Abstract
Melanocortin 1 receptor (MC1R), a Gs protein-coupled receptor expressed in melanocytes, is a major determinant of skin pigmentation, phototype and cancer risk. Upon stimulation by αMSH, MC1R triggers the cAMP and ERK1/ERK2 MAPK pathways. In mouse melanocytes, ERK activation by αMSH binding to Mc1r depends on cAMP, and melanocytes are considered a paradigm for cAMP-dependent ERK activation. However, human MC1R variants associated with red hair, fair skin [red hair color (RHC) phenotype], and increased skin cancer risk display reduced cAMP signaling but activate ERKs as efficiently as wild type in heterologous cells, suggesting independent signaling to ERKs and cAMP in human melanocytes. We show that MC1R signaling activated the ERK pathway in normal human melanocytes and melanoma cells expressing physiological levels of endogenous RHC variants. ERK activation was comparable for wild-type and mutant MC1R and was independent on cAMP because it was neither triggered by stimulation of cAMP synthesis with forskolin nor blocked by the adenylyl cyclase inhibitor 2′,5′-dideoxyadenosine. Stimulation of MC1R with αMSH did not lead to protein kinase C activation and ERK activation was unaffected by protein kinase C inhibitors. Conversely, pharmacological interference, small interfering RNA studies, expression profiles, and functional reconstitution experiments showed that αMSH-induced ERK activation resulted from Src tyrosine kinase-mediated transactivation of the stem cell factor receptor, a receptor tyrosine kinase essential for proliferation, differentiation, and survival of melanocyte precursors, thus demonstrating a functional link between the stem cell factor receptor and MC1R. Moreover, this transactivation phenomenon is unique because it is unaffected by natural mutations impairing canonical MC1R signaling through the cAMP pathway.
In human melanocytes αMSH activates ERK1/2 by MC1R-mediated, cAMP-independent transactivation of cKIT tyrosine kinase, probably via Src. ERK activation is efficient for melanoma-associated MC1R variants.
Melanocytes are skin cells specialized in the biosynthesis of photoprotective melanin pigments. Their proliferation and the synthesis of melanins are tightly controlled by interacting chemical and physical cues (1,2). Among the signaling cascades triggered by these signals, the cAMP and the MAPK ERK1 and ERK2 pathways have been intensively analyzed. cAMP induces melanocyte differentiation in vitro (3) and in vivo (4), and ERK signaling is crucial for the control of both proliferation (5) and melanogenesis, through the activation of the cAMP response element binding protein (CREB) (6) and the microphthalmia-associated transcription factor (MITF), a master regulator of melanocyte development, differentiation, and proliferation (7).
cAMP production in melanocytes is strongly stimulated by αMSH and related peptide hormones collectively named melanocortins (MCs). MCs activate the melanocortin 1 receptor (MC1R), a G protein-coupled receptor (GPCR) that regulates the amount and type of melanin pigments and is a major determinant of skin phototype, sensitivity to ultraviolet radiation, and skin cancer risk (8). cAMP is responsible for key melanogenic effects of αMSH (3), namely activation of the rate-limiting enzyme tyrosinase and a switch from production of light-colored and poorly photoprotective pheomelanins to darker and more photoprotective eumelanins (9). Many of these cAMP-dependent differentiation effects are due to transcriptional induction of MITF.
On the other hand, the MAPK module involving ERK1 and ERK2 is a major intracellular signaling pathway that controls key cellular decisions such as proliferation, differentiation, or migration (10). This pathway is normally initiated by binding of growth factors to cell surface tyrosine kinase receptors [receptor tyrosine kinases (RTKs)] and recruitment of Grb2 and the guanine nucleotide exchange factor Sos, leading to activation of RAt sarcoma (RAS) GTPase. The GTP-bound active form of RAS then activates members of the rapid accelerated fibrosarcoma (RAF) family of protein kinases, which in turn phosphorylate and thereby activate the MAPK kinase (MEK) (11,12). MEK phosphorylates and activates ERK1 and ERK2. Active ERKs phosphorylate cytoplasmic and cytoskeletal proteins and translocate to the nucleus to regulate the activity of several transcription factors (13). The crucial role of this pathway in melanocytes is demonstrated by the occurrence of mutations in BRAF or NRAS in approximately 50 or 20–30% of human melanomas, respectively (14,15,16,17). Because NRAS and BRAF mutations are mutually exclusive in melanoma (18), they collectively account for ERK hyperactivation in more than 75% of these tumors.
