Abstract
Serum- and glucocorticoid-inducible kinase 3 (SGK3) is a protein kinase of the AGC family of protein kinase A, protein kinase G, and protein kinase C and functions downstream of phosphatidylinositol 3-kinase (PI3K). Recent study revealed that SGK3 plays a pivotal role in Akt/protein kinase B independent signaling downstream of oncogenic PI3KCA mutations in breast cancer. Here we report that SGK3 is an estrogen receptor (ER) transcriptional target and promotes estrogen-mediated cell survival of ER-positive breast cancer cells. Through a meta-analysis on 22 microarray studies of breast cancer in the Oncomine database, we found that the expression of SGK3 is significantly higher (5.7-fold, P < 0.001) in ER-positive tumors than in ER-negative tumors. In ER-positive breast cancer cells, SGK3 expression was found to be induced by 17β-estradiol (E2) in a dose- and time-dependent manner, and the induction of SGK3 mRNA by E2 is independent of newly synthesized proteins. We identified two ERα-binding regions at the sgk3 locus through chromatin immunoprecipitation with massively parallel DNA sequencing. Promoter analysis revealed that ERα stimulates the activity of sgk3 promoters by interaction with these two ERα-binding regions on E2 treatment. Loss-of-function analysis indicated that SGK3 is required for E2-mediated cell survival of MCF-7 breast carcinoma cells. Moreover, overexpression of SGK3 could partially protect MCF-7 cells against apoptosis caused by antiestrogen ICI 182,780. Together, our study defines the molecular mechanism of regulation of SGK3 by estrogen/ER and provides a new link between the PI3K pathway and ER signaling as well as a new estrogen-mediated cell survival mechanism mediated by SGK3 in breast cancer cells.
SGK3, a downstream kinase of phosphatidylinositol 3’-kinase (PI3K), is transcriptionally upregulated by estrogen receptor (ER) and promotes estrogen-mediated cell survival in breast cancer cells.
Estrogen plays a pivotal role in the development and progression of estrogen receptor (ER)-positive breast cancer by exerting its biological effects primarily via ER (ER has two isoforms: ERα and ERβ; in this paper, ER refers to ERα unless otherwise indicated). Estrogen, such as 17β-estradiol (E2), stimulates proliferation of normal and transformed mammary epithelial cells by inducing expression of immediate and delayed hormone-responsive genes important for cell cycle progression (1). E2 is also suggested to promote cell survival by inhibiting apoptosis (2). Although many genes such as bcl-2 (3), c-myc (4), and c-Jun N-terminal kinase (jnk) (5) have been suggested to be involved in antiapoptosis of breast cancer cells, the molecular mechanisms of E2-mediated survival are still not fully characterized.
Aberrant phosphatidylinositol 3-kinase (PI3K) signaling occurs commonly in cancer including breast cancer, which has been implicated in tumorigenesis and tumor progression (6). PI3K is activated by growth factors and catalyzes the formation of the second messenger phosphatidylinositol 3,4,5-triphosphate (PIP3). PIP3 binds to the pleckstrin homology domain of the protein kinases such as phosphoinositide-dependent kinase-1 (PDK1) and protein kinase B (also known as Akt), thus regulating their activity. PDK1 in turn phosphorylates and activates the protein kinases including Akt, serum- and glucocorticoid-inducible kinases (SGKs), p70 S6-kinase, and protein kinase Cζ, thus mediating a variety of important cell functions including cell growth and survival (7).
Although Akt is the PI3K effector that is most widely implicated in cell survival of cancers, emerging data suggest that other effectors of PI3K also play an important role in cancers (8,9,10). Notably, Vasudevan et al. (8) recently reported that many PI3KCA (activating mutations of the catalytic subunit-α of PI3K) mutant cancer cell lines and human breast tumors exhibit only minimal Akt activation and a diminished reliance on Akt for anchorage-independent growth; instead these cells retain robust PDK1 activation and exhibit dependency on the PDK1 substrate SGK3. SGK3 is a member of SGKs that belongs to the AGC kinase family (protein kinase A, protein kinase G, and protein kinase C). SGKs have three isoforms in mammals (SGK1, SGK2, and SGK3), which are coded by three distinct genes. All SGK isoforms show extensive sequence homology to Akt in the kinase domain. Moreover, they are downstream targets of PI3K and are phosphorylated by PDK1 (11). SGKs lack the pleckstrin homology domain, but SGK3 contains an extra Phox homology domain that also binds PIP3 and is required for its activation and endosomal localization (12,13). The mouse homologue of SGK3, termed cytokine-independent survival kinase, was first identified as the factor mediating IL-3-dependent survival of 32D cells (14). Several lines of evidence suggest that SGK3 plays an important role in breast cancer (8,15,16). For example, Slagsvold et al. reported that SGK3 attenuates the degradation of the chemokine receptor CXCR4 by phosphorylating the E3 ubiqutin ligase AIP4 and thus promotes breast cancer metastasis (15). Recently Xu et al. (16) have showed that SGK3 can phosphorylate Flightless-I, a coactivator of nuclear receptors including ER and enhance ER transactivaction.
Estrogen/ER signaling and PI3K pathways exhibit a regulatory crosstalk in breast cancer cells. E2 up-regulates PI3K/Akt signaling in an ER-dependent manner (17,18), whereas Akt phosphorylates ER and thus enhances its ligand-independent activity, which has been implicated in endocrine resistance of breast cancer (19). Here we report that SGK3 is transcriptionally up-regulated by E2/ER and promotes E2-mediated cell survival, using MCF-7 breast carcinoma cells as a model system. Our study provides a new molecular link between the estrogen/ER and PI3K pathway and suggests a novel mechanism of E2-mediated cell survival mediated by SGK3 in breast cancer cells.
Results
SGK3 expression is higher in ER-positive breast tumors than ER-negative breast tumors
Vasudevan et al. (8) reported that SGK3 is critical for cell viability of breast cancer cells with the PI3KCA mutations and low level of phosphorylated Akt. It is notable that PI3KCA mutations are usually associated with expression of ER and progesterone receptor in breast cancer (20), indicating that there might be a functional association between SGK3 and ER. Microarray data from this (21) and others’ laboratories (22,23,24) have shown that SGK3 expression is up-regulated in ER-positive breast cancer cells after E2 treatment, suggesting that sgk3 might be an ER-regulated gene. To investigate the association between SGK3 and ER, we first determined whether there is a correlation between SGK3 expression and ER status in clinical breast tumor specimens. We integrated data from 22 microarray studies in the Oncomine database that reported on SGK3 expression in both ER-positive and ER-negative breast cancer specimens (n = 2366). A meta-analysis was performed on these data by using Fisher’s method for microarrays as proposed by Rhodes et al. (25) and Smith et al. (26). We estimated means and sd values from Oncomine with approximations. The meta-analysis showed a statistically significant difference in SGK3 expression between ER-positive and ER-negative specimens (P < 0.0001). Figure 1 is a shadow plot of the 22 studies from Oncomine with a quantile and box plot in the upper panel. The fold change of SGK3 expression of ER-positive tumors vs. ER-negative tumors was 5.7. The result indicates that SGK3 expression is much higher in ER-positive breast tumors than in ER-negative breast tumors, supporting that SGK3 is an ER target in vivo.
Figure 1.
Meta-analysis of the data of 22 microarray studies from the Oncomine database on the association with SGK3 and ER in breast tumor specimens. Twenty-two microarray data of breast cancer specimens (n = 2366) from Oncomine (http://www.oncomine.org) were collected and meta-analyzed using the Fisher’s method as described in the Materials and Methods. The majority of the microarray studies in ER+/ER− breast tumors cluster above 0, indicating the expression of SGK3 is higher in ER+ breast tumors when compared with ER− breast tumors. The box plot shows that the center mass of the studies is to the right of zero, suggesting a positive fold change of ER+ relative to ER−. The mean expression of SGK3 in ER+ breast tumors is 5.7-fold higher than in ER− breast tumors (P < 0.0001).
E2 stimulates sgk3 gene transcription via ER
To reveal the molecular basis for the increase in SGK3 expression by E2/ER in breast cancer, a thorough characterization of the E2 response was performed using MCF-7, an ER-positive breast carcinoma cell line as the model. MCF-7 cells were hormone stripped for 48 h and then treated either with different dosages of E2 for 48 h or with fixed concentration (10 nm) of E2 for different lengths of time. SGK3 mRNA level was measured by quantitative RT-PCR (note: the primers used in quantitative PCR are nontranscript variant specific and are able to amplify all three of the SGK3 transcript variants). E2 was found to stimulate SGK3 expression in a dose-dependent manner (Fig. 2A), and the enhancement of SGK3 mRNA levels in response to E2 was evident as early as 1 h after treatment (Fig. 2B), suggesting that sgk3 gene is a direct transcriptional target of ER. This was confirmed by the results that enhancement of SGK3 mRNA level in response to E2 could be blocked by general transcription inhibitor actinomycin D but not affected by protein synthesis inhibitor cycloheximide (Fig. 2C). Furthermore, up-regulation of SGK3 by E2 was blocked by ER pure antagonist ICI 182,780 (Fig. 2D).
Figure 2.
E2 regulates SGK3 expression via ER at the transcription level. MCF-7 cells were hormone stripped for 48 h before the treatment. After treatment, SGK3 expression was assessed by quantitative RT-PCR (A–D) or Western blotting (E and F). Quantitative RT-PCR was carried out in triplicate and the results were normalized to β-actin. A, The cells were treated with different concentrations of E2 as indicated for 48 h. B, The cells were treated with 10 nm E2 for the indicated time. C, The cells were pretreated with 1 μg/ml actinomycin (ActD) or 100 μg/ml cycloheximide (CHX) or the solvent for 1 h and then treated with 50 nm E2 for 4 h. D, The cells were pretreated with 100 nm ICI182,780 for 1 h and then treated with 10 nm E2 for 9 h. E, The cells were treated with the different concentrations of E2 as indicated for 24 h. F, The cells were pretreated with 100 nm ICI182,780 for 1 h and then treated with 10 nm E2 for 9 h. NS, Nonspecific band.
Western blot analysis was also performed to test the up-regulation of SGK3 protein level by E2 via ER. As shown in Fig. 2, E and F, E2 increased SGK3 protein level in a dose-dependent manner, and ICI 182,780 blocked the enhancement of SGK3 protein level by E2.
To show that the up-regulation of SGK3 expression by ER was not a single cell-type phenomenon, we treated another ER-positive breast cancer cell line, BT474, as well as two ER-negative breast cancer cell lines, MDA-MB-231 and SK-BR3, with E2. As expected, SGK3 expression was dose dependently increased after E2 treatment in ER-positive BT474 cells but not in ER-negative MDA-MB-231 and SK-BR3 cells (Fig. 3).
Figure 3.
E2 up-regulates SGK3 expression in ER-positive cells but not in ER-negative cells. ER-positive BT474 cells and ER-negative SK-BR3 and MDA-MB-231 cells were hormone stripped for 48 h and then treated with different concentrations of E2 as indicated for 48 h. Total RNA was extracted and SGK3 mRNA level was assessed by quantitative RT-PCR. The experiments were performed in triplicate and the results were normalized to β-actin.
Identification of ER-binding regions at the sgk3 locus
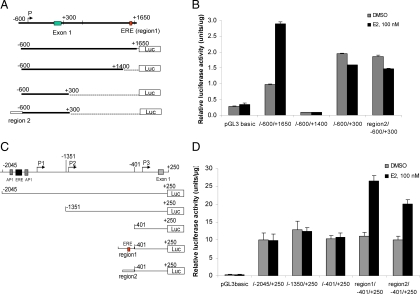
To understand the molecular basis for up-regulation of SGK3 expression by ER in breast cancer cells, we first identified the elements that ER binds at the sgk3 locus. According to the gene database of NCBI, human sgk3 gene has three transcript variants, as illustrated in Fig. 4A. The first exon of transcript variant 3 is located 63.5 kb upstream to the exon 1 of transcript variants 1 and 2, suggesting that there are two different promoters to drive the transcription of these three SGK3 transcript variants, one for transcript variant 3 and the other for transcript variants 1 and 2. In our separate ongoing study of genome-wide identification of ER-binding elements in MCF-7 cells using chromatin immunoprecipitation (ChIP) in combination with massively parallel DNA sequencing (ChIP-Seq), two ER-binding regions designated region 1 and region 2 were detected at the sgk3 locus when the cells were treated with E2 (upper panel in Fig. 4A). The sequences of these two ER-binding regions are shown in Fig. 4B. These two regions are in the first intron of SGK3 transcript variant 3 (GenBank: NM_001033578) and in the far upstream (62 and 33 kb, respectively) of the transcription start site of SGK3 transcript variants 1 (GenBank: NM_013257) and 2 (GenBank: NM_170709) (lower panel in Fig. 4A). Our quantitative RT-PCR analysis using transcript variants 1 and 2- or 3-specific primers indicated that all the transcripts were up-regulated after E2 treatment in MCF-7 cells (Fig. 4C), suggesting that E2 stimulates both of the sgk3 promoters. To verify that the two regions identified by ChIP-Seq analysis are true ER-binding elements, we performed an independent ChIP assay. MCF-7 cells were hormone stripped for 72 h and then treated with 100 nm E2 or dimethylsulfoxide (DMSO) for 45 min. As shown in Fig. 4D, both of the bindings of ER to region 1 and to region 2 were significantly increased after E2 treatment compared with DMSO control, demonstrating that they are true ERα-binding regions.
Figure 4.
Identification of ER-binding regions at sgk3 locus. A, UCSC Genome Browser illustrations of our ChIP-Seq data on ER-binding regions at the human sgk3 locus (upper panel) and schematic representation of SGK3 transcript variants (lower panel). In our ChIP-Seq approach, two clear ER-binding peaks, referred to as region 1 and region 2, were detected in the sgk3 locus in MCF7 cells treated with E2 compared with DMSO control. The human sgk3 gene encodes three transcript variants. Transcript variant 3 has a longer 5′-untranslational region than transcript variants 1 and 2; transcript variant 2 is missing one exon compared with transcript variant 1 due to alternative splicing. Both of two ChIP-Seq-identified ER-binding regions, region 1 and region 2, are in the intron 1 of transcript variant 3 and far upstream (62 and 33 kb, respectively) to the transcriptional start site of transcript variants 1 and 2. B, Sequences and the putative transcription factor binding sites (underlined) of ER-binding regions 1 and 2. C, E2 up-regulates the expression of all the transcript variants in MCF7 cells. After hormone stripped for 48 h, MCF-7 cells were treated with DMSO or different dosages of E2 as indicated for 24 h. Total RNA was isolated from cells, and the mRNA levels of transcript variants 1 and 2 and 3 were measured by quantitative RT-PCR using transcript variant-specific primer sets. The data are expressed as the mean ± sd of the relative expression compared with the levels of β-actin mRNA in triplicate samples. D, ChIP-PCR analysis of the recruitments of ER to regions 1 and 2. MCF-7 cells were hormone stripped for 72 h and then treated with DMSO or 100 nm E2 for 45 min. ChIP-PCR assays were performed as described in Materials and Methods.
ER transactivates the promoter of transcript variant 3 via region 1
To reveal the molecular mechanism of transcriptional regulation of sgk3 by estrogen/ER, we carried out a thorough study of the sgk3 promoters. Because the two ER-binding regions are located in intron 1 of the transcript variant 3, we first investigated whether the promoter of transcript variant 3 is regulated by estrogen via these two ER-binding regions. Sequences of the 2.25 kb fragment that includes the 5′-flanking region, exon 1 and partial intron 1 harboring ER-binding region 1 (from +1401 to +1650) was subjected to promoter prediction analysis (Neural Network Promoter Prediction, http://www.fruitfly.org/seq_tools/promoter.html) and transcription factor binding site analysis (software TRANSFAC 8.1). A putative promoter (P) was found in the 5′-flanking region and a perfect full estrogen response element (ERE), AGGGTCAGATTGAACT was found within the ER-binding region 1 (Figs. 4B and 5A). To map the minimal promoter region and the estrogen regulatory sequences, a series of luciferase reporter constructs containing different fragments of the 5′-flanking region of transcript variant 3, as illustrated in Fig. 5A, were generated and transfected into MCF-7 cells. As shown in Fig. 5B, the promoter activity of the longest fragment (pGL3hSGK3-600/+1650) was 3.5-fold higher than that of the empty pGL3 basic vector control (no promoter, no enhancer) in the absence of E2. Furthermore, E2 treatment resulted in a 3-fold increase of the promoter activity of this fragment, confirming that −600/+1650 region contains a true promoter and an ERE. Deletion of region 1 (pGL3hSGK3-600/+1400) resulted in a dramatic reduction of the basal promoter activity and a complete loss of estrogen responsiveness, suggesting that region 1 is critical for the promoter and also responsible for its E2 responsiveness. Further deletion of the region from +301 to +1400 (pGL3hSGK3-600/+300) resulted in a 20-fold increase of the basal promoter activity but no E2 responsiveness (Fig. 5B), further confirming that region 1 is critical for estrogen responsiveness of the promoter. The result also suggests that the region from +301/+1400 contains a negative regulatory element(s), and the region −600/+300 contains the minimal promoter.
Figure 5.
Identification of the promoters and estrogen-responsive regions of the sgk3 gene. A, Schematic illustration of the 5′-flanking region as well as partial intron 1 of SGK3 transcript variant 3. The location of the P, exon 1 and the ER-binding region 1 that contains an ERE, and the structure of luciferase reporters driven by different fragments of sgk3 promoter are indicated. The nucleotides are numbered from the transcriptional start site that is assigned as +1. B, MCF-7 cells were transfected with the luciferase reporters as indicated and then cultured in the presence or absence of 100 nm E2. Twenty-four hours after transfection, luciferase activity was measured and normalized to protein concentration. Results represent three independent experiments performed in triplicates. C, Schematic illustration of the 5′-flanking region of SGK3 transcript variants 1 and 2. P1, P2, and P3 are three putative promoters as predicated by promoter predication analysis. The location of two putative AP-1 sites, one putative ERE, and exon 1, and the structure of luciferase reporters driven by different fragments of sgk3 promoter are indicated. D, The promoter activity of the sgk3 gene fragments as indicated was determined by luciferase assays. The procedures for transfection and luciferase assays are the same as described in B.
To examine whether the promoter of transcript variant 3 is also regulated by ER-binding region 2 in response to E2, region 2 was PCR amplified and inserted in front of the region −600/+300 containing the minimal promoter (pGL3hSGK3-region2/−600/+300). Region 2 does not contain ERE, but it contains the putative binding sites for CCAAT/enhancer-binding protein, paired box transcription factor-3, Forkhead, and activator protein (AP)-2αA (Fig. 4B). It has been reported that ER could be recruited to these DNA sites via interaction with those transcription factors (27). As shown in Fig. 5B, region 2 had no effect on the basal and E2-inducible promoter activity, implying that region 2 is not responsible for E2 responsiveness of this promoter. Taken together, our results indicate that ER stimulates the transcription of transcript variant 3 via region 1 on E2 treatment.
ER stimulates the promoter of transcript variants 1 and 2 via regions 1 and 2 on E2 treatment
To study the regulation of the promoter driving transcription of SGK3 transcript variants 1 and 2, a 2250-bp 5′-flanking genomic sequence of transcript variants 1 and 2 was subjected to promoter prediction analysis and transcription factor binding site analysis. Three putative promoters, P1, P2, and P3, were predicted, and two putative AP-1 sites and one ERE were suggested (Fig. 5C). The luciferase reporters driven by the different fragments of 5′-flanking region of transcript variants 1 and 2, as illustrated in Fig. 4C, were generated and transfected into MCF-7 cells. Our result revealed that P3 in region −401/+250 is the minimal promoter, and the putative AP-1 sites and ERE site within −2045/+250 region did not confer the E2 responsiveness to this promoter (Fig. 5D).
We then tested whether the two ChIP-Seq-identified ER-binding regions, located at 62 and 33 kb upstream of the transcriptional start site of variants 1 and 2, respectively, are responsible for the E2 induction of the promoter of transcript variants 1 and 2 (P3 in Fig. 5C). Because these regions are too far apart to be cloned as a single fragment, region 1 and region 2 were PCR amplified and inserted separately in front of the region −401/+250 containing P3 (pGL3hSGK3 region1/−401/+250 and pGL3hSGK3 region 2/−401/+250), as illustrated in Fig. 5C. As shown in Fig. 5D, the addition of either region 1 or region 2 conferred P3 to E2 responsiveness, indicating that the activated ER stimulates the promoter of transcript variants 1 and 2 via distal ER-binding regions 1 and 2.
SGK3 depletion reduces E2-mediated survival of MCF-7 cells
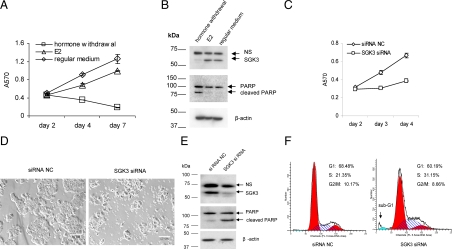
Most breast cancer cells rely on estrogen for survival and growth. As shown in Fig. 6, A and B, hormone deprivation of MCF-7 cells reduced cell proliferation and induced apoptosis [as indicated by apoptosis hallmark poly(ADP-ribose) polymerase (PARP) cleavage, Fig. 6B], whereas E2 addition could promote cell proliferation and inhibit apoptosis. Coincidently, hormone deprivation decreased SGK3 protein level, whereas E2 addition increased SGK3 protein level (Fig. 6B).
Figure 6.
SGK3 depletion reduces E2-mediated cell survival of MCF-7 cells. A and B, MCF-7 cells were seeded into either 24-well plates at 5 × 104 cells/well (A) or in 10-cm dishes at 1.5 × 106 cells/dish (B) and cultured in regular medium (MEM with 10% FBS). After 40 h incubation, the cells were replaced with fresh regular medium or switched to phenol red-free MEM with 10% CD FBS. The cells in the phenol red-free MEM with 10% CD FBS were then treated with DMSO (hormone withdrawal) or 50 nm E2 (E2). Cell proliferation was measured by the MTT assay at the indicated time (A). Forty-eight hours after treatment, cells were harvested for Western blotting in which antibodies against PARP and SGK3 were used, respectively (B). PARP cleavage indicates apoptosis. NS, Nonspecific band. C and D, MCF-7 cells were plated into 24-well plates at 5 × 104 cells/well and transfected with siRNA NC or SGK3 siRNA using reverse transfection method as described in Materials and Methods. The transfected cells were cultured in phenol red-free medium with 10% CD FBS plus 10 nm E2. The cell proliferation was measured by the MTT assay at the indicated time (C). The cells were visualized by microscopy 72 h after transfection (D). Scale bars, 50 μm. E and F, MCF-7 cells were plated into 6-cm dishes at 5 × 105 cells/dish and transfected with siRNA NC or SGK3 siRNA. The transfected cells were cultured in phenol red-free medium with 10% CD FBS plus 10 nm E2. After 72 h, both the attached and the floating cells were collected for Western blotting (E) or cell cycle analysis (F). The antibodies against PARP and SGK3 were used in the Western blotting, respectively. Cell cycle analysis was performed using propidium iodide staining, and the percentages of the phases were determined using ModFIT LT software (Verity Software House,Topsham, ME).
Because SGK3 is an estrogen-inducible kinase, we tested whether SGK3 is involved in E2-mediated survival and growth of ER-positive breast cancer cells. To this end, MCF-7 cells were transfected with SGK3 small interfering RNA (siRNA) or siRNA negative control (NC), and were kept in phenol red-free medium with 10% charcoal/dextran-treated (CD) fetal bovine serum (FBS) plus 10 nm E2. SGK3 siRNA transfection caused a dramatic reduction in SGK3 protein expression compared with siRNA NC transfection (Fig. 6E). In parallel, SGK3 siRNA-transfected cells significantly decreased cell proliferation (Fig. 6C). It was observed under microscope that many cells became round and floated in the medium after SGK3 siRNA transfection but not after siRNA NC transfection (Fig. 6D), which indicates that decrease of cell proliferation by SGK3 siRNA were due, at least in part, to the increase of cell death. As expected, PARP cleavage was found to significantly increase in SGK3 siRNA-transfected cells compared with siRNA NC-transfected cells (Fig. 6E). To further investigate the effect of SGK3 depletion, flow cytometry analysis was carried out 72 h after SGK3 siRNA or siRNA NC was introduced into the cells. As shown in Fig. 6F, no G1 arrest but an increase in the S-phase plus a minor sub-G1 (an indicator of apoptosis) was observed in SGK3 siRNA-transfected cells compared with siRNA NC-transfected cells, indicating that the decrease of cell proliferation by SGK3 siRNA was not mainly due to cell cycle arrest. Together our results suggest that SGK3 promotes E2-mediated survival of MCF-7 cells, at least in part, by inhibiting apoptosis.
Overexpression of SGK3 partially protects MCF-7 cells against apoptosis caused by antiestrogen ICI 182,780
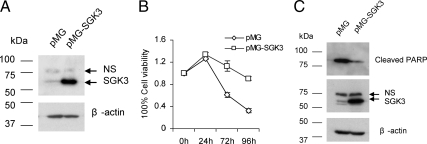
It is documented that antiestrogen ICI 182,780 induces apoptosis of estrogen-dependent MCF-7 cells (28,29). To further provide the evidence for the involvement of SGK3 in E2-mediated cell survival of breast cancer cells, we tested whether forced overexpression of SGK3 could prevent MCF-7 cells, at least in part, from death caused by antiestrogen ICI 182,780 treatment. To this end, we first generated MCF-7 cell lines stably transfected with empty vector (MCF-7/pMG) or SGK3 expression vector (MCF-7/pMG-SGK3) [note: only the cDNA coding the SGK3 isoform with 496 amino acid (aa) residues was used to transfect into MCF-7 cells since the 496 aa isoform is dominant in MCF-7 cells; for more, see Discussion]. Western blotting confirmed that SGK3 was highly expressed in MCF-7/pMG-SGK3 cells compared with MCF-7/pMG cells (Fig. 7A). Then we treated these two cell lines with ICI 182,780 and measured the cell viability using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. As shown in Fig. 7B, the viability of MCF-7/pMG-SGK3 cells was significantly less affected by ICI 182,780 treatment compared with MCF-7/pMG cells. Moreover, cleaved PARP protein level was significantly lower in MCF-7/pMG-SGK3 cells than in MCF-7/pMG cells after ICI 182,780 treatment (Fig. 7C). Together these results indicate that overexpression of SGK3 could partially but significantly protect MCF-7 cells against apoptosis caused by antiestrogen ICI 182,780.
Figure 7.
Overexpression of SGK3 protects MCF-7 cells against apoptosis caused by ICI 182,780 treatment. A, Western blot analysis of SGK3 protein level in the MCF-7 cell lines stably transfected with control vector (pMG) or SGK3 expression vector (pMG-SGK3). B, MCF-7/pMG and MCF-7/pMG-SGK3 cells were plated into 24-well plates at 1 × 105 cells/well and cultured in the regular medium with 10% FBS. After overnight incubation, the cells were treated with 10 nm ICI 182,780. The cell viability was assessed by MTT assay after treatment for the indicated time. C, MCF-7/pMG and MCF-7/pMG-SGK3 cells were cultured in regular medium with 10% FBS and treated with 10 nm ICI 182,780 for 48 h before Western blotting in which the antibodies against cleaved PARP and SGK3 were used. NS, Nonspecific band.
Discussion
SGK1, SGK2, and SGK3 are coded by three distinct genes but share about 80% sequence identity in their catalytic domains (11). Sgk1 was originally cloned as an immediate early gene transcriptionally stimulated by serum and glucocorticoids in rat mammary tumor cells (30); sgk2 and sgk3 were cloned by virtue of their similarity to the sgk1 sequence (11,31). Unlike other kinases of the AGC family, SGK1 is predominantly regulated at the transcriptional level by a huge number of extracellular stimuli including growth factors and hormones (32,33,34). In contrast, very little is known about the transcriptional regulation of sgk2 and sgk3. SGK3 mRNA was not induced by serum or glucocorticoids in rat2 fibroblasts (11). It has been even considered that SGK3 expression is constitutive and is not regulated at the transcriptional level (9). In the current study, we demonstrated for the first time that sgk3 is transcriptionally regulated by estrogen/ER in breast cancer cells. Therefore, SGK3 is an estrogen-inducible kinase. To our knowledge, SGK3 is the only kinase of AGC family that has been demonstrated to be induced by estrogen/ER in breast cancer cells.
The sgk3 gene encodes three different transcript variants and uses two promoters, as found in our study. Transcript variants 1 and 3 encode the same protein with 496 aa (molecular mass 57.1 kDa), whereas transcript variant 2 encodes the shorter protein with 464 aa (53.3 kDa) because transcript variant 2 misses one exon compared with transcript variant 1 due to alternative splicing (Fig. 4A). According to our RT-PCR result (our unpublished data) and Western blot analysis, the major SGK3 mRNA in MCF-7 cells is from transcript variant 1, and the dominant isoform of SGK3 is 496 aa long. It is reasonable to consider that alternative splicing alters the kinase activity of SGK3 because the shorter isoform of SGK3 is missing 32 aa (aa 327–358) in the kinase domain compared with the longer isoform due to alternative splicing. In this study, we functionally mapped the two promoters of sgk3 and found that the promoter driving the transcription of transcript variants 1 and 2 is much stronger than the one driving the transcription of transcript variant 3 in MCF-7 cells. Our results revealed that ER stimulates the promoter of transcript variants 1 and 2 through a very long-range activation on E2 treatment. For example, the ER-binding regions 1 and 2, located at 62 and 33 kb upstream of the transcription start site of variants 1 and 2, respectively, are responsible for E2 induction of the promoter of these two transcript variants. Genome-wide ChIP profiling studies of ER including our owns (unpublished data) revealed that only a small percentage of ER-binding sites are located within the proximal promoter regions of target genes and that the majority are located in an intron at great distance (>20 kb) from the transcription start site of genes (27,35,36). It has also been reported that multiple ER-binding sites on the ER target genes, including pS2, GREB1, and bcl-2 interact via looping to regulate transcription (23,37,38). In contrast, ER regulates the promoter of transcript variant 3 only via ER-binding region 1 that is 1.4 kb downstream to the transcription start site.
According to the Oncomine data, as shown in Fig. 1, SGK3 expression is significantly higher (5.7-fold) in ER-positive breast tumors than in ER-negative breast tumors. Moreover, SGK3 expression is found to be down-regulated in the tissues from postmenopausal women treated with letrozole, an aromatase inhibitor that is used to block estrogen synthesis (39). All of these clinic data support our finding that sgk3 is an ER direct target gene.
SGK3 is an estrogen-inducible kinase and is up-regulated in ER-positive breast cancer cells, suggesting that it may play an important role in ER-positive breast cancer. Our study indicates that SGK3 is required for estrogen-mediated survival of ER-positive breast cancer cells, although it seems not to be sufficient for estrogen-mediated cell survival. It is documented that deregulation of the PI3K pathway either through loss of PTEN (phosphatase and tensin homolog deleted on chromosome 10) or activating mutations of PI3KCA occurs frequently in human cancer including breast cancer (40,41). As described earlier, SGK3 has been found to play a pivotal role in Akt/protein kinase B-independent signaling downstream of oncogenic PI3KCA mutations in breast cancer (8). Based on the findings from this and other laboratories (8,14,16), we hypothesize that in some of PI3KCA mutated and ER-positive breast cancers, one important cell survival mechanism is that E2 up-regulates the expression of SGK3 via ER, thus enhancing PI3K/SGK3 pathway activated by PI3KCA (Fig. 8).
Figure 8.
A diagram of the proposed cell survival signaling pathway in ER+ breast cancer cells with PI3KCA mutations and low phosphorylated Akt. SGK3 is induced by estrogen/ER through transcriptional mechanism and phosphorylated by PDK1 activated by PI3KCA mutations. SGK3 exerts cell survival signaling by up-regulating Bcl-xL expression, inhibiting the proapoptotic proteins [Bcl-2 antagonist of cell death (Bad) and Forkhead box O3 (FoxO3)] and phosphorylating Flightless I (FLII), thus enhancing nuclear receptor (NR)-mediated transcriptions.
Together with the previous study from Xu et al. (16) in which SGK3 phosphorylates coactivator Flightless-I, thus enhancing ER activity and promoting cell proliferation of ER-positive breast cancer cells, we suggest that there is a positive feedback loop between SGK3 and ER, and the feedback loop may play an important role in estrogen signaling in ER-positive breast cancer. Ongoing experiments are to further define the role of SGK3 in ER-positive breast cancer cells.
Materials and Methods
Cell culture
MCF-7 cells were routinely cultured in MEM containing 10% FBS, 2 mm l-glutamine, 1 mm sodium pyruvate, 1% nonessential aas, and 100 U/ml penicillin-streptomycin. To evaluate the effect of E2 on SGK3 expression, cells were switched to phenol red-free MEM with 10% CD FBS 48 h before E2 treatment. MCF-7 cells stably transfected with empty vector or SGK3 expression vector were selected at 150 μg/ml hygromicin B for 5 wk and maintained at 50 μg/ml hygromicin B.
Plasmid constructs
The different fragments of sgk3 promoters were amplified by PCR using genomic DNA from HeLa cells as the template. PCR products were digested and cloned into the NheI/XhoI and KpnI/HindIII sites, respectively, in pGL3 basic vector (Promega, Madison, WI). SGK3 cDNA was generated by RT-PCR using RNA from MCF-7 cells as the template and the following are the sequences of the PCR primers (forward primer: 5′-CTGAGATCTCACCATGCAAAGAGATCACACC-3′; reverse primer: 5′-GGGGCTAGCTCACAAAAATAAGTCTTCT-3′). The coding sequence of sgk3 was cloned into the mammalian expression vector pMG-H2 using BglI-NheI restriction sites. All the inserts were verified by DNA sequencing.
Quantitative RT-PCR
Quantitative RT-PCR was performed as described previously (42). The primers used in real-time PCR were as follows: for SGK3 (nonsplicing variant specific), 5′-CCAGGAGTGAGTCTTACAG-3′ and 5′-CCAGCCACATTAGGATTA-3′; for SGK3 transcript variants 1and 2, 5′-GAGGAGCCGCCAGCGGCCGTGA-3′ and 5′-CGTTACACTTGGGCAGCTTTCC-3′; for SGK3 transcript variant 3, 5′-GCAGGTCCCGGCCGAGTGTGGC-3′ and 5′-CGTTACACTTGGGCAGCTTTCC-3′; and for β-actin, 5′-CACCAACTGGGACGACAT-3′ and 5′-GCACAGCCTGGATAGCAAC-3′.
Western blotting
Western blotting was performed as described previously (43). Primary antibodies for immunoblotting included mouse monoclonal SGK3 antibody (MCA5480Z; AbD SeroTec, Oxford, UK), rabbit polyclonal PARP antibodies (no. 9542; Cell Signaling Technology, Danvers, MA), and rabbit polyclonal cleaved PARP antibodies (no. 9541; Cell Signaling Technology).
ChIP assay
MCF-7 cells were cultured in phenol red-free medium containing 10% CD FBS for 72 h and then treated with DMSO or 100 nm E2 for 45 min. ChIP assay was performed as described previously (44), except that the antibodies used here are anti-ER rabbit polyclonal antibody (HC-20; Santa Cruz Biotechnology, Santa Cruz, CA). The sequences of the primers used in subsequent PCR are the following: for ER-binding region 1, 5′-CAGAACTTCCCCTTCTTTAACTGC-3′ (forward primer) and 5′-GCTGACCTACCAATTCAATGTTTC-3′ (reverse primer); for region 2, 5′-CCCAAGGGATAGGCACAGTA-3′ (forward primer) and 5′-TTCCCAAGGACTGACATCAA-3′ (reverse primer).
Luciferase reporter assays
MCF-7 cells were seeded in 12-well plates at 3 × 105 cells/well and cultured in phenol red-free MEM medium containing 5% CD FBS overnight. The cells in each well were transfected with 2 μg of Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and 0.5 μg reporter plasmids. Four hours after transfection, the cells were replaced with phenol red-free MEM medium with 5% CD FBS and treated with DMSO or E2 (100 nm). Luciferase activity of the cell lysates was assayed 24 h after transfection using luciferase assay substrate (Promega). Each transfection was performed in triplicate.
siRNA transfection
siRNA transfection was performed using siPORT NeoFX transfection agent (Ambion, Austin, TX) according to the manufacturer’s instruction on reverse transfection and carried out in either 24-well plates or 6-cm dishes. Briefly, cells were trypsinized and diluted in the medium at 1 × 105 cells/ml. For each well of a 24-well plate, 1 μl siPORT NeoFX transfection agent and 1.5 μl 10 μm SGK3 siRNA (Santa Cruz Biotechnology) or siRNA NC (Ambion) were diluted in 25 μl OPTI-MEM I medium (Invitrogen), respectively. For each 6-cm dish, 10 μl siPORT NeoFX transfection agent and 15 μl 10 μm SGK3 siRNA or siRNA NC were diluted in 250 μl OPTI-MEM I medium, respectively. After being mixed and incubated for 10 min, the mixtures of siRNA and transfection agent were dispersed into a 24-well plate or 6-cm dishes. Cell suspensions (1 × 105 cells/ml) were overlaid onto the transfection complexes at 0.5 ml cells/well or 5 ml cells/dish.
MTT assay
Cells were seeded into 24-well plates at 5 × 104 cells/well (for regular proliferation assay) or 1 × 105 cells/well (for cell viability assay after ICI182,780 treatment). At the indicated time after treatment, the medium in each well was removed and replaced with 0.5 ml fresh phenol red-free medium containing 0.5 mg/ml MTT and then incubated at 37 C for 1 h. The medium was discarded, and 0.5 ml DMSO was added to the well to dissolve the formazan dye trapped in the living cells. One hundred microliters of the supernatant were then transferred into a 96-well plate and read in the plate reader at A570.
Statistics analysis
Twenty-two microarray studies in the Oncomine database (http://www.oncomine.org) that reported on SGK3 expression in both ER-positive and ER-negative breast cancer specimens (n = 2366) were considered for a meta-analysis. The microarray studies were meta-analyzed using Fisher’s method as proposed by Rhodes et al. (25) and Smith et al. (26). We estimated means and sd values from Oncomine with approximations.
Acknowledgments
We thank Haiqing Li and Dr.Yate-Ching Yuan from Division of Information Science for the assistance in analyzing the ChIP-Seq data of ER and Lucy Brown (Analytical Cytometry Core) for flow cytometry analysis.
Footnotes
This work was supported by National Institutes of Health Grant CA44735 (to S.C.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 17, 2010
Abbreviations: aa, Amino acid; AGC, kinase family of protein kinase A, protein kinase G, and protein kinase C; AP, activator protein; CD, charcoal/dextran treated; ChIP, chromatin immunoprecipitation; ChIP-Seq, ChIP in combination with massively parallel DNA sequencing; DMSO, dimethylsulfoxide; E2, 17β-estradiol; ER, estrogen receptor; ERE, estrogen response element; FBS, fetal bovine serum; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NC, negative control; P, putative promoter; PARP, poly(ADP-ribose) polymerase; PDK1, phosphoinositide-dependent kinase-1; PI3K, phosphatidylinositol 3-kinase; PIP3, phosphatidylinositol 3,4,5-triphosphate; SGK, serum- and glucocorticoid-inducible kinase; siRNA, small interfering RNA.
References
- Foster JS, Henley DC, Ahamed S, Wimalasena J 2001 Estrogens and cell-cycle regulation in breast cancer. Trends Endocrinol Metab 12:320–327 [DOI] [PubMed] [Google Scholar]
- Parton M, Dowsett M, Smith I 2001 Studies of apoptosis in breast cancer. BMJ 322:1528–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perillo B, Sasso A, Abbondanza C, Palumbo G 2000 17β-estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol Cell Biol 20:2890–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrik V, Zheng Y, Harrow F, Chen Y, Foster DA 2005 Survival signals generated by estrogen and phospholipase D in MCF-7 breast cancer cells are dependent on Myc. Mol Cell Biol 25:7917–7925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Lein ES, Firestone GL 2001 Tissue-specific expression of the transcriptionally regulated serum and glucocorticoid-inducible protein kinase (Sgk) during mouse embryogenesis. Mech Dev 103:177–181 [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL 2002 The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2:489–501 [DOI] [PubMed] [Google Scholar]
- Cantley LC 2002 The phosphoinositide 3-kinase pathway. Science 296:1655–1657 [DOI] [PubMed] [Google Scholar]
- Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, Hennessy BT, Tseng H, Pochanard P, Kim SY, Dunn IF, Schinzel AC, Sandy P, Hoersch S, Sheng Q, Gupta PB, Boehm JS, Reiling JH, Silver S, Lu Y, Stemke-Hale K, Dutta B, Joy C, Sahin AA, Gonzalez-Angulo AM, Lluch A, Rameh LE, Jacks T, Root DE, Lander ES, Mills GB, Hahn WC, Sellers WR, Garraway LA 2009 AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell 16:21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier M, Woodgett JR 2006 Serum and glucocorticoid-regulated protein kinases: variations on a theme. J Cell Biochem 98:1391–1407 [DOI] [PubMed] [Google Scholar]
- Lang F, Böhmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V 2006 (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 86:1151–1178 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Deak M, Morrice N, Cohen P 1999 Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J 344(Pt 1):189–197 [PMC free article] [PubMed] [Google Scholar]
- Virbasius JV, Song X, Pomerleau DP, Zhan Y, Zhou GW, Czech MP 2001 Activation of the Akt-related cytokine-independent survival kinase requires interaction of its phox domain with endosomal phosphatidylinositol 3-phosphate. Proc Natl Acad Sci USA 98: 12908–12913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liu D, Gill G, Songyang Z 2001 Regulation of cytokine-independent survival kinase (CISK) by the Phox homology domain and phosphoinositides. J Cell Biol 154:699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Yang X, Songyang Z 2000 Identification of CISK, a new member of the SGK kinase family that promotes IL-3-dependent survival. Curr Biol 10:1233–1236 [DOI] [PubMed] [Google Scholar]
- Slagsvold T, Marchese A, Brech A, Stenmark H 2006 CISK attenuates degradation of the chemokine receptor CXCR4 via the ubiquitin ligase AIP4. EMBO J 25:3738–3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liao L, Qin J, Xu J, Liu D, Songyang Z 2009 Identification of Flightless-I as a substrate of the cytokine-independent survival kinase CISK. J Biol Chem 284:14377–14385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YR, Park J, Yu HN, Kim JS, Youn HJ, Jung SH 2005 Up-regulation of PI3K/Akt signaling by 17β-estradiol through activation of estrogen receptor-α, but not estrogen receptor-β, and stimulates cell growth in breast cancer cells. Biochem Biophys Res Commun 336:1221–1226 [DOI] [PubMed] [Google Scholar]
- Stoica GE, Franke TF, Wellstein A, Czubayko F, List HJ, Reiter R, Morgan E, Martin MB, Stoica A 2003 Estradiol rapidly activates Akt via the ErbB2 signaling pathway. Mol Endocrinol 17:818–830 [DOI] [PubMed] [Google Scholar]
- Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H 2001 Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor α: a new model for anti-estrogen resistance. J Biol Chem 276:9817–9824 [DOI] [PubMed] [Google Scholar]
- Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, Yu JS, Malmström PO, Mansukhani M, Enoksson J, Hibshoosh H, Borg A, Parsons R 2005 PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res 65:2554–2559 [DOI] [PubMed] [Google Scholar]
- Itoh T, Karlsberg K, Kijima I, Yuan YC, Smith D, Ye J, Chen S 2005 Letrozole-, anastrozole-, and tamoxifen-responsive genes in MCF-7aro cells: a microarray approach. Mol Cancer Res 3:203–218 [DOI] [PubMed] [Google Scholar]
- Reid G, Métivier R, Lin CY, Denger S, Ibberson D, Ivacevic T, Brand H, Benes V, Liu ET, Gannon F 2005 Multiple mechanisms induce transcriptional silencing of a subset of genes, including oestrogen receptor α, in response to deacetylase inhibition by valproic acid and trichostatin A. Oncogene 24:4894–4907 [DOI] [PubMed] [Google Scholar]
- Bourdeau V, Deschênes J, Laperrière D, Aid M, White JH, Mader S 2008 Mechanisms of primary and secondary estrogen target gene regulation in breast cancer cells. Nucleic Acids Res 36:76–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi F, Sonderegger B, Wirapati P, Delorenzi M, Naef F 2009 Relationship between estrogen receptor α location and gene induction reveals the importance of downstream sites and cofactors. BMC Genomics 10:381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DR, Barrette TR, Rubin MA, Ghosh D, Chinnaiyan AM 2002 Meta-analysis of microarrays: interstudy validation of gene expression profiles reveals pathway dysregulation in prostate cancer. Cancer Res 62:4427–4433 [PubMed] [Google Scholar]
- Smith DD, Saetrom P, Snove Jr O, Lundberg C, Rivas GE, Glackin C, Larson GP 2008 Meta-analysis of breast cancer microarray studies in conjunction with conserved cis-elements suggest patterns for coordinate regulation. BMC Bioinformatics 9:63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhmer C, Palmada M, Kenngott C, Lindner R, Klaus F, Laufer J, Lang F 2007 Regulation of the epithelial calcium channel TRPV6 by the serum and glucocorticoid-inducible kinase isoforms SGK1 and SGK3. FEBS Lett 581:5586–5590 [DOI] [PubMed] [Google Scholar]
- Diel P, Smolnikar K, Michna H 1999 The pure antiestrogen ICI 182780 is more effective in the induction of apoptosis and down regulation of BCL-2 than tamoxifen in MCF-7 cells. Breast Cancer Res Treat 58:87–97 [DOI] [PubMed] [Google Scholar]
- Lim KB, Ng CY, Ong CK, Ong CS, Tran E, Nguyen TT, Chan GM, Huynh H 2001 Induction of apoptosis in mammary gland by a pure anti-estrogen ICI 182780. Breast Cancer Res Treat 68:127–138 [DOI] [PubMed] [Google Scholar]
- Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL 1993 Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol 13:2031–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai F, Yu L, He H, Zhao Y, Yang J, Zhang X, Zhao S 1999 Cloning and mapping of a novel human serum/glucocorticoid regulated kinase-like gene, SGKL, to chromosome 8q12.3-q13.1. Genomics 62:95–97 [DOI] [PubMed] [Google Scholar]
- Lang F, Cohen P 2001 Regulation and physiological roles of serum- and glucocorticoid-induced protein kinase isoforms. Sci STKE 2001:re17 [DOI] [PubMed] [Google Scholar]
- Shanmugam I, Cheng G, Terranova PF, Thrasher JB, Thomas CP, Li B 2007 Serum/glucocorticoid-induced protein kinase-1 facilitates androgen receptor-dependent cell survival. Cell Death Differ 14:2085–2094 [DOI] [PubMed] [Google Scholar]
- Sherk AB, Frigo DE, Schnackenberg CG, Bray JD, Laping NJ, Trizna W, Hammond M, Patterson JR, Thompson SK, Kazmin D, Norris JD, McDonnell DP 2008 Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res 68:7475–7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M 2006 Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- Welboren WJ, van Driel MA, Janssen-Megens EM, van Heeringen SJ, Sweep FC, Span PN, Stunnenberg HG 2009 ChIP-Seq of ERα and RNA polymerase II defines genes differentially responding to ligands. EMBO J 28:1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M 2005 Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- Perillo B, Ombra MN, Bertoni A, Cuozzo C, Sacchetti S, Sasso A, Chiariotti L, Malorni A, Abbondanza C, Avvedimento EV 2008 DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science 319:202–206 [DOI] [PubMed] [Google Scholar]
- Kendall A, Anderson H, Dunbier AK, Mackay A, Dexter T, Urruticoechea A, Harper-Wynne C, Dowsett M 2008 Impact of estrogen deprivation on gene expression profiles of normal postmenopausal breast tissue in vivo. Cancer Epidemiol Biomarkers Prev 17:855–863 [DOI] [PubMed] [Google Scholar]
- Dunlap J, Le C, Shukla A, Patterson J, Presnell A, Heinrich MC, Corless CL, Troxell ML 2010 Phosphatidylinositol-3-kinase and AKT1 mutations occur early in breast carcinoma. Breast Cancer Res Treat 120:409–418 [DOI] [PubMed] [Google Scholar]
- Wee S, Wiederschain D, Maira SM, Loo A, Miller C, deBeaumont R, Stegmeier F, Yao YM, Lengauer C 2008 PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA 105:13057–13062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li Y, Chen B, Zhang Y, Lou G, Chen S, Zhou D 2008 Identification and characterization of PNRC splicing variants. Gene 423:116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri S, Phung S, Wang X, Wu X, Yuan YC, Wagman L, Chen S 2008 Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer Res 68:4910–4918 [DOI] [PubMed] [Google Scholar]
- Zhou D, Masri S, Ye JJ, Chen S 2005 Transcriptional regulation of the mouse PNRC2 promoter by the nuclear factor Y (NFY) and E2F1. Gene 361:89–100 [DOI] [PubMed] [Google Scholar]