Abstract
Dopamine agonist resistance or intolerance is encountered in approximately 20% of prolactinoma patients. Because human epidermal growth factor receptor 2 (HER2)/ErbB2 is overexpressed in prolactinomas and ErbB receptor ligands regulate prolactin (PRL) gene expression, we tested the role of HER2/ErbB2 in prolactinoma hormone regulation and adenoma cell proliferation to assess the rationale for targeting this receptor for prolactinoma therapy. As we showed prolactinoma HER2 overexpression, we generated constitutively active HER2-stable GH3 cell transfectants (HER2CA). PRL mRNA levels were induced approximately 250-fold and PRL secretion was enhanced 100-fold in HER2CA cells, which also exhibited increased proliferation. Lapatinib, a dual tyrosine kinase inhibitor (TKI) of both epidermal growth factor receptor (EGFR)/ErbB1 and HER2, blocked receptor signaling, and suppressed PRL expression more than gefitinib, a TKI of EGFR/ErbB1. Lapatinib also suppressed colony formation in soft agar more than gefitinib. Oral lapatinib treatment caused tumor shrinkage and serum PRL suppression both in HER2CA transfectant-inoculated Wistar-Furth rats and in estrogen-induced Fischer344 rat prolactinomas. In cultured human cells derived from resected prolactinoma tissue, lapatinib suppressed both PRL mRNA expression and secretion. These results demonstrate that prolactinoma HER2 potently induces PRL and regulates experimental prolactinoma cell proliferation. Because pituitary HER2 signaling is abrogated by TKIs, this receptor could be an effective target for prolactinoma therapy.
This study demonstrates a role for functional HER2/ErbB2 in lactotroph adenoma and a strategy for novel targeted therapy of lapatinib in prolactinoma.
Pituitary tumors are detected in up to 25% of random autopsies (1), and prolactinomas constitute the most prevalent hormone-secreting pituitary adenomas (2,3). Prolactinomas are benign monoclonal adenomas that hypersecrete prolactin (PRL) and usually present with amenorrhea, galactorrhea, and infertility in females, and sexual dysfunction, and sellar mass effects including headaches, visual dysfunction, and/or hypopituitarism, in males (4). Both sexes are at increased risk of osteoporosis (5). Drug treatment for this commonly encountered tumor is limited to dopamine agonists. If dopamine agonists are not effective in normalizing PRL levels or in shrinking tumor size, or if the patient cannot tolerate medication side effects, trans-sphenoidal adenoma resection may be considered (6,7). However, surgical cure rates for patients with invasive macroprolactinomas are poor, and even if resected, large prolactinomas tend to recur postoperatively (5).
Because PRL gene expression is regulated by the ErbB family receptor ligands, epidermal growth factor (EGF) and heregulin (HRG) (8,9,10), we hypothesized that EGF receptor (EGFR) inhibition would be effective for control of PRL secretion and tumor load in prolactinomas (8). Human epidermal growth factor receptor 2 (HER2)/ErbB2 is an orphan receptor that amplifies signaling by ErbB-containing heterodimers including the EGFR, by enhancing ligand-binding affinity and/or receptor recycling and stability (11,12,13). HER2/ErbB2 gene amplification or overexpression is associated with poor clinical outcomes in breast and non-small-cell lung cancers (14,15,16), and transgenic mice overexpressing HER2/ErbB2 develop mammary tumors and lung metastases (17,18). HER2/ErbB2 receptors are expressed in pituitary tumors (19,20,21), including prolactinomas (9). However, HER2/ErbB2 receptor function in pituitary tumors remains unknown. Lapatinib, a small-molecule tyrosine kinase inhibitor (TKI), targets both EGFR/ErbB1 and HER2/ErbB2 and reversibly binds cytoplasmic receptor kinase ATP-binding sites abrogating both MAPK and Akt pathway signaling (22).
Here we demonstrate functional in vitro and in vivo roles of HER2/ErbB2 in rat prolactinoma hormone regulation and cell proliferation and also show effects on HER2/ErbB2 overexpressing rat prolactinoma cells and on primary human prolactinoma cells derived from surgically resected prolactinoma tissue. The results support a rationale for ErbB targeted therapy in patients harboring PRL-secreting pituitary adenomas.
Results
HER2/ErbB2 overexpression enhances PRL expression and secretion and cell proliferation
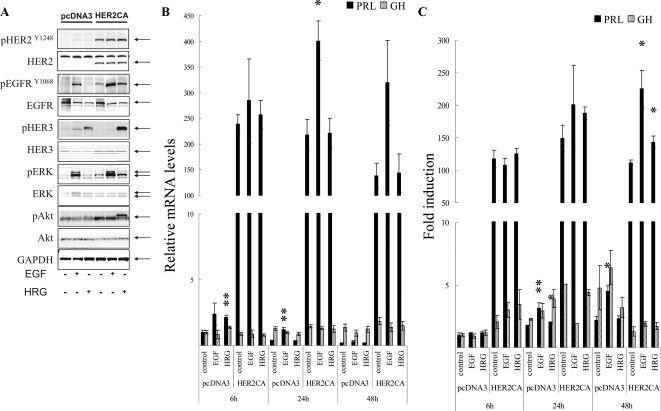
GH3 rat lactosomatotroph pituitary tumor cells (GH3) were stably transfected with an expression vector containing the constitutively active form (V654E) of HER2/ErbB2 cDNA (HER2CA) or empty vector (pcDNA3). Western blot results showed that HER2 and phospho-HER2 protein were induced approximately 10-fold in HER2CA transfectants (Fig. 1A). Cells expressing HER2CA also contained higher levels of phosphorylated EGFR, MAPK, and Akt, but less phospho-HER3 than pcDNA3 transfectants (Fig. 1A). EGF induction of both phosphorylated EGFR and MAPK, and HRG induction of both phosphorylated HER3 and Akt, was also enhanced in HER2CA cells (Fig. 1A). HER2CA cells exhibited a marked and selective induction (∼250 fold) of PRL mRNA (P < 0.0001), with no observed effects on GH mRNA expression (Fig. 1B). PRL, but not GH, secretion into the HER2CA cell medium was enhanced about 100-fold (Fig. 1C). As shown previously (8,9), both EGF and HRG induced PRL expression and secretion approximately 2-fold in each respective transfectant (Fig. 1, B and C).
Figure 1.
HER2 enhances PRL mRNA expression and secretion. A, GH3 cells stably expressing HER2CA or pcDNA3 (empty vector) were treated with 5 nm EGF or 6 nm HRG for 10 min, and Western blotting was performed. B, GH3 cells stably expressing HER2CA or pcDNA3 were treated with 5 nm EGF or 6 nm HRG for the indicated times, and real-time PCR analysis was performed. C, GH and PRL secretion in the culture medium was determined by RIA. Hormone secretion levels were normalized for cell numbers. Values are mean ± sem. *, P < 0.05; **, P < 0.01. Representative results are from triplicate samples in at least two or more independent experiments.
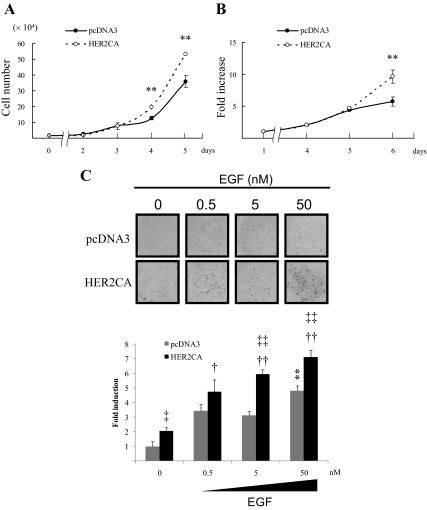
HER2CA cells proliferated faster than pcDNA3 transfectants as assessed by both water-soluble tetrazolium salt (WST-1) assays (1.8-fold on d 6; P < 0.01), and by cell counts (1.5-fold on d 5; P < 0.01) (Fig. 2, A and B). Furthermore, colony formation in soft agar was enhanced in HER2CA cells, and addition of EGF further enhanced dose-dependent colony formation 10 d after seeding both pcDNA3 (∼5-fold, P < 0.01) and HER2CA transfectants (∼8 fold, P < 0.01) (Fig. 2C).
Figure 2.
HER2 enhances cell proliferation. A, GH3 cells stably expressing HER2CA or pcDNA3 were plated (20,000 per well) in 12-well plates for the indicated times, and cells were counted. B, GH3 cells stably expressing HER2CA or pcDNA3 were plated (4000 per well) in 96-well plates for the indicated times, and WST-1 assay was performed. C, Stably transfected GH3 cells were seeded (4000 per well) for colony-forming assays, and EGF was added to the upper layer with cells and to the soft agar surface with serum-depleted media every third day. Colonies were counted from five randomly selected fields. Values are mean ± sem. *, P < 0.05; **, P < 0.01; ‡, P < 0.05 vs. pcDNA3 control; †, P < 0.05 vs. HER2CA control; ‡‡, P < 0.05 vs. pcDNA3 control; ††, P < 0.05 vs. HER2CA control. Representative results are from triplicate samples in at least two or more independent experiments.
Lapatinib suppresses PRL expression and secretion and cell growth
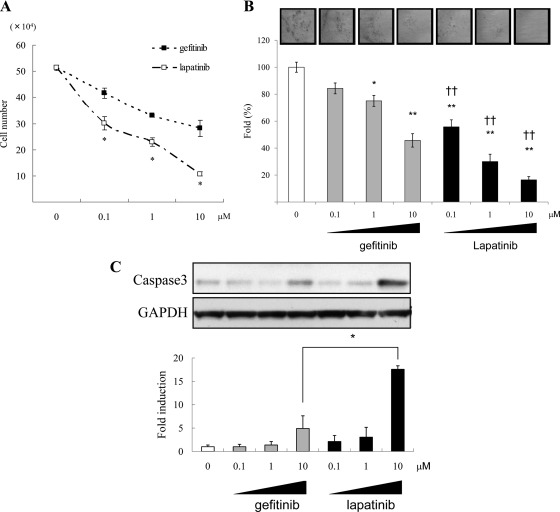
Because prolactinoma hormone secretion and cell proliferation were enhanced in constitutively active HER2/ErbB2 transfectants, we tested effects of lapatinib, a dual TKI, for both EGFR/ErbB1 and HER2/ErbB2 and compared these with effects of gefitinib, an EGFR/ErbB1 TKI (23). Lapatinib attenuated EGF-induced HER2 and MAPK autophosphorylation more markedly than gefitinib, and intracellular PRL levels were decreased by lapatinib but not by gefitinib (Fig. 3A). PRL mRNA levels were decreased by both drugs, although lapatinib elicited more marked suppression (Fig. 3B). In contrast, GH mRNA levels were not altered by either drug. Lapatinib also specifically suppressed PRL secretion by 40% at 24 (P < 0.05) and 48 h (P < 0.01), whereas treatment with gefitinib suppressed PRL secretion only at 24 h (Fig. 3C). These results indicate that lapatinib is more effective than gefitinib in suppressing PRL synthesis and secretion in these PRL-secreting adenoma cells.
Figure 3.
Lapatinib attenuates HER2 signaling and suppresses PRL more than gefitinib. A, GH3 cells stably expressing HER2CA were pretreated with gefitinib or lapatinib (0.1–10 μm) for 45 min before induction with EGF (5 nm) for 10 min, and Western blotting was performed. B, GH3 cells stably expressing HER2CA were treated with gefitinib or lapatinib (1 μm) for the indicated times, and real-time PCR analysis was performed. C, GH and PRL secretion in the culture medium was determined by RIA. Hormone secretion levels were normalized for cell numbers, Values are mean ± sem. *, P < 0.05; **, P < 0.01. Representative results are from triplicate samples in at least two or more independent experiments.
After 24 h treatment, cell number was decreased in a dose-dependent manner by lapatinib more than gefitinib (Fig. 4A). Colony formation in soft agar was also decreased in a dose-dependent manner by the TKIs, and fewer HER2CA colonies were observed with lapatinib than gefitinib treatment (Fig. 4B). As shown in Fig. 4C, lapatinib more than gefitinib dose-dependently induced cleaved caspase3, a pro-apoptotic mechanism for the observed inhibitory effects on cell growth.
Figure 4.
Dose-dependent effects of gefitinib or lapatinib on HER2CA GH3 proliferation and apoptosis. A, GH3 cells stably expressing HER2CA were treated with gefitinib or lapatinib (0.1–10 μm) for 24 h, and cells were counted. B, Stably transduced GH3 cells were seeded (4000 per well) for colony-forming assay, and gefitinib or lapatinib (0.1–10 μm) was added with serum-depleted media every third day. Colonies were counted from five randomly selected fields. C, GH3 cells stably expressing HER2CA were treated with gefitinib or lapatinib (0.1–10 μm) for 24 h, and Western blotting was performed. The ratio of cleaved caspase-3 vs. GAPDH was calculated by densitometric analysis of each treatment group. Values are mean ± sem. *, P < 0.05; **, P < 0.01; ††, P < 0.01 vs. same dose gefitinib treatment. Representative results are from triplicate samples in at least two or more independent experiments.
Lapatinib action on HER2CA GH3 tumors in vivo
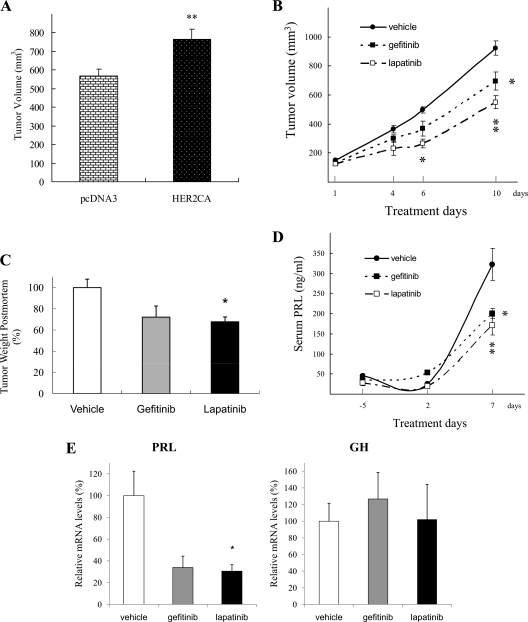
To evaluate in vivo effects of HER2 overexpression on lactosomatotroph tumor growth, HER2CA or pcDNA3 transfectants were inoculated sc into female Wistar-Furth rats. As shown in Fig. 5A, HER2CA tumors thus generated were larger than controls (766 ± 53 vs. 568 ± 38 mm3; P < 0.05). Next we examined effects of lapatinib administration on tumor growth and hyperprolactinemia. Rats were randomly assigned 3 d after tumor cell inoculation to receive daily lapatinib (100 mg/kg body weight), gefitinib (100 mg/kg body weight), or vehicle (0.5% methylcellulose, 0.5% Tween80/PBS; 100 μl) by oral gavage (n = 10 rats per group) for 10 d. As shown in Fig. 5B, HER2CA tumor volume was attenuated by lapatinib more than gefitinib (vehicle, 924 ± 48 mm3; gefitinib, 695 ± 63 mm3, P < 0.05; and lapatinib, 549 ± 45 mm3, P < 0.01). After euthanasia, tumors were excised and immediately weighed and processed. Postmortem tumor weights were suppressed by lapatinib approximately 40% (P = 0.019), whereas gefitinib suppressed tumor mass about 30% (P = 0.052). Serum PRL levels were also attenuated by both drugs, with lapatinib treatment resulting in approximately 50% suppression (P < 0.01), and gefitinib treatment about 40% suppression (P < 0.05) (Fig. 5D). Tumor PRL mRNA levels were also attenuated by the treatments (∼70%, P < 0.05), whereas tumor GH mRNA levels were unaltered (Fig. 5E).
Figure 5.
Lapatinib attenuates HER2CA GH3 tumor growth and hormone secretion in vivo more than gefitinib. A, GH3 cells stably expressing HER2CA (3 × 106 cells per rat, 0.2 ml with matrigel) or pcDNA3 were inoculated sc in WF rats (4–5 wk of age). Tumor volumes were measured 8 d after cell inoculation. **, P < 0.01 vs. pcDNA3. B and C, Rats were divided into three groups 3 d after inoculation; vehicle (0.5% methylcellose, and 0.5% tween 80/PBS), gefitinib (100 mg/kg), and lapatinib (100 mg/kg). B, Tumor weights were measured after euthanasia (C). Serum PRL levels were measured by RIA (D), and ex vivo real-time PCR was performed (E). Tumor volumes were calculated using the formula, π/6 × large diameter × small diameter2. Values are mean ± sem. *,P < 0.05; **, P < 0.01 vs. vehicle group.
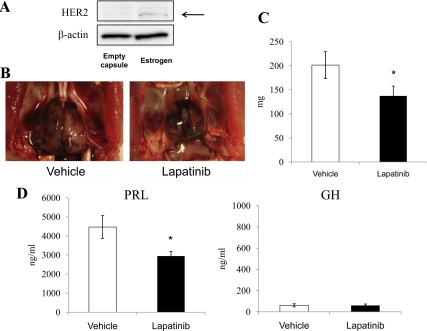
Next, we tested Fischer-344 rats treated with 17β-estradiol as a model for pituitary prolactinomas (24). One month after inoculation of 17β-estradiol-filled capsules, HER2 expression was elevated in pituitary tumors (Fig. 6A). Serum PRL levels were elevated, 2 months after inoculation, to 3330 ± 185 ng/ml (data not shown). We then extracted the capsule and initiated oral lapatinib or vehicle treatment for a subsequent 2 wk. As shown in Fig. 6B, pituitary tumor growth induced by estrogen was attenuated by lapatinib. Postmortem tumor measurements showed that lapatinib suppressed tumor weight by approximately 35% (P < 0.05; Fig. 6C), and serum PRL levels by about 35% (P < 0.05), whereas serum GH levels were not altered (Fig. 6D).
Figure 6.
Lapatinib attenuates estrogen-induced pituitary lactotroph tumor growth and hormone secretion in vivo. 17β-Estradiol-filled capsules or empty capsule were inoculated sc in F344 rats (4–5 wk of age) for 1 month. A, Western blotting analysis was performed using collected pituitary or pituitary tumor. Next, 17β-estradiol-filled capsules were inoculated sc in F344 rats (4–5 wk of age) for 2 months. After capsule excision, oral vehicle or lapatinib was administered for 2 wk. B, Representative pituitary tumor induced by estrogen was attenuated by lapatinib treatment. C, Tumor weights were measured after euthanasia. D, Serum PRL and GH levels were measured by RIA. Values are mean ± sem. *, P < 0.05 vs. vehicle group.
Lapatinib attenuates PRL mRNA expression and PRL secretion in human prolactinoma cells
To further support the rationale for clinical use of lapatinib in patients with prolactinoma, we tested drug effects in primary cell cultures derived from two surgically resected prolactinomas. Tumor A prolactinoma cultures showed that PRL mRNA levels, as measured by real-time PCR, were markedly suppressed by lapatinib (∼90%, P < 0.01), whereas gefitinib had no effect (Fig. 7A). PRL secretion into the culture medium was also suppressed about 70% by lapatinib (P < 0.01), whereas gefitinib exhibited more modest PRL suppression (∼50%, Fig. 7B). In cultured prolactinoma cells derived from tumor B, PRL mRNA levels were suppressed by lapatinib (∼45%, P < 0.01), and gefitinib by approximately 45% (P < 0.05, Fig. 7D). PRL secretion into the culture medium was also suppressed about 60% by lapatinib (P < 0.01), whereas gefitinib exhibited more modest PRL suppression (∼40%, Fig. 7E). Because rat PRL induction by EGF is mediated by MAPK (8), we tested U0126, a MAP kinase inhibitor in tumor B cultured cells. In the absence of added EGF, human PRL expression was suppressed approximately 55% by U0126 (P < 0.001, Fig. 7G).
Figure 7.
Lapatinib attenuates PRL secretion and mRNA expression in human prolactinoma cell cultures. A, B, D, and E, After trans-sphenoidal surgery of human prolactinomas, tumor cells were cultured. Prolactinoma cells (tumor A) were treated with lapatinib (0.1–10 μm) or gefitinib (10 μm) for 24 h, and real-time PCR of PRL was performed (A). PRL levels in culture media were measured using RIA (B). Hematoxylin and eosin (H&E) and PRL staining of tumor and confocal immunocytochemistry of EGFR and HER2 (tumor A) (C), or for tumor B (F). Magnification of these figures was ×100. Prolactinoma cells (tumor B) were treated with lapatinib (0.01–10 μm) or gefitinib (0.01–10 μm) for 24 h, and real-time PCR was performed (D). PRL levels in culture media were measured using RIA (E). Prolactinoma cells (tumor B) were treated with U0126 (0.1–5 μm) for 24 h, and real-time PCR of PRL was performed (G). Values are mean ± sem. *, P < 0.05; **, P < 0.01 vs. control; ***, P < 0.001 vs. control.
Using confocal immunofluorescence microscopy, we confirmed expression of both EGFR and HER2 in both prolactinoma tissues, and EGFR appeared localized to cell nuclei. Tumor A expressed HER2 on the membrane and cytoplasm, whereas in tumor B, both EGFR and HER2 were detected in nuclei (Fig. 7, C and F). Because both sensitivity and resistance of TKI for ErbB has been associated with an EGFR mutation (25,26,27), we subjected the patients’ pituitary adenoma DNA to sequencing of the EGFR and the HER2 TK domains including exons 18–24. No mutation was found in the TK domain exons of either receptors in tumor A, whereas two polymorphisms were detected in exon 20 (162093G > A) and exon 23 (179447T > C) of the EGFR in tumor B (data not shown). These results suggest that lapatinib suppresses PRL in the absence of such prolactinoma EGFR and HER2 mutations.
Discussion
We show that HER2/ErbB2 overexpression markedly induces PRL expression and secretion and cell growth in rat lactotroph tumor cells. EGF is known to activate PRL transcription (10), and subsequently EGF binding to prolactinoma tissue was reported (28), and EGF and its receptor expression were demonstrated in these tumors (29,30,31,32). HER2/ErbB2 expression has also been demonstrated in human prolactinomas (9,19,33). Here, we show that prolactinoma HER2/ErbB2 overexpression induces EGFR but not HER3 phosphorylation, with marked increase of PRL, suggesting that PRL induction is mainly mediated by HER2-EGFR heterodimerization rather than HER2-HER3 heterodimerization in these benign hormone-secreting tumors. Interestingly, HER2-HER3 has been shown as the most potently transforming and mitogenic receptor complex of this family in some cancers (34,35). Mechanisms for PRL induction by EGF have been reported as including MAPK-dependent cell pathways (8,36,37), and we also reported that HRG, a ligand for HER3, induced PRL in a HER2- and/or HER3-dependent manner (9). In the present study, both PRL mRNA expression and secretion and ERK phosphorylation were more markedly induced by EGF than by HRG in HER2CA cells. Because EGF only binds to EGFR, and HER2/ErbB2 heterodimerizes with all ErbB family members, these results support that HER2-EGFR heterodimers function to induce PRL.
HER2CA overexpression also induced tumor cell proliferation, at least partially, due to MAPK, although the mammalian target of rapamycin pathway has also been implicated in HER2-proliferative actions (13). EGF has been reported to either enhance or attenuate GH3 proliferation (38,39), and EGF treatment is here shown to enhance colony formation in soft agar. Furthermore, HER2CA-inoculated tumor growth was enhanced, suggesting that this receptor overexpression induces prolactinoma cell proliferation.
Because HER2/ErbB2 exhibits such marked selective functions in prolactinoma cells, we focused on this receptor as a therapeutic target for patients with prolactinomas. To determine effects of lapatinib as a novel targeted drug for prolactinoma, we employed four experimental approaches. These included in vitro experiments using HER2CA transfectants, an in vivo allograft model using Wistar-Furth rats inoculated with HER2CA transfectants, another in vivo lactotroph tumor model using Fisher344 rats inoculated with 17β-estradiol, and primary human prolactinoma cell cultures. We used concentrations of lapatinib and gefitinib (0.1 to 10 μm) for in vitro and 100 mg/kg for in vivo experiments as previously shown by others (40,41,42,43,44,45). The respective IC50 values of lapatinib and gefitinib for EGFR or HER2 have been demonstrated by Fabian et al. (46). Because high drug concentration could down-regulate transcriptional activity and induce apoptosis, we assessed GH expression as an internal negative control. We also showed previously that gefitinib treatment of AtT20 corticotroph cells that do not express the EGFR did not alter proliferation (8). The maximal nonlethal dose of gefitinib is 150 mg/kg (47). Although concentrations of gefitinib were high, the lapatinib doses used here were comparable to serum drug levels achieved in animal models and humans (43).
Both PRL gene expression and secretion were suppressed by lapatinib in all these experiments. Lapatinib effects were stronger and longer lasting than effects of gefitinib, supporting the critical function of HER2 to induce PRL expression and secretion. Lapatinib also showed superior inhibition of both cell proliferation and tumor growth. Moreover, induction of caspase-3 activity and reduction of soft agar colony formation, together with observed tumor shrinkage in vivo, support an antitumorigenic effect of lapatinib in HER2-overexpressing prolactinomas. To assess whether antitumorigenic effects of lapatinib are dependent on HER2 expression levels, we also treated pcDNA3 transfectants and tested cell number showing that lapatinib suppression of cell number is indeed enhanced in HER2-overexpressing transfectants (data not shown), suggesting that for prolactinoma, lapatinib is more effective when the tumor overexpresses HER2.
Human prolactinoma HER2 expression was confirmed by immunofluorescence, and nuclear EGFR location was also detected. Nuclear EGFR localization has been reported in breast, ovarian, and thyroid cancers (48,49,50), likely due to ligand-dependent nuclear translocation of the EGFR (51). Nuclear EGFR may act as a transcription factor (52) and as a direct inducer of proliferating cell nuclear antigen phosphorylation (53). Importantly, nuclear EGFR has been reported to be associated with acquired resistance to cetuximab, a monoclonal antibody directed against the human EGFR ligand-binding site (54). However, our results showed marked attenuation of PRL gene expression and secretion by lapatinib, suggesting that nuclear EGFR does not abrogate lapatinib inhibition of PRL in human prolactinomas expressing HER2. Recently blocking effects of lapatinib were reported on EGFR nuclear translocation (55), consistent with our findings of PRL suppression in the human prolactinoma culture experiments. To assess PRL-inhibitory effects of these drugs, RNA levels might be a more accurate indicator, because of more rigorous experimental normalization, suggesting that lapatinib clearly exhibits stronger effects in both tumor A and tumor B. Lower HER2 expression levels and predominant HER2 nuclear localization could therefore be consistent with less potent suppressive effects of lapatinib in tumor B than in tumor A. Taken together with our previous reports, the HER2 receptor is expressed in nine of 10 prolactinomas (9). In eight of nine tumors, HER2 was located on the membrane, suggesting the atypical HER2 nuclear location in tumor B. Furthermore, we did not identify an EGFR or HER2 tyrosine kinase domain missense mutation in these tumor cells, yet lapatinib suppressed PRL, suggesting drug efficacy in the absence of such mutations. Unfortunately, the availability of human prolactinoma tissue is extremely limited, and tumor tissue samples are fragmented and minute. These factors preclude large ex vivo human cell culture studies.
The in vitro, in vivo, and human ex vivo results indicate that lapatinib is a promising targeted drug as another treatment option for patients with prolactinomas, especially in HER2-overexpressing adenoma. Medical therapies for prolactinomas include the dopamine receptor agonists, bromocriptine and cabergoline (4). Because of its longer lasting and more potent effect, cabergoline is the preferable treatment choice for most patients with prolactinoma (56). Several approaches for treatment of prolactinoma patients resistant to or intolerant of cabergoline include dose increase with rise of concomitant side effects, surgical therapy, radiotherapy, or experimental treatments (4,57,58). Temozolomide has been reported as useful for malignant prolactinomas with variable results reported in a few cases (57,59,60).
In conclusion, we show that HER2/ErbB2 potently induces PRL secretion and regulates experimental prolactinoma cell proliferation. Because HER2/ErbB2 pituitary signaling is abrogated by TKIs, especially lapatinib, this receptor could be an effective target for medical therapy of prolactinomas.
Materials and Methods
Materials
DMEM/F12 (phenol red free), and penicillin/streptomycin were purchased from Invitrogen (Carlsbad, CA). EGF was from Sigma Chemical Co. (St. Louis, MO), and NRG1-β1/HRG1-β1 was from R&D Systems (Minneapolis, MN). Lapatinib (Tykarb) was from LC Laboratories (Woburn, MA), and gefitinib (Iressa) was purchased from Biaffin GmbH & Co. (Kassel, Germany). U0126 was purchased from Promega Corp. (Madison, WI).
Stable transfected cells
GH3 rat lactosomatotroph tumor cells secreting PRL and GH were purchased from the American Type Culture Collection (Manassas, VA). HER2CA cells were generated by transfection with pcDNA3-HER2CA (V654E; Addgene plasmid 16259) purchased from Addgene, Inc. (Cambridge, MA). Stable colonies were selected in the presence of 500 μg/ml G418 (Invitrogen). A vector control cell line pcDNA3 was simultaneously established by transfecting pcDNA3 that lacked inserted cDNA. After selection and propagation of stable transfectants, cells were cultured in DMEM/F12 medium containing 15% horse serum, 2.5% fetal bovine serum, penicillin/streptomycin, and 500 μg/m G418. After synchronization by serum starvation (medium containing 0.2% BSA for ∼24 h), cells with treatment agents was grown in fresh serum-depleted medium (0.2% BSA), and samples were collected at the indicated times.
Quantitative PCR
Total RNA was extracted with Trizol reagent (Invitrogen) according to instructions of the manufacturer. The amount and the integrity of RNA were assessed by measurement of absorbance at 260 and 280 nm. Total RNA was reverse transcribed into first-strand cDNA using iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer. Quantitative PCRs were carried out in the iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) as described elsewhere (8). Certified reverse transcription (2) primer assays for rat GH, rat PRL, and human PRL were purchased from SuperArray Biosciences Corp. (Frederick, MD). Primer sequences (Invitrogen) were as follows: 5′-GGACATCTAAGGGCATCACA-3′ [18S rRNA forward (F)]; 5′-TCAAGAACGAAAGTC-GGAGG-3′ [18S rRNA reverse (R)]; 5′-CATGTACGTTGCTATCCAGGC-3′ (human β-actin, F); 5′-CTCCTTAATGTCACGCACGAT-3′ (human β-actin, R); 5′-ACAACTTTGGTATCGTGGA-AGGA-3′ [human glyceraldehyde-3-phosphatase (GAPDH), F]; and 5′-GCCATCACGCCACAGTTTC-3′ (human GAPDH, R).
Western blotting
After completion of treatments, cells were placed on ice and washed with cold PBS. For protein extraction, cells were lysed in 100 μl radioimmune precipitation assay buffer (Sigma) containing complete protease inhibitor cocktail tablets (Roche Molecular Biochemicals, Indianapolis, IN) and phosphatase inhibitor cocktail 2 (Sigma). Lysates were centrifuged at 13,000 × g for 10 min at 4 C, and protein concentrations were determined by BCA protein assay reagent (Thermo Scientific, Rockford, IL). Western blot analysis was performed according to the guidelines of NuPAGE electrophoresis system protocol (Invitrogen). In brief, whole-cell lysates (∼50 μg protein per lane) were heated for 5 min at 100 C, respectively. Proteins were separated on 4–12% NuPAGE Bis-Tris gels and electrotransferred for 3 h to polyvinylidene difluoride membranes (Invitrogen). Membranes were blocked for 1 h in 5% nonfat dry milk or 5% BSA in Tris-buffered saline-Tween 20 (TBS-T) buffer, and incubated overnight with primary antibody. The following primary antibodies were used: anti-pErk1/2, anti-Erk1/2, anti-Akt, anti-pEGFR (Tyr1068), anti-EGFR, anti-pHER3/ErbB3 (Tyr1289), and anti-Cleaved Caspase-3 from Cell Signaling Technology (Danvers, MA), anti-pNeu (Tyr1248), anti-Neu (C-18), anti-HER3/ErbB3, anti-PRL, and anti-GAPDH from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and anti-pAkt (Ser473) from Abcam (Cambridge, MA). After washing with TBS-T, membranes were incubated with peroxidase-conjugated secondary antibody for 1 h (5% nonfat dry milk or 5% BSA in TBS-T buffer). Blots were washed and hybridization signals measured by enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, NJ).
Soft agarose colony-forming assay
Base layers consisting of growth medium containing 0.6% low-melting point agarose (Invitrogen) were poured onto six-well plates and allowed to solidify. Cells (8 ×103 per well) were plated in triplicate in top layers consisting of growth medium containing 0.3% agarose. Cells were stained 7–10 d later with 0.2% iodonitrotetrazolium chloride (Invitrogen), and colonies composed of more than or equal to 50 cells were counted manually in five randomly selected fields.
Cell proliferation assay
pcDNA3 and HER2CA cells were plated at a density of 2 × 104 per well in 12-well plates or 4 × 103 cells per well in 96-well plates with growth medium. For 12-well plates, cells were counted by hemocytometer at the indicated times. For 96-well plates, premixed WST-1 cell proliferation reagent (Roche Molecular Biochemicals) was added (1:10) at the indicated times and incubated for 4 h at 37 C in a humidified atmosphere maintained at 5% CO2, after which absorbance was measured at 450 nm. WST-1 is a colorimetric assay for quantification of cell proliferation and cell viability, based on the cleavage of the tetrazolium salt by mitochondrial dehydrogenases in viable cells.
Hormone assay
RIAs for rat GH and PRL were performed in duplicate, using reagents provided by the National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases (Harbor-UCLA Medical Center, Torrance, CA). Iodination of GH and PRL (5 μg) with iodine-125 (500 μCi) (PerkinElmer Life & Analytical Sciences, Boston, MA) mixed with 0.1 mg Iodo-Gen (Pierce Chemical Co., Rockford, IL) was performed using 10-ml columns prepared by G-75 Sephadex (Sigma Chemical Co.). Low interspecies cross-reactivity of the GH and PRL assays was previously shown (8).
Animals
In accordance with Institutional Animal Care and Use Committee guidelines, 4- to 5-wk-old female Wistar-Furth rats (Harlan Sprague Dawley, Inc., Indianapolis, IN) were inoculated with pcDNA3 (n = 6) or HER2CA (n = 6) cells (3 × 106 cells per rat). Rats were fed with a commercial pelleted diet ad libitum and tap water. Tumor volumes were measured with a caliper and calculated using the formula, π/6 × large diameter × small diameter2 as previously described (8). Next, 4- to 5-wk-old female Wistar-Furth rats were inoculated sc with HER2CA cells and from d 3 after sc inoculation, rats were divided into three groups (n = 10 per group) and treated with gefitinib (100 mg/kg), lapatinib (100 mg/kg), or vehicle (0.5% methylcellose, 0.5% Tween 80/PBS; 100 μl) via oral gavage daily for 1 wk. Blood (500 μl) was collected twice for hormone assessment (before inoculation, 2 d after inoculation) by retroorbital bleeding. This procedure was performed under isoflurane inhalational anesthesia via a nose cone connected to a rodent anesthesia machine (Kent Scientific Corp., Torrington, CT). On the last treatment day (d 7), rats were euthanized within 3 h of drug administration. Cardiac blood was collected with 18-gauge syringes and tumors were excised and weighed. Fragments of each tumor were fixed in formalin and embedded in paraffin for immunohistochemical staining, preserved in RNA later solution (Ambion, Inc., Austin, TX) for subsequent RNA extraction and frozen in liquid nitrogen for subsequent protein extraction.
Next, 4- to 5-wk-old ovariectomized female Fischer-344 rats (Harlan Sprague Dawley, Inc.) were sc implanted with an 17β-estradiol-filled SILASTIC brand capsule [Dow Corning Corp. (Midland, MI), Medical Grade Tubing Special; length, 3 cm; outer diameter, 0.125 in.; inner diameter, 0.062 in.] under isoflurane inhalational anesthesia. Two months thereafter, when serum PRL levels reached 3000 ng/ml, 17β-estradiol-filled capsules were extracted, and animals were treated with lapatinib (100 mg/kg) or vehicle (0.5% methylcellose, 0.5% Tween 80/PBS; 100 μl) via oral gavage twice a day for 2 wk. Blood (500 μl) was collected for hormone assessment by retroorbital bleeding under isoflurane inhalational anesthesia. On the last treatment day (d 14), rats were euthanized within 3 h of drug administration. Cardiac blood was collected and pituitary tumors were excised and weighed. Fragments of each tumor were fixed in formalin and embedded in paraffin, preserved in RNA later solution, or frozen in liquid nitrogen.
Cultures of prolactinoma-derived cells
Human prolactinoma tissues were obtained at the time of surgery (Pituitary Center, Cedars-Sinai Medical Center) and transferred in 0.3% BSA containing DMEM according to an IRB-approved protocol. After washing with medium, tumor tissues were chopped with a sterile scalpel into approximately 1- to 2-mm pieces. Tissues were rinsed and digested with DMEM containing 0.3% BSA, 0.35% collagenase, and 0.15% hyaluronidase at 37 C for 30 min. The mixture was centrifuged at 1,500 rpm for 5 min at 4 C, and the cell pellet was resuspended in an appropriate volume of culture medium containing 10% fetal bovine serum and antibiotics in 48-well plates. After 24 h incubation with serum-depleted starvation medium (DMEM with 0.3% BSA), treatment agents were added with fresh serum-depleted medium (0.3% BSA), and medium was collected for RIA. RNA was extracted after 24 h treatment. Medium was also collected at baseline. To normalize for cell number effect, the PRL value of treated medium was divided by that of pretreatment starvation medium to obtain a treated value for each well (n = 4).
Immunofluorescence
Tumor specimens were fixed in 10% formalin and embedded in paraffin. After deparaffinization and antigen retrieval, slides were blocked in 10% goat serum in 1% BSA/PBS and then incubated overnight with primary antibody at 4 C. The following antibodies were used: rabbit polyclonal anti-EGFR (ab2430; 1:50; Abcam), anti-Neu (C-18; 1:100; Santa Cruz Biotechnology). After washes, slides were incubated with Alexa Fluor goat antirabbit 488 (H+L) secondary antibodies (1:500; Invitrogen) and with Topro3 (Invitrogen) for 2 h at room temperature after which slides were mounted with Prolong Gold antifade reagent (Invitrogen). Confocal microscope images were obtained using TCS-SP confocal scanner (Leica Microsystems, Deerfield, IL) in a dual-emission mode to separate autofluorescence from specific staining. A spectral window from 500–550 nm wavelength detected emission of Alexa 488. A second window from 560–620 nm detected the autofluorescence contribution to the signal. In the final images, Alexa 488 appears green. Autofluorescence appears red. The two images were merged, so that all autofluorescence appears yellow, and true signals appear green.
DNA extraction and sequencing analysis
Genomic DNA was isolated by QiAamp DNA Micro Kit (QIAGEN, Chatsworth, CA) from frozen tissues following the manufacturer’s instructions. EGFR and HER2 sequences of the seven exons of the TK domain (exons 18–24) were detected using PCR-based direct sequencing. PCR amplification was done in 50 μl volume containing genomic DNA using Expand High Fidelity PCR System (Roche). DNA was amplified for one cycle at 94 C for 2 min, 30 cycles at 94 C for 20 sec, 55–65 C for 30 sec, and 68 C for 15 sec, followed by 7 min extension at 68 C. Primer Sequences for EGFR were 5′-GAGGTGACCCTTGTCTCTGTGT-3′ (exon 18 F); 5′-AGCCCAGAGGCCTGTGCCA-3′ (exon 18 R); 5′-CCAGATCACTGGGCAGCATGTGGCACC-3′ (exon 19 F); 5′-AGCAGGGTCTAGAGCAGAGCAGCTGCC-3′ (exon 19 R); 5′-ACTGACGTGCCTCTCCCTCC-3′ (exon 20 F); 5′-CCGTATCTCCCTTCCCTGATT-3′ (exon 20 R); 5′-ATCTGTCCCTCACAGCAGGGTC-3′ (exon 21 F); 5′-GGCTGACCTAAAG-CCACCT-3′ (exon 21 R); 5′-AATTAGGTCCAGAGTGAGTTAAC-3′ (exon 22 F); 5′-ACTTGCATGTCAGAGGATATAATG-3′ (exon 22 R); 5′-CATCAAGAAACAGTAACCAGTAATG-3′ (exon 23 F); 5′-AAGGCCTCAGCTGTTTGGCTAAG-3′ (exon 23 R); 5′-TTGACTGGAAGTGTCGCATCACC-3′ (exon 24 F); 5′-CATGTGACAGAACACAGTGACATG-3′ (exon 24 R); and for HER2 were 5′-GTGAAGTCCTCCCAGCCCGC-3′ (exon 18 F); 5′-CTCCCATCAGA-ACTGCCGACC-3′ (exon 18 R); 5′-TGGAGGACAAGTAATGATCTCCTGG-3′ (exon 19 F); 5′-AAGAGAGACCAGAGCCCAGACCTG-3′ (exon 19 R); 5′-GCCATGGCTGTGGTTTGTGATGG-3′ (exon 20 F); 5′-ATCCTAGCCCCTTGTGGACATAGG-3′ (exon 20 R); 5′-GGACTCTTGCTGGGCATGTGG-3′ (exon 21 F); 5′-CCACTCAGAGTTCTCCCATGG-3′ (exon 21 R); 5′-CCATGGGAGAACTCTGAGTGG-3′ (exon 22 F); 5′-TCCCTTCACATGCTGAGGTGG-3′ (exon 22 R); 5′-AGACTCCTGAGCAGAACCTCTG-3′ (exon 23 F); 5′-AGCCAGCACAGCTCAGCCAC-3′ (exon 23 R); 5′-ACTGTCTAGACCAGACTGGAGG-3′ (exon 24 F); and 5′-GAGGGTGCTCTTAGCCACAGG-3′ (exon 24 R). Sequencing was performed by Sequetech DNA Sequencing Service.
Statistical analysis
Results are expressed as mean ± sem. Differences were assessed by one-way ANOVA following by Scheffe’s F test. P < 0.05 was considered significant.
Acknowledgments
We thank Dr. Mark Greene from Cedars-Sinai Medical Center for helpful discussions, Dr. James Mirocha from the Cedars Sinai Biostatistics Core, and Dr. Kolja Wawrowsky from the Imaging Core.
Footnotes
This work was supported by National Institutes of Health Grants CA07597 (to S.M.) and K23DK085148-01 (to O.C.) and the Doris Factor Molecular Endocrinology Laboratory.
Disclosure Summary: Authors have no conflicts to disclose.
Abbreviations: EGF, epidermal growth factor; EGFR, EGF receptor; GAPDH, glyceraldehyde-3-phosphatase; HER2, human epidermal growth factor receptor 2; HRG, heregulin; PRL, prolactin; TBST, Tris-buffered saline-Tween20; TKI, tyrosine kinase inhibitor; WSR-1, water-soluble tetrazolium salt.
First Published Online November 24, 2010
References
- Melmed S 2003 Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest 112:1603–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A, Karavitaki N, Wass JA 2009 Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf) [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, LaPensee CR, LaPensee EW 2008 What can we learn from rodents about prolactin in humans? Endocr Rev 29:1–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam MP, Molitch ME, Lombardi G, Colao A 2006 Advances in the treatment of prolactinomas. Endocr Rev 27:485–534 [DOI] [PubMed] [Google Scholar]
- Klibanski A 2010 Clinical practice. Prolactinomas. N Engl J Med 362:1219–1226 [DOI] [PubMed] [Google Scholar]
- Olafsdottir A, Schlechte J 2006 Management of resistant prolactinomas. Nat Clin Pract Endocrinol Metab 2:552–561 [DOI] [PubMed] [Google Scholar]
- Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, Brue T, Cappabianca P, Colao A, Fahlbusch R, Fideleff H, Hadani M, Kelly P, Kleinberg D, Laws E, Marek J, Scanlon M, Sobrinho LG, Wass JA, Giustina A 2006 Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf) 65:265–273 [DOI] [PubMed] [Google Scholar]
- Vlotides G, Siegel E, Donangelo I, Gutman S, Ren SG, Melmed S 2008 Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res 68:6377–6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlotides G, Cooper O, Chen YH, Ren SG, Greenman Y, Melmed S 2009 Heregulin regulates prolactinoma gene expression. Cancer Res 69:4209–4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch GH, Potter E, Nicolaisen AK, Evans RM, Rosenfeld MG 1982 Epidermal growth factor rapidly stimulates prolactin gene transcription. Nature 300:192–194 [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, Press MF 1989 Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707–712 [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX 2001 Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2:127–137 [DOI] [PubMed] [Google Scholar]
- Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, Greene MI 2007 ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest 117:2051–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire WL 1987 Prognostic factors for recurrence and survival in human breast cancer. Breast Cancer Res Treat 10:5–9 [DOI] [PubMed] [Google Scholar]
- Varley JM, Swallow JE, Brammar WJ, Whittaker JL, Walker RA 1987 Alterations to either c-erbB-2(neu) or c-myc proto-oncogenes in breast carcinomas correlate with poor short-term prognosis. Oncogene 1:423–430 [PubMed] [Google Scholar]
- Hirsch FR, Varella-Garcia M, Cappuzzo F 2009 Predictive value of EGFR and HER2 overexpression in advanced non-small-cell lung cancer. Oncogene 28 (Suppl 1):S32–S37 [DOI] [PubMed] [Google Scholar]
- Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P 1988 Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell 54:105–115 [DOI] [PubMed] [Google Scholar]
- Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ 1992 Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA 89:10578–10582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzat S, Zheng L, Smyth HS, Asa SL 1997 The c-erbB-2/neu proto-oncogene in human pituitary tumours. Clin Endocrinol (Oxf) 46:599–606 [DOI] [PubMed] [Google Scholar]
- Roncaroli F, Nosé V, Scheithauer BW, Kovacs K, Horvath E, Young Jr WF, Lloyd RV, Bishop MC, Hsi B, Fletcher JA 2003 Gonadotropic pituitary carcinoma: HER-2/neu expression and gene amplification. Report of two cases. J Neurosurg 99:402–408 [DOI] [PubMed] [Google Scholar]
- Nose-Alberti V, Mesquita MI, Martin LC, Kayath MJ 1998 Adrenocorticotropin-producing pituitary carcinoma with expression of c-erbB-2 and high PCNA index: a comparative study with pituitary adenomas and normal pituitary tissues. Endocr Pathol 9:53–62 [DOI] [PubMed] [Google Scholar]
- Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, Keith BR, Murray DM, Knight WB, Mullin RJ, Gilmer TM 2001 The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Therap 1:85–94 [PubMed] [Google Scholar]
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M 2009 Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957 [DOI] [PubMed] [Google Scholar]
- Heaney AP, Horwitz GA, Wang Z, Singson R, Melmed S 1999 Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med 5:1317–1321 [DOI] [PubMed] [Google Scholar]
- Wang Q, Greene MI 2008 Mechanisms of resistance to ErbB-targeted cancer therapeutics. J Clin Invest 118:2389–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sánchez JM, Bastus R, Mayo C, Bertran-Alamillo J, et al. 2009 Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 361:958–967 [DOI] [PubMed] [Google Scholar]
- Cappuzzo F, Varella-Garcia M, Shigematsu H, Domenichini I, Bartolini S, Ceresoli GL, Rossi E, Ludovini V, Gregorc V, Toschi L, Franklin WA, Crino L, Gazdar AF, Bunn Jr PA, Hirsch FR 2005 Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non-small-cell lung cancer patients. J Clin Oncol 23:5007–5018 [DOI] [PubMed] [Google Scholar]
- Jaffrain-Rea ML, Petrangeli E, Lubrano C, Minniti G, Di Stefano D, Sciarra F, Frati L, Tamburrano G, Cantore G, Gulino A 1998 Epidermal growth factor binding sites in human pituitary macroadenomas. J Endocrinol 158:425–433 [DOI] [PubMed] [Google Scholar]
- Kontogeorgos G, Stefaneanu L, Kovacs K, Cheng Z 1996 Localization of epidermal growth factor (EGF) and epidermal growth factor receptor (EGFr) in human pituitary adenomas and nontumorous pituitaries: an immunocytochemical study. Endocr Pathol 7:63–70 [DOI] [PubMed] [Google Scholar]
- LeRiche VK, Asa SL, Ezzat S 1996 Epidermal growth factor and its receptor (EGF-R) in human pituitary adenomas: EGF-R correlates with tumor aggressiveness. J Clin Endocrinol Metab 81:656–662 [DOI] [PubMed] [Google Scholar]
- Theodoropoulou M, Arzberger T, Gruebler Y, Jaffrain-Rea ML, Schlegel J, Schaaf L, Petrangeli E, Losa M, Stalla GK, Pagotto U 2004 Expression of epidermal growth factor receptor in neoplastic pituitary cells: evidence for a role in corticotropinoma cells. J Endocrinol 183:385–394 [DOI] [PubMed] [Google Scholar]
- Onguru O, Scheithauer BW, Kovacs K, Vidal S, Jin L, Zhang S, Ruebel KH, Lloyd RV 2004 Analysis of epidermal growth factor receptor and activated epidermal growth factor receptor expression in pituitary adenomas and carcinomas. Mod Pathol 17:772–780 [DOI] [PubMed] [Google Scholar]
- Chaidarun SS, Eggo MC, Sheppard MC, Stewart PM 1994 Expression of epidermal growth factor (EGF), its receptor, and related oncoprotein (erbB-2) in human pituitary tumors and response to EGF in vitro. Endocrinology 135:2012–2021 [DOI] [PubMed] [Google Scholar]
- Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y 1996 A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol 16:5276–5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin BJ, Sela M, Yarden Y 1996 Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J 15:2452–2467 [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N, Chen S, Dunckley JA, LaPensee C, Kansra S 2009 Estrogen receptor-α mediates the epidermal growth factor-stimulated prolactin expression and release in lactotrophs. Endocrinology 150:795–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Bangaru ML, Sneade L, Dunckley JA, Ben-Jonathan N, Kansra S 2009 Epidermal growth factor receptor cross-talks with ligand-occupied estrogen receptor-α to modulate both lactotroph proliferation and prolactin gene expression. Am J Physiol Endocrinol Metab 297:E331–E339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LK, Vlodavsky I, Baxter JD, Gospodarowicz D 1980 Nuclear accumulation of epidermal growth factor in cultured rat pituitary cells. Nature 287:340–343 [DOI] [PubMed] [Google Scholar]
- Schonbrunn A, Krasnoff M, Westendorf JM, Tashjian Jr AH 1980 Epidermal growth factor and thyrotropin-releasing hormone act similarly on a clonal pituitary cell strain. Modulation of hormone production and inhbition of cell proliferation. J Cell Biol 85:786–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CF, Leggas M, Schuetz JD, Panetta JC, Cheshire PJ, Peterson J, Daw N, Jenkins III JJ, Gilbertson R, Germain GS, Harwood FC, Houghton PJ 2004 Gefitinib enhances the antitumor activity and oral bioavailability of irinotecan in mice. Cancer Res 64:7491–7499 [DOI] [PubMed] [Google Scholar]
- Leggas M, Panetta JC, Zhuang Y, Schuetz JD, Johnston B, Bai F, Sorrentino B, Zhou S, Houghton PJ, Stewart CF 2006 Gefitinib modulates the function of multiple ATP-binding cassette transporters in vivo. Cancer Res 66:4802–4807 [DOI] [PubMed] [Google Scholar]
- Nahta R, Yuan LX, Du Y, Esteva FJ 2007 Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol Cancer Ther 6:667–674 [DOI] [PubMed] [Google Scholar]
- Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, Liewehr DJ, Steinberg SM, Merino MJ, Rubin SD, Steeg PS 2008 Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst 100:1092–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu I, Blackwell K, Chen S, Slingerland J 2005 The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res 65:18–25 [PubMed] [Google Scholar]
- Ueda S, Basaki Y, Yoshie M, Ogawa K, Sakisaka S, Kuwano M, Ono M 2006 PTEN/Akt signaling through epidermal growth factor receptor is prerequisite for angiogenesis by hepatocellular carcinoma cells that is susceptible to inhibition by gefitinib. Cancer Res 66:5346–5353 [DOI] [PubMed] [Google Scholar]
- Fabian MA, Biggs III WH, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lélias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, et al. 2005 A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol 23:329–336 [DOI] [PubMed] [Google Scholar]
- Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG 2000 Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res 6:4885–4892 [PubMed] [Google Scholar]
- Lo HW, Xia W, Wei Y, Ali-Seyed M, Huang SF, Hung MC 2005 Novel prognostic value of nuclear epidermal growth factor receptor in breast cancer. Cancer Res 65:338–348 [PubMed] [Google Scholar]
- Marti U, Ruchti C, Kämpf J, Thomas GA, Williams ED, Peter HJ, Gerber H, Bürgi U 2001 Nuclear localization of epidermal growth factor and epidermal growth factor receptors in human thyroid tissues. Thyroid 11:137–145 [DOI] [PubMed] [Google Scholar]
- Xia W, Wei Y, Du Y, Liu J, Chang B, Yu YL, Huo LF, Miller S, Hung MC 2009 Nuclear expression of epidermal growth factor receptor is a novel prognostic value in patients with ovarian cancer. Mol Carcinog 48:610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia W, Wei Y, Bartholomeusz G, Shih JY, Hung MC 2005 Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell 7:575–589 [DOI] [PubMed] [Google Scholar]
- Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC 2001 Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol 3:802–808 [DOI] [PubMed] [Google Scholar]
- Wang SC, Nakajima Y, Yu YL, Xia W, Chen CT, Yang CC, McIntush EW, Li LY, Hawke DH, Kobayashi R, Hung MC 2006 Tyrosine phosphorylation controls PCNA function through protein stability. Nat Cell Biol 8:1359–1368 [DOI] [PubMed] [Google Scholar]
- Li C, Iida M, Dunn EF, Ghia AJ, Wheeler DL 2009 Nuclear EGFR contributes to acquired resistance to cetuximab. Oncogene 28:3801–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HP, Yoon YK, Kim JW, Han SW, Hur HS, Park J, Lee JH, Oh DY, Im SA, Bang YJ, Kim TY 2009 Lapatinib, a dual EGFR and HER2 tyrosine kinase inhibitor, downregulates thymidylate synthase by inhibiting the nuclear translocation of EGFR and HER2. PLoS One 4:e5933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlechte JA 2003 Clinical practice. Prolactinoma. N Engl J Med 349:2035–2041 [DOI] [PubMed] [Google Scholar]
- Goffin V, Bernichtein S, Touraine P, Kelly PA 2005 Development and potential clinical uses of human prolactin receptor antagonists. Endocr Rev 26:400–422 [DOI] [PubMed] [Google Scholar]
- Passos VQ, Fortes MA, Giannella-Neto D, Bronstein MD 2009 Genes differentially expressed in prolactinomas responsive and resistant to dopamine agonists. Neuroendocrinology 89:163–170 [DOI] [PubMed] [Google Scholar]
- Kovacs K, Horvath E, Syro LV, Uribe H, Penagos LC, Ortiz LD, Fadul CE 2007 Temozolomide therapy in a man with an aggressive prolactin-secreting pituitary neoplasm: morphological findings. Hum Pathol 38:185–189 [DOI] [PubMed] [Google Scholar]
- McCormack AI, McDonald KL, Gill AJ, Clark SJ, Burt MG, Campbell KA, Braund WJ, Little NS, Cook RJ, Grossman AB, Robinson BG, Clifton-Bligh RJ 2009 Low O6-methylguanine-DNA methyltransferase (MGMT) expression and response to temozolomide in aggressive pituitary tumours. Clin Endocrinol (Oxf) 71:226–233 [DOI] [PubMed] [Google Scholar]