Within melanocytes, the ERK module is activated by the stem cell factor receptor (cKIT), a RTK crucial for melanogenesis, proliferation, migration, and survival of the pigment-producing cells (19). In mice, cKIT maps to the dominant white spotting (w) locus, and its endogenous ligand stem cell factor (SCF; KIT ligand, mast cell growth factor) to the sl locus. Mutations in either one of these loci cause a pleitropic phenotype with white spotting of the fur due to absence of melanocytes, lack of mast cells, and defects in hematopoiesis and germ cell development (20). cKIT mutations have also been identified in human piebaldism (21,22) and at low frequency in human melanomas (23), and cKIT expression is often lost in these tumors (24,25).
Several GPCRs can activate the ERK module by a variety of mechanisms that rely on the activity of second-messenger regulated protein kinases such as protein kinase C (PKC), non-RTKs such as Src, or on the generation of intracellular signaling complexes formed by binding of β-arrestins or other scaffolds to the activated GPCRs (26,27,28). Moreover, GPCRs can stimulate cytosolic or membrane-bound metalloproteases that cleave the membrane-anchoring domain of RTK ligands and release the active agonist, thus promoting receptor activation (28,29,30).
In mouse melanoma cells, MC1R activation by MCs is positively coupled to cAMP and ERK signaling. Based on data from the B16 mouse melanoma model, it has been proposed that ERK activation in melanocytic cells is due to cAMP-dependent but protein kinase A (PKA)-independent activation of NRAS and BRAF (31,32,33). However, recent findings suggest that the situation in human melanocytes might be different. The human MC1R gene is extremely polymorphic (34), and several variant alleles are associated with red hair and fair skin (the RHC phenotype) (8,35,36,37) and increased risk for melanoma and nonmelanoma skin cancer (38,39). Three frequent and highly penetrant RHC alleles, R151C, R160W, and D294H (36,40), are diminished function forms with reduced functional coupling to the cAMP cascade (41,42,43,44,45,46,47). At least for R151C and R160W, this functional impairment results mainly from aberrant trafficking with intracellular retention and reduced cell surface expression, rather than from binding or signaling defects (41,44,46). However, these hypomorphic variants retain normal signaling to the ERKs when expressed in heterologous PC12 cells (48). Moreover, attempts to detect ERK activation in normal human melanocytes (NHMs) treated with the strong cAMP inducer forskolin (FSK) have been unsuccessful (49). In addition, activation of CREB by ERK-dependent phosphorylation has been reported in NHMs treated with mitogenic RTK ligands but not with cAMP analogs (6). These observations suggest that activation of the ERK module by human MC1R might be due to unknown cAMP-independent events.
Here we show that the RHC variants of the MC1R with reduced or absent signaling to cAMP activate the ERKs as efficiently as wild-type (WT) MC1R in a physiological setting, both in human melanoma cells and NHMs. We also show that ERK activation is independent on cAMP, PKA, PKC, or Ca2+. Instead, positive functional coupling of MC1R to the ERKs relies on the transactivation of cKIT. Moreover, our data show that αMSH activates the Src non-RTK in melanoma cells independently on cAMP and that Src is involved in activation of the ERKs downstream of MC1R, most likely by mediating αMSH-induced cKIT transactivation. These findings might have important and unexpected implications for our understanding of the functional connections of the main signaling pathways controlling human melanocyte proliferation and differentiation. Moreover, they provide new insights for the rational design of photoprotective strategies (4).
Results
ERK activation in NHMs expressing variant MC1R
It has been reported that activation of the ERK pathway in mouse melanoma cells stimulated with αMSH is mediated by cAMP (31,32). Concerning human MC1R, it has been shown that the melanoma-associated RHC mutants R151C, R160W, and D294H are hypomorphic in signaling via the cAMP pathway, in either melanocytic (44,45) or heterologous cells (43,44,45), but they are as effective as WT in triggering ERK activation when expressed in heterologous PC12 cells (48). Because this result could be affected by receptor overexpression or depend on the cell type, we tested variant MC1R signaling to ERK in NHM cultures of defined MC1R genotype, at physiological levels of endogenous receptor expression and hormone concentration. No cultures homozygous for any of the RHC variants were found, but we identified two compound heterozygotes derived from Caucasian donors (R160W/D294H, culture 830c, and R151C/R160W, culture 1307c). These cultures do not respond to αMSH with detectable increases in intracellular cAMP or tyrosinase activity as opposed to a control culture (1377b) WT for MC1R (49,50). For these control cells, preliminary experiments showed a detectable ERK activation, as shown by increased levels of phosphorylated ERK (pERK) comparable for αMSH and its synthetic analog [Nle4, d-Phe7]-α (NDP)-MSH (not shown). In 1377b (WT for MC1R) or 830c (R160W/D294H) NHMs, the ERKs were activated after a 15-min stimulation with 10−9 m αMSH (Fig. 1A). ERK activation was at least as intense in 830c mutant cells as in WT cells (Fig. 1B). A second heterozygote NHM culture (1307c, MC1R genotype R151C/R160W) yielded similar results with higher pERK levels than WT cells after hormonal stimulation (Fig. 1, A and B). Therefore, activation of the ERKs by physiological concentrations of MC1R agonists was comparable in NHMs expressing endogenous WT or variant MC1R. Notably, the potent adenylyl cyclase stimulator FSK did not activate the ERKs in 1307 cells (Fig. 1, A and B) or in several other NHM cultures of different MC1R genotype tested to date (not shown), whereas endothelin 1 treatment caused the expected strong stimulation of ERK phosphorylation (49).
Figure 1.
Melanocortin signaling to ERK in NHMs and melanoma cells of defined MC1R genotype. A, NHM expressing WT MC1R (1377b culture) or variant heterozygotes for the R160W and D294H alleles (830c culture) or the R151C and R160W variants (culture 1307c) were stimulated for 15 min with αMSH (10−9 m), 10−9 m endothelin 1 (ET-1), or 10−5 m FSK, as indicated. Cell extracts were analyzed for pERK by Western blot and for total ERK2 as loading control. A representative blot of two independent experiments is shown. B, Quantification of two independent experiments, normalized to the intensity of the pERK signal in control cells. Results are given as mean ± range. C, ERK activation by NDP-MSH in HBL human melanoma cells. Cells were serum starved for 3 h and then treated with NDP-MSH (10−7 m, 5 min) with or without the MEK inhibitor PD98059 (50 μm). Cell lysates were analyzed for ERK1/2 phosphorylation and total ERK2 as loading control. D, ERK-dependent morphological changes induced by NDP-MSH in HBL cells. Cells grown in six-well plates were treated with NDP-MSH for 48 h in the presence or absence of PD98059 and photographed. The boxed areas in the central micrographs are enlarged and shown at a higher magnification.
The results presented above strongly suggest that in human melanocytic cells MC1R signals to the ERK module independently on cAMP. Because NHMs are extremely difficult to grow in the absence of potentially interfering cytokines and growth factors, we looked for a suitable human melanoma cell line to study the mechanism of ERK activation by MC1R. The tyrosinase-positive pigmented HBL melanoma cells are WT for the MC1R (47,51) as well as for NRAS and BRAF (our unpublished data). HBL cells stimulated with NDP-MSH showed increased ERK phosphorylation that was blocked by the MEK inhibitor PD98059 (Fig. 1C). A MC-induced change in cell shape with increased dendricity was equally abolished by PD98059 (Fig. 1D). Consistent with their WT MC1R genotype and with previous results (47), HBL cells responded to NDP-MSH with a strong time-dependent increase in intracellular cAMP with maximal levels between 15 and 30 min after addition of the MC agonist to the cultures (not shown). Given these normal and robust responses, HBL cells were subsequently used as models.
MC1R signaling to ERK in human melanoma cells is independent of cAMP
The RHC variants R151C, R160W, and D294H have been shown to act as partial dominant-negative mutants for WT MC1R signaling through the cAMP pathway (41,44,45). Therefore, it was of interest to analyze their effects on MC signaling to the ERKs in HBL cells. Cells were stably transfected with WT, R151C, R160W, or D294H, and one representative clone for each variant expressing near-physiological levels of the receptor was selected (46) and analyzed. Cells expressing WT MC1R and challenged with NDP-MSH showed a marked increase in pERK, comparable with the response to the PKC activator phorbol-12-myristate-13-acetate (PMA) used as a positive control (Fig. 2A). pERK levels were maximal 5 min after stimulation and then returned to control levels. FSK failed to trigger detectable ERK activation. Clones expressing variant MC1R behaved similarly in terms of the strength and kinetics of ERK activation (Fig. 2A). Conversely, MC-induced cAMP production was strongly repressed in HBL cells expressing variant MC1R (Fig. 2B), consistent with a dominant-negative behavior of the mutants on signaling through the cAMP pathway (41,44,45). Overall, these data showed that: 1) MC1R activated the ERK module, even at the low levels of cell surface expression found in parental HBL melanoma cells (45,46); 2) the RHC variants expressed in melanoma cells did not exert dominant-negative effects on WT MC1R signaling to ERK but apparently contributed to agonist-dependent ERK phosphorylation; and 3) a positive effect of cAMP on MC1R-dependent ERK activation was unlikely. On the other hand, the R151C and R160W receptor forms are strongly retained in intracellular compartments with cell surface expression levels approximately 5 times lower than WT (44,46), a feature that accounts for their decreased functional coupling to the cAMP pathway and for their dominant negative behavior. Thus, the comparable stimulation of the ERK shown in Fig. 2A was surprising.
Figure 2.
ERK activation by NDP-MSH in human melanoma cells expressing variant MC1R. A, Clones of HBL cells stably expressing the MC1R forms indicated on top of each blot were stimulated with NDP-MSH (10−7 m) for the times shown, PMA (0.1 ng/μl, 15 min), or FSK (10−5 m, 15 min). Cell extracts were blotted for pERK. The experiment was repeated at least three times with similar results. B, Agonist-induced cAMP levels after stimulation with NDP-MSH in HBL cells expressing WT or variant MC1R. Cells were serum deprived for 3 h before agonist challenge for 30 or 180 min. Results are given as mean ± sem (n ≥ 4). C, Dose dependence of NDP-MSH-induced ERK phosphorylation in HBL cells expressing endogenous WT MC1R. Cells were serum deprived and challenged with the indicated concentrations of NDP-MSH for 5 min before estimation of pERK levels by Western blot. A representative blot of three is shown. D, Comparison of the changes in pERK levels (squares, left axis) and cAMP accumulation (triangles, right axis) in HBL cells treated with the indicated concentrations of NDP-MSH. Results are the mean ± sem (n ≥ 3).
We addressed this point by comparing the dose-response curves for agonist-induced stimulation of the cAMP and ERK pathways in HBL cells expressing physiological levels of the MC1R (∼0.3 fmol of NDP-MSH binding sites per microgram of protein) (44,46). The saturation curve for ERK activation was strongly left shifted relative to cAMP production, with EC50 values approximately 2 orders of magnitude lower (Fig. 2, C and D). Similar results have been previously reported in a heterologous cellular model (48), although in this case the strong overexpression of the receptor protein did not allow for an adequate interpretation of the data. Therefore, much lower levels of hormone receptor complexes were required to achieve maximal ERK stimulation compared with cAMP production, thus accounting for the smaller effects of RHC mutations on signaling to the ERK pathway. This suggests that the plasma membrane levels of MC1R, which are limiting for stimulation of the cAMP pathway (52), are not the limiting factor for ERK activation.
However, because functional coupling of MC1R to the ERK pathway is currently attributed to cAMP (31,32), we performed additional experiments to address this issue. First, we incubated HBL cells with FSK for times ranging from 5 to 30 min to exclude that failure to detect cAMP-dependent ERK activation could be due to inadequate selection of the time window (Fig. 3A). FSK did not activate the ERKs at the time points shown or at longer times (1 or 3 h, not shown) and even caused a small reduction in the levels of phosphorylated ERK. Although this inhibitory effect was difficult to quantify owing to the already low basal levels of ERK phosphorylation, it seemed reproducible (see, for instance, Fig. 3B). These data are in line with the inhibition of ERK signaling by cAMP in most cell types (53,54) and with reports of suppression of ERK activity after stimulation of cAMP production with FSK in RAS-mutated human melanoma cells (55). Next we compared the levels of cAMP and active ERKs in cells stimulated with NDP-MSH or FSK in the presence or absence of the specific adenylyl cyclase inhibitor 2′, 5′-dideoxyadenosine (DDA). As shown in Fig. 3, B and C, DDA had no effect on MC-dependent ERK activation but effectively abolished MC or FSK-induced production of cAMP. Overall, these results show that cAMP is neither sufficient nor necessary to trigger ERK activation in human melanocytic cells.
Figure 3.
ERK activation in HBL cells is independent of cAMP. A, Inability of FSK to induce ERK phosphorylation. Serum-starved HBL cells were incubated with FSK (10−5 m) for the times indicated or with the positive controls NDP-MSH (10−7 m, 5 min) or PMA (0.1 ng/μl, 15 min). Cell lysates were analyzed for pERK or ERK2 as loading control. B, The adenylyl cyclase inhibitor DDA does not block MC1R-induced ERK activation. HBL cells were challenged with NDP-MSH (10−7 m, 5 or 30 min) or FSK (10–5 m, 30 min), with or without DDA (2.5 mm, 30 min). ERK phosphorylation was detected by Western blot. C, HBL cells were treated as above and intracellular cAMP levels were determined. Results are given as mean ± sem (n ≥ 4).
Functional coupling of MC1R to the ERK module involves transactivation of a RTK
Regulation of ERK signaling by GPCRs is complex and dependent on the cellular context. In addition to the cAMP pathway, the PKC cascade links several GPCRs to the ERKs, either by direct PKC-dependent phosphorylation of members of the ERK module or indirectly via activation of non-RTKs such as Src (27,28,30). ERK activation can also depend on Gβγ complexes dissociated from the Gα subunit on activation of heterotrimeric G proteins (56) or on the formation of signaling complexes recruited by the GPCRs on phosphorylation by G protein-coupled receptor kinases followed by binding of β-arrestins, which act as scaffolds for proteins of the ERK module (26). Moreover, several GPCRs trigger ERK signaling via transactivation of RTKs such as the epidermal growth factor receptor (EGFR) by incompletely defined mechanisms (27,28,30). We tested these possible modes of coupling using HBL cells as a model for cells of the melanocytic lineage.
To study the role of the diacylglicerol/Ca2+-activated PKC, we analyzed both PKC activation by NDP-MSH and the effects of the specific PKC inhibitor Ro31–8425 on MC-dependent ERK activation. PKC activity was estimated by detection of phosphorylation of the myristoylated alanine-rich PKC substrate (MARCKS), a major and ubiquitous PKC substrate (57). No evidence of PKC activation was obtained in cells treated with NDP-MSH, as shown by lack of detectable MARCKS phosphorylation as opposed to rapid and strong phosphorylation in cells stimulated with PMA (Fig. 4A). This response was specific for PKC activation because it was abolished by preincubation with Ro31-8425 but was not sensitive to the PKA inhibitor isomer (Rp)-cAMP or the MEK inhibitor PD98059. Moreover, Rp-cAMP and Ro31-8425 failed to decrease MC-mediated activation of the ERKs (Fig. 4B). In addition, because PKC activation by GPCRs is dependent on transient increases in cytosolic Ca2+, we checked the effects of nifedipine, a dihydropyridine Ca2+ channel blocker, and (±)-Bay K8644, an L-type Ca2+ channel activator. Neither of these agents had any significant effect on basal or MC-induced pERK levels (Fig. 4C). Overall, these data show that ERK activation in melanocytic cells stimulated with NDP-MSH is independent on Ca2+ fluxes or PKC. Moreover, they strongly suggest that MC1R does not activate PKC in melanocytic cells, as opposed to other receptors of the MCR family (58,59).
Figure 4.
MC1R-mediated ERK activation in HBL cells is independent of PKC, calcium fluxes, or PKA. A, HBL cells were preincubated for 1 h in the presence or absence of the protein kinase inhibitors PD98059 (50 μm), Ro31–8425 (25 nm), or Rp-cAMP (20 μm) and then challenged with either NDP-MSH (10−7 m, 5 min) or PMA (0.1 ng/μl, 15 min). Cell lysates were analyzed by Western blot for detection of phosphorylation of a PKC substrate (MARCKS, upper blot) and total ERK2 as loading control. B, HBL cells were challenged with NDP-MSH (10−7 m, 5 min) with or without preincubation (1 h) with Ro31–8425 or Rp-cAMP. Cell extracts were blotted for pERK. C, HBL cells were challenged with NDP-MSH (10−7 m, 5 min) with or without (±)-Bay K8644 (1 μm, 1 h) or nifedipine (10 μg/ml, 1 h). ERK phosphorylation was analyzed by Western blot.
Given that we did not find evidence of cAMP, PKA, PKC, or Ca2+ involvement in MC-induced ERK activation, we considered the possibility of transactivation of a RTK. When HBL cells were challenged with NDP-MSH, a rapid and transient increase in tyrosine (Tyr) phosphorylation was detected by Western blot, which was maximal slightly sooner than maximal pERK levels were reached (Fig. 5A). Conversely, FSK did not mediate a similar stimulation of Tyr phosphorylation (Fig. 5B). Therefore, NDP-MSH stimulated Tyr kinase activity in HBL cells independently of cAMP, with kinetics compatible with a role in activation of the ERKs. We next tested the effect of AG1478 (tyrphostin), a strong and fairly specific EGFR inhibitor (nanomolar IC50 values) that also inhibits other RTKs at much higher concentrations. Preincubation of HBL cells with AG1478 effectively inhibited basal and NDP-MSH-induced ERK phosphorylation only at micromolar concentrations (Fig. 5C) and occurred upