-366 | 0.05 ± 0.02 (4) | 0.56 ± 0.09 | 0.39 | ||
| Δ307-371 | 0.11 ± 0.03 (3) | 1.53 ± 0.26 | 0.42 | ||
| Δ307-376 | 0.20 ± 0.03 (5) | 2.02 ± 0.23 | 0.52 | 1.26 ± 0.20 | 147.2 ± 6.5 |
| Δ303-366 | 0.13 ± 0.04 (4) | 1.51 ± 0.40 | 1.08 | ||
| Δ303-371 | 0.43 ± 0.14 (3) | 5.11 ± 0.38 | 0.58 | 1.01 ± 0.15 | 79.6 ± 5.7 |
| Δ303-376 | 0.42 ± 0.16 (4) | 5.34 ± 1.56 | 0.48 | 1.62 ± 0.59 | 151.95 ± 19.7 |
| Δ367-371 | 0.04 ± 0.01 (4) | 0.53 ± 0.10 | 0.81 | ||
| Δ372-376 | 0.04 ± 0.02 (4) | 0.47 ± 0.09 | 0.82 | ||
| Δ377-384 | 0.73 ± 0.42 (4) | 7.99 ± 2.83 | 0.47 | 0.56 ± 0.04 | 34.35 ± 1.8 |
EC50 values represent the TSH concentration [mean ± sem; (n) = number of experiments] required to elicit a half-maximal cAMP response. Each EC50 ratio (mutant/wild-type) was calculated from the wild-type data determined in parallel in each experiment, not from the mean value for all experiments shown in the table. TSH binding kinetics were determined on TSHR deletion mutants whose sensitivity to TSH stimulation was reduced more than twofold (EC50 > twofold that of the wild-type TSHR; shown in bold). TSH binding data (Kd and Bmax) represent the mean ± range of values from two experiments. TSH binding to TSHRD287–305 was too low for kinetic analysis (unable).
We also used the flow cytometry data to determine the specific constitutive activities (see Materials and Methods) for the foregoing TSHR. The specific constitutive activities of TSHR with deletions beginning at amino acid residue 317 were similar to that of the wild-type TSHR with the exception of TSHR Δ317–376, which was consistently greater than the wild-type TSHR (Fig. 3C). However, in our view, despite statistical significance, the relatively small amplitude of this increase (approximately twofold) reduces the likelihood of its biological importance. Taken together, these data indicate that TSHR hinge downstream cleavage sites at amino acid residue 371 and 376, but not at residue 384, are compatible with a physiologically normal, or near normal, receptor.
Deletions in the TSHR hinge region beginning upstream of amino acid residue 317
Having established the downstream limits of hinge region cleavage with retention of normal, or near normal, TSHR function at residues 371 and 376 (present study) and previously at residue 366 (21,22), we extended the hinge deletions further upstream from residue 317 (Fig. 1B). We chose residues 303 and 307 as the upstream sites for TSHR hinge deletions based on previous evidence that smaller deletions involving these residues (residues 303–306 and 307–316) were well tolerated (5) and that deletions further upstream would disrupt TSHR disulfide bonding. These experiments (with one exception) were performed with transiently transfected CHO cells because of difficulties experienced with the Flp-In system for generating stable transfectants, as described in Materials and Methods.
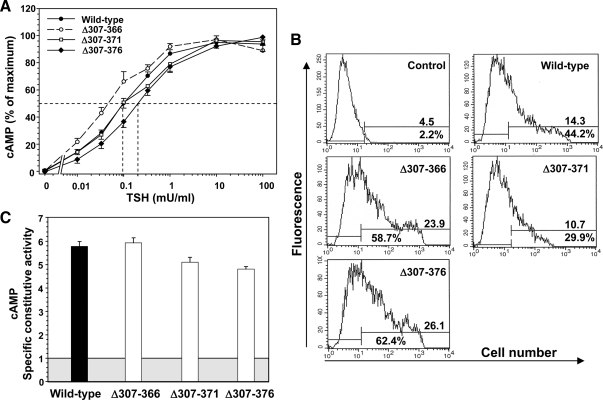
Hinge deletions creating TSHR Δ307–366 and TSHR Δ307–371 did not reduce sensitivity to TSH stimulation of cAMP levels, with EC50 values identical to, or even less than, the wild-type TSHR (Fig. 4A). In contrast, TSHR Δ307–376 was twofold less sensitive to TSH stimulation than the wild-type TSHR, a very small but consistent observation in five separate experiments (Fig. 4A). TSH binding to TSHR Δ307–376 tested in two of these experiments (Kd of 1.26 ± 0.2; mean ± range) was not significantly different to the Kd for the wild-type TSHR studied in parallel (0.86 ± 0.16). Based on the level of expression of these TSHR as determined by flow cytometry (Fig. 4B), specific constitutive activities of all three hinge region deletions originating at residue 307 were similar to that of the wild-type TSHR (Fig. 4C).
Figure 4.
Deletions in the TSHR hinge region downstream of residue 307. TSHR with deletion of amino acid residues 307–366, 307–371, and 307–376 (TSHR Δ307–366, Δ307–371 and Δ307–376) were transiently expressed in CHO cells (see Materials and Methods). A, TSH stimulation of intracellular cAMP levels in the wild-type TSHR and (TSHR Δ307–366, Δ307–371, and Δ307–376 (see Materials and Methods). For each TSHR cAMP values are expressed as a percent of maximum levels attained. Each point represents the mean ± sem. cAMP value from seven experiments for the wild-type TSHR, four experiments for TSHR Δ307–366, three experiments for TSHR Δ307–371, and four experiments for TSHR Δ307–376 (duplicate determinations in each experiment). The dashed lines indicate the effective TSH concentration required for half-maximal stimulation (EC50). B, Flow cytometry of TSHR-expressing CHO cells used for TSH stimulation (A). The top number in each panel indicates the fluorescence geometric mean, whereas the lower number indicates the percentage of cells (M2) that express the TSHR. Control, wild-type TSHR cells incubated with normal mouse IgG. C, TSHR-specific constitutive activities. The experimental protocol and data analysis is the same as described in the legends to Figs. 2 and 4. The shaded box represents cAMP levels in untransfected CHO cells, and the extension of the bars above this box indicates the contribution from the TSHR to cellular cAMP levels. Each bar indicates the mean ± sem of intracellular cAMP determinations in quadruplicate wells of cells for each TSHR. The data are representative of three experiments with similar results.
With similar TSHR hinge region deletions beginning slightly further upstream (at amino acid residue 303 rather than 307), the EC50 for TSH stimulation of TSHR Δ303–366 was unchanged relative to the wild-type TSHR, whereas the EC50s for TSHR Δ303–371 and TSHR Δ303–376 were both increased five-fold compared with the wild-type TSHR (Fig. 5A). The Kds for TSH binding to the two deletion mutants with reduced sensitivity to TSH stimulation were not increased to the same extent (Table 1), indicating a dissociation between TSH binding to and activation of the TSHR. Flow cytometric determination of TSHR levels of expression on the cell surface are shown in Fig. 5B. Specific constitutive activities of TSHR with deletions originating at upstream residue 303 were not significantly increased relative to the wild-type TSHR (Fig. 5C).
Figure 5.
Deletions in the TSHR hinge region downstream of residue 303. TSHR with deletion of amino acid residues 303–366, 303–371, and 303–376 (TSHR Δ303–366, Δ303–371, and Δ303–376) were expressed in CHO cells (TSHR Δ303–371, Δ303–376 transiently and Δ303–366 stably; see Materials and Methods). The description of these experiments is identical to that for Fig. 4. A, TSH stimulation of intracellular cAMP levels in the wild-type TSHR and TSHR Δ303–366, Δ303–371, and Δ303–376 (see Materials and Methods). For each TSHR cAMP values are expressed as a percent of maximum levels attained. Each point represents the mean cAMP value from seven experiments for the wild-type TSHR, four experiments for TSHR Δ303–366, three experiments for TSHR Δ303–371, and four experiments for TSHR Δ303–376 (duplicate determinations in each experiment). B, Flow cytometry of TSHR-expressing CHO cells used for TSH stimulation (A). The top number in each panel indicates the fluorescence geometric mean, whereas the lower number indicates the percentage of cells (M2) that express the TSHR. Control, wild-type TSHR cells incubated with normal mouse IgG. C, TSHR specific constitutive activities. Each bar indicates the mean ± sem of intracellular cAMP determinations in quadruplicate wells of cells for each TSHR. The data are representative of three experiments with similar results.
Small deletions in the C-terminal region of the TSHR hinge
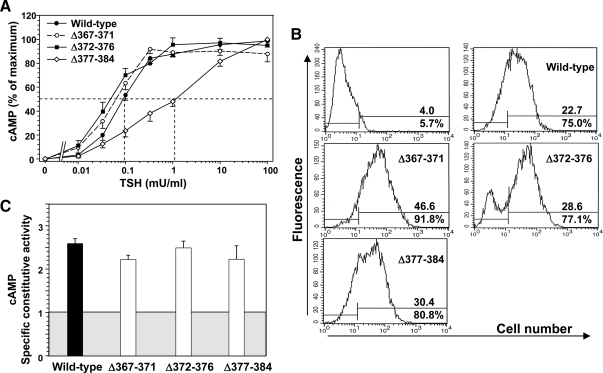
We observed previously that progressive downstream extensions of deletions in the TSHR hinge region from amino acid residues 366 to 384 had the dual effect of diminishing TSH binding and function and increasing ligand independent constitutive activity (Fig. 1B) (10). To explore further the role of this small region (residues 367–384) in TSHR structure and function we generated sequential deletions (residues 367–371, 372–376, and 377–384; Fig. 1B). The two more upstream deletions (Δ367–371 and Δ372–376) did not significantly alter the cAMP response to TSH stimulation compared with the wild-type TSHR (EC50 of approximately 0.1 mU TSH per ml) (Fig. 6A). However, the most downstream deletion (Δ377–384) reduced sensitivity to TSH stimulation (increased EC50) by approximately one order of magnitude. Despite the reduced sensitivity of TSHR Δ377–384 to TSH stimulation, the Kd for TSH binding to this receptor was similar to that with the wild-type TSHR (Table 1), as was its level of expression on the cell surface (Fig. 6B). That is, deleting TSHR hinge residues 377–384 resulted in uncoupling between normal TSH binding and signal transduction. Surprisingly, in view of our previous observations (10), summarized in Fig. 1B, none of the short deletions in this region, including TSHR residues 377-384, enhanced ligand-independent TSHR signaling (specific constitutive activity; Fig. 6C).
Figure 6.
Effect of small sequential deletions in the C-terminal region of the TSHR hinge on receptor function. TSHR with deletion of amino acid residues 367–371, 372–376, and 377–384 (Δ367–371, Δ372–376, and Δ377–384) were stably expressed in CHO cells (see Materials and Methods). A, TSH stimulation of intracellular cAMP levels in the wild-type TSHR and TSHR Δ367–371, Δ372–376, and Δ377–384 (see Materials and Methods). To facilitate comparison, cAMP values are expressed as a percent of maximum levels attained for each TSHR. Each point represents the mean ± cAMP value from duplicate determinations in three separate experiments. The dashed lines indicate the effective TSH concentration required for half-maximal stimulation (EC50). B, Flow cytometry of TSHR-expressing CHO cells used for TSH stimulation (Panel A). The top number in each panel indicates the fluorescence geometric mean whereas the lower number indicates the percentage of cells (M2) that express the TSHR. Control, wild-type TSHR cells incubated with normal mouse IgG. C, TSHR constitutive activities. The same stably transfected CHO cell lines used in the foregoing experiments were incubated for 2 h at 37 C in medium lacking TSH but supplemented with IBMX (see Materials and Methods). For comparison among cell lines, cyclic AMP values were normalized (‘Specific constitutive activity’) to the level of TSHR expression determined by flow cytometry using monoclonal antibody CS-17 whose epitope is upstream of TSHR residue 289. The shaded box represents cAMP levels in untransfected CHO cells, and the extension of the bars above this box indicates specific TSHR constitutive activity. Each bar indicates the mean ± sem of intracellular cAMP determinations in quadruplicate wells of cells for each TSHR-expressing cell line. The data are representative of two experiments with similar results.
Discussion
The hinge region is the ‘black box’ in the GPHR, limiting understanding of how ligands bind and activate these receptors. In contrast to the well-established three-dimensional structures of the FSHR (2) and TSHR (3) LRDs, homology of the GPHR hinge regions with other known proteins is insufficient to permit molecular modeling with any degree of confidence. Homology is poor even among the GPHR family members (17). Their linear amino acid sequences vary greatly in length, from 152 residues in the TSHR to 101 residues in the LH receptor (estimated using the boundaries corresponding to TSHR residues 261-412). The marmoset LH receptor hinge lacking exon 10 is even smaller (24). The TSHR hinge region is the most enigmatic in that, unlike the other GPHR, it undergoes posttranslational cleavage on the cell surface into disulfide-linked A- and B-subunits (reviewed in Ref. 25). An unexpected finding was that this cleavage process resulted in the deletion from the hinge region of a polypeptide fragment containing, at least in part, the component in the TSHR hinge region lacking in the LH receptor (13). Because of the established TSHR subunit terminology (A- and B-) (26) and similarity with the posttranslational modification of insulin, the missing fragment was termed a C-peptide (13). Subsequent TSHR structural analysis indicated that the putative C-peptide was not released intact, but in a piecemeal manner beginning at the N terminus (14,15). The lack of an intact C-peptide led to change in the terminology to ‘C-peptide region.’
The important questions as to the boundaries of the C-peptide region and the functional implications of TSHR proteolytic posttranslational modification remain unanswered. Direct microsequencing of TSHR purified from thyroid tissue revealed A-subunit C termini at residues 314, 322, and 325 and B-subunit N termini at residues 366, 370, 378, and 388 (aside from intermediate fragments with N termini at residues 332, 353, 356, and 357 (14). However, no functional correlation was possible with this structural information on purified TSHR protein. Moreover, it is unclear whether, at least some, of the aforementioned putative cleavage sites are physiological or artifacts consequent to TSHR degradation upon purification, to which this receptor is particularly susceptible (15,18,27). Therefore, experimental deletion mutagenesis is, at present, the only possible approach to correlate the boundaries of the deleted hinge region with TSHR function. As a starting point, it is known that experimental deletion of TSHR C-peptide region residues 317–366 generates a TSHR that traffics to the cell surface of transfected cells and that is perfectly normal in terms of TSH binding affinity, TSH-mediated cAMP signal transduction, and ligand-independent constitutive activity (23).
In previous studies, we observed that very large TSHR hinge region deletions, beginning at residue 287 and terminating at residues 371, 376, and 384, were not associated with normal receptor function. These deletions progressively lost TSH binding affinity and TSH-mediated signal transduction (10). Also of interest was an increase in ligand-independent constitutive activity (10), consistent with the unoccupied TSHR ectodomain being an inverse agonist (28). In the present study, we generated less extensive TSHR hinge region deletions to determine the approximate limit to which these deletions were compatible with retention of normal, physiological receptor function. The data also permit the first correlation of TSHR function with structural data obtained from TSHR protein analysis (described above). Based on the previously established normal function of TSHR Δ317–366 (21,22) we performed progressive deletions of residues upstream and downstream of amino acids 317 and 366, respectively. Our data indicate that TSHR Δ303–366 and TSHR Δ307–371, but not further downstream deletions, retain normal, or very near normal, TSH-induced cAMP signal transduction, as well as ligand-independent constitutive activity. We can conclude, therefore, that observations of B-subunit N termini at residues 378 and 388 (14) are incompatible with normal TSHR function and represent either artifacts of receptor purification or intermediary physiological degradation. B-subunit degradation further downstream with removal of cysteine residues at positions 390, 398, and 408 (15; Fig. 1A) could lead to A-subunit shedding, a process that may contribute to the pathogenesis of Graves’ disease (29). Regarding the upstream cleavage site, our functional data are consistent with the structural observation of residue 314 being the C terminus of the A-subunit; Fig. 1A). However, our data indicate also that deletions further upstream, to TSHR residues 303 or 307, are compatible with a normally functioning receptor.
As mentioned above, an unusual feature of the TSHR relative to the other GPHR is its high ligand-independent constitutive activity. Vlaeminck et al. (28) reported that deletion of TSHR residues upstream of residue 287 (1–21 being the signal peptide) increased TSHR constitutive activity, indicating that the TSHR ectodomain (primarily the LRD) functioned as an inverse agonist. Deletions extending further downstream had no additive effect. On the other hand, we observed that deletions downstream of TSHR amino acid residue 287 extending to residues 371 and 384 (entirely within the hinge region; Fig. 1B) enhanced constitutive activity and reduced TSH binding and concomitant receptor activation (10). These data led us to examine this small component at the C-terminal portion of the hinge region, namely TSHR residues 367–384. We now report that deletion of TSHR residues 377–384 is associated with a partial dissociation, or uncoupling, between normal TSH binding and cAMP signal transduction without alteration in constitutive activity. These unexpected data reinforce and emphasize the under appreciated concept that the TSHR hinge region is a conduit for ligand-induced signaling, as summarized in recent reviews (1,12), and is not simply an inert scaffold for the LRD. Besides residues 377–384, TSHR juxtamembrane hinge residues E409 (30) and D410 (31), as well as E251 at the C-terminal end of the LRD (32), are also involved in coupling TSH binding to cAMP generation.
In summary, the present data provide novel insight into the structure and function of the TSHR hinge region (estimated to extend from amino acid residues 261–412), a major portion of which is redundant. We report the first comparison of TSHR function with previously described proteolytic posttranslational hinge region modifications. Deletion of TSHR hinge amino acids 303–366 (64 residues) or 307–371 (65 residues) are the maximum hinge region deletions compatible with normal TSHR function. Downstream hinge residues 377–384 also appear to play a role in coupling TSH binding with cAMP signal transduction.
Materials and Methods
Construction and expression of TSHR deletion mutations
Transfer of the human TSHR cDNA (33) with three introduced restriction sites (34) and the H601 polymorphism converted to Y601 into the vector pcDNA5/FRT (Invitrogen, Carlsbad, CA) have been described previously (10). Deletions in the hinge region of TSHR of residues 367–371, 372–376, and 377–384 (TSHRΔ367–371, TSHRΔ372–376, and TSHRΔ377–384; Fig. 1B) were introduced using the QuickChange site-directed mutagenesis kit (Stratagene, San Diego, CA). Mutations were confirmed by nucleotide sequencing. The larger deletions beginning at amino acid residues 317, 307, and 303 (Fig. 1B) were made by overlap PCR using Pfu Ultra (Stratagene, La Jolla, CA). The cDNA was restricted with Afl II (codon 260) and Xba I (after 3′-end stop codon) and substituted for the same fragment in pcDNA5/FRT-TSHR.
TSHR cDNA encoding the series of deletions beginning at upstream residue 317, as well as smaller deletions for residues 367–371, 372–376, and 377–384, were transfected using FuGENE HD (Roche, Indianapolis, IN) into Flp-In CHO host cells and selected with Hygromycin-B (75–150 μg/ml) according to the protocol of the manufacturer (Invitrogen). Stably transfected cells were expanded and aliquots were frozen. As we experienced previously, stable transfections using the Flp-In system are not uniformly successful. For this reason, the TSHR deletion mutants beginning at upstream residue 303 and 307 (with one exception, TSHRΔ303–366), were studied using transient transfections. For the latter, cells were tested 48 h after transfection. All cells were cultured in F-12 medium supplemented with 10% fetal bovine serum, penicillin (100 U/ml), gentamycin (50 μg/ml), and fungizone (2.5 μg/ml).
Cultured cell cAMP assays
Stably transfected CHO cells expressing the wild-type and mutant TSHR were plated in 10-cm dishes overnight, transferred to 96-well plates, and studied approximately 24 h later. Similarly, for transient transfections, cells were transferred into 96-well plates ∼24 h after transfection and 24 h before testing. Cells from the same transfection were also plated in 6-well culture plates to monitor the transfection efficiency by flow cytometry (see below). For bioassay, the culture medium described above was replaced with F12 medium supplemented with 1 mm isobutyl methylxanthine, 10 mm HEPES, 0.3% BSA, and where indicated in the text, bovine TSH (Sigma-Aldrich, St. Louis, MO). CHO cells expressing no TSHR were also included as controls. After 2 h at 37 C, the medium was aspirated and intracellular cAMP was extracted with 0.2 ml 95% ethanol. The extracts were evaporated to dryness and resuspended in 0.2 ml Dulbecco’s PBS (pH 7.5), and assayed as described previously (10) using the LANCE cAMP kit according to the protocol of the manufacturer (Perkin-Elmer, Shelton, CT). Concentrations of TSH eliciting half maximal stimulation of cAMP levels were calculated with GraphPad Prism (La Jolla, CA) using nonlinear regression. For inter- and intraassay comparisons of basal, unstimulated cAMP generation by different TSHR, we determined the specific constitutive activities as described by the Vassart laboratory (28). For this analysis basal cAMP values were normalized using the flow cytometric geometric mean for each receptor (see below). As a benchmark, the specific constitutive activity of the wild-type TSHR was designated as 1.0. Statistical analysis among groups was performed by one-way ANOVA using SigmaPlot (SYSTAT Software, Chicago, IL).
Flow cytometry
TSHR expressing and control CHO cells were harvested from six-well plates using 1 mm EDTA, 1 mm EGTA in PBS. In each experiment involving transiently tranfected cells, flow cytometry was performed in parallel. Because cells stably expressing the TSHR were frozen in identical aliquots repetition of flow cytometry was not necessary. After washing with PBS containing 10 mm HEPES, pH 7.4 and 2% fetal bovine serum, the cells were incubated for 60 min at room temperature in 100 μl of the same buffer containing 1 μg of either normal mouse IgG or TSHR monoclonal antibody CS-17 (35). For all TSHR mutants and the wild-type TSHR we used CS-17 (epitope upstream of TSHR residue 289) (35). After rinsing, the cells were incubated for 60 min with 100 μl fluorescein isothiocyanate-conjugated goat antimouse IgG (1:100) (Caltag, Burlingame, CA), washed with the buffer, and analyzed using a FACScan flow cytofluorimeter (Becton-Dickinson, Franklin Lakes, NJ). Cells stained with propidium iodide (1 μg/ml final concentration) were excluded from analysis. In theory, all cells stably transfected using the Flp-In system should express a single cDNA transgene and the cells should be clonal (M2 of 90–100%). Our experience (for example, Fig. 3) is that 100% clonality is not attained in all cell lines. However, using the fluorescence geometric mean for the cell pool provides a quantitative indication of TSHR expression in each experimental well. It should be noted that, unlike with the Flp-In system, transient transfections express a variable number of transgenes. Therefore, even though the percentage of TSHR expressing cells (M2) is typically lower, the range of expression per cell is wider and includes some highly expressing cells. Again, the geometric mean provides a quantitative basis for determining TSHR constitutive activity.
TSH binding to transfected cells
CHO cells stably or transiently transfected with plasmids expressing the wild-type TSHR or TSHR deletion mutations were transferred plated in 24-well plates and cultured overnight. The medium was then aspirated and replaced with 250 μl binding buffer (Hanks’ buffer with 250 mm sucrose substituting for NaCl to maintain isotonicity and 0.25% BSA) containing approximately 10,000 cpm 125I-TSH (BRAHMS, Berlin Germany). After incubation for 4 h at room temperature, cells were rapidly rinsed four times with ice cold binding buffer, solubilized with 250 μl 1 N NaOH, and radioactivity was then measured in a gamma-counter. The radiolabeled TSH was supplemented with the concentrations of unlabeled bovine TSH (Sigma-Aldrich) indicated in the text. Nonspecific TSH binding was determined using untransfected CHO cells. TSH binding kinetics (Kd and Bmax) were analyzed using GraphPad Prism. Because, in our experience, nonlinear regression analysis using multiple programs (including that for two site specific binding) is unable to adequately process the data in all experiments, we used linear regression with Scatchard analysis, excluding the low-affinity high-capacity nonspecific binding site to which TSH (unlike hCG) is susceptible.
Acknowledgments
We thank Dr. J. Froehlich at BRAHMS, Hennigsdorf, Germany for generously providing radiolabeled TSH. We are also grateful for financial contributions by Dr. Boris Catz, Los Angeles.
Footnotes
This work was supported by National Institutes of Health Grants DK 19289 (to B.R.) and DK 54684 (to S.M.M.).
Present address for Y.M.-S.: Kuma Hospital, Kobe, 650-0011 Japan.
Disclosure Summary: The authors have nothing to declare.
Abbreviations: GPHR, Glycoprotein hormone receptor; LRD, leucine-rich repeat domain; TSHR, thyrotropin receptor.
First Published Online November 24, 2010
References
- Kleinau G, Krause G 2009 Thyrotropin and homologous glycoprotein hormone receptors: structural and functional aspects of extracellular signaling mechanisms1. Endocr Rev 30:133–151 [DOI] [PubMed] [Google Scholar]
- Fan QR, Hendrickson WA 2005 Structure of human follicle-stimulating hormone in complex with its receptor. Nature 433:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J, Chirgadze DY, Sanders P, Baker S, Sullivan A, Bhardwaja A, Bolton J, Reeve M, Nakatake N, Evans M, Richards T, Powell M, Miguel RN, Blundell TL, Furmaniak J, Smith BR 2007 Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid 17:395–410 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Russo D, Chazenbalk GD, Wadsworth HL, Rapoport B 1990 Extracellular domain chimeras of the TSH and LH/CG receptors reveal the mid-region (amino acids 171-260) to play a vital role in high affinity TSH binding. Biochem Biophys Res Comm 173:1150–1156 [DOI] [PubMed] [Google Scholar]
- Kosugi S, Ban T, Akamizu T, Kohn LD 1991 Site-directed mutagenesis of a portion of the extracellular domain of the rat thyrotropin receptor important in autoimmune thyroid disease and nonhomologous with gonadotropin receptors. Relationship of functional and immunogenic domains. J Biol Chem 266:19413–19418 [PubMed] [Google Scholar]
- Nagayama Y, Rapoport B 1992 Role of the carboxyl-terminal half of the extracellular domain of the human thyrotropin receptor in signal transduction. Endocrinology 131:548–552 [DOI] [PubMed] [Google Scholar]
- Moyle WR, Xing Y, Lin W, Cao D, Myers RV, Kerrigan JE, Bernard MP 2004 Model of glycoprotein hormone receptor ligand binding and signaling. J Biol Chem 279:44442–44459 [DOI] [PubMed] [Google Scholar]
- Costagliola S, Panneels V, Bonomi M, Koch J, Many MC, Smits G, Vassart G 2002 Tyrosine sulfation is required for agonist recognition by glycoprotein hormone receptors. EMBO J 21:504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinau G, Jäschke H, Neumann S, Lättig J, Paschke R, Krause G 2004 Identification of a novel epitope in the thyroid-stimulating hormone receptor ectodomain acting as intramolecular signaling interface. J Biol Chem 279:51590–51600 [DOI] [PubMed] [Google Scholar]
- Mizutori Y, Chen CR, McLachlan SM, Rapoport B 2008 The thyrotropin receptor hinge region is not simply a scaffold for the leucine-rich domain but contributes to ligand binding and signal transduction. Mol Endocrinol 22:1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Kleinau G, Jaeschke H, Paschke R, Krause G 2008 Extended hormone binding site of the human thyroid stimulating hormone receptor: distinctive acidic residues in the hinge region are involved in bovine thyroid stimulating hormone binding and receptor activation. J Biol Chem 283:18048–18055 [DOI] [PubMed] [Google Scholar]
- Mueller S, Jaeschke H, Günther R, Paschke R 2010 The hinge region: an important receptor component for GPHR function. Trends Endocrinol Metab 21:111–122 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Tanaka K, Nagayama Y, Kakinuma A, Jaume JC, McLachlan SM, Rapoport B 1997 Evidence that the thyrotropin receptor ectodomain contains not one, but two, cleavage sites. Endocrinology 138:2893–2899 [DOI] [PubMed] [Google Scholar]
- de Bernard S, Misrahi M, Huet JC, Beau I, Desroches A, Loosfelt H, Pichon C, Pernollet JC, Milgrom E 1999 Sequential cleavage and excision of a segment of the thyrotropin receptor ectodomain. J Biol Chem 274:101–107 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Chazenbalk GD, McLachlan SM, Rapoport B 1999 Subunit structure of thyrotropin receptors expressed on the cell surface. J Biol Chem 274:33979–33984 [DOI] [PubMed] [Google Scholar]
- Graves P, Pritsker A, Davies TF 1999 Post-translational processing of the natural human thyrotropin receptor: demonstration of more than two cleavage sites. J Clin Endocrinol Metab 84:2177–2181 [DOI] [PubMed] [Google Scholar]
- Van Durme J, Horn F, Costagliola S, Vriend G, Vassart G 2006 GRIS: glycoprotein-hormone receptor information system. Mol Endocrinol 20:2247–2255 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Chazenbalk GD, McLachlan SM, Rapoport B 1999 The shed component of the TSH receptor is primarily a carboxyl terminal truncated form of the A subunit, not the entire A subunit. Molec Cell Endocrinol 150:113–119 [DOI] [PubMed] [Google Scholar]
- Kakinuma A, Chazenbalk GD, Tanaka K, Nagayama Y, McLachlan SM, Rapoport B 1997 An N-linked glycosylation motif from the non-cleaving luteinizing hormone receptor substituted for the homologous region (Gly-367 to Glu-369) of the thyrotropin receptor prevents cleavage at its second, downstream site. J Biol Chem 272:28296–28300 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Chazenbalk GD, McLachlan SM, Rapoport B 1998 Thyrotropin receptor cleavage at site 1 does not involve a specific amino acid motif but instead depends on the presence of the unique, 50 amino acid insertion. J Biol Chem 273:1959–1963 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Chazenbalk GD, McLachlan SM, Rapoport B 2000 Evidence that cleavage of the thyrotropin receptor involves a “molecular ruler’ mechanism: deletion of amino acid residues 305-320 causes a spatial shift in cleavage Site 1 independent of amino acid motif. Endocrinology 141:3573–3577 [DOI] [PubMed] [Google Scholar]
- Wadsworth HL, Chazenbalk GD, Nagayama Y, Russo D, Rapoport B 1990 An insertion in the human thyrotropin receptor critical for high affinity hormone binding. Science 249:1423–1425 [DOI] [PubMed] [Google Scholar]
- Chazenbalk GD, Tanaka K, McLachlan SM, Rapoport B 1999 On the functional importance of thyrotropin receptor intramolecular cleavage. Endocrinology 140:4516–4520 [DOI] [PubMed] [Google Scholar]
- Zhang FP, Rannikko AS, Manna PR, Fraser HM, Huhtaniemi IT 1997 Cloning and functional expression of the luteinizing hormone receptor complementary deoxyribonucleic acid from the marmoset monkey testis: absence of sequences encoding exon 10 in other species. Endocrinology 138:2481–2490 [DOI] [PubMed] [Google Scholar]
- Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM 1998 The thyrotropin receptor: Interaction with thyrotropin and autoantibodies. Endocr Rev 19:673–716 [DOI] [PubMed] [Google Scholar]
- Buckland PR, Rickards CR, Howells RD, Jones ED, Rees Smith B 1982 Photo-affinity labelling of the thyrotropin receptor. FEBS Lett 145:245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo D, Chazenbalk GD, Nagayama Y, Wadsworth HL, Seto P, Rapoport B 1991 A new structural model for the thyrotropin (TSH) receptor as determined by covalent crosslinking of TSH to the recombinant receptor in intact cells: evidence for a single polypeptide chain. Mol Endocrinol 5:1607–1612 [DOI] [PubMed] [Google Scholar]
- Vlaeminck-Guillem V, Ho SC, Rodien P, Vassart G, Costagliola S 2002 Activation of the cAMP pathway by the TSH receptor involves switching of the ectodomain from a tethered inverse agonist to an agonist. Mol Endocrinol 16:736–746 [DOI] [PubMed] [Google Scholar]
- Chen CR, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM 2003 The thyrotropin receptor autoantigen in Graves’ disease is the culprit as well as the victim. J Clin Invest 111:1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Kleinau G, Jaeschke H, Neumann S, Krause G, Paschke R 2006 Significance of ectodomain cysteine boxes 2 and 3 for the activation mechanism of the thyroid-stimulating hormone receptor. J Biol Chem 281:31638–31646 [DOI] [PubMed] [Google Scholar]
- de Roux N, Misrahi M, Brauner R, Houang M, Carel JC, Granier M, Le Bouc Y, Ghinea N, Boumedienne A, Toublanc JE, Milgrom E 1996 Four families with loss of function mutations of the thyrotropin receptor. J Clin Endocrinol Metab 81:4229–4235 [DOI] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2010 TSH receptor residue E251 in the extracellular leucine-ric repeat domain is critical for linking TSH binding to receptor activation. Endocrinology 151:1940–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama Y, Kaufman KD, Seto P, Rapoport B 1989 Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochem Biophys Res Comm 165:1184–1190 [DOI] [PubMed] [Google Scholar]
- Nagayama Y, Wadsworth HL, Chazenbalk GD, Russo D, Seto P, Rapoport B 1991 Thyrotropin-luteinizing hormone/chorionic gonadotropin receptor extracellular domain chimeras as probes for TSH receptor function. Proc Natl Acad Sci USA 88:902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CR, McLachlan SM, Rapoport B 2007 Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology 148:2375–2382 [DOI] [PubMed] [Google Scholar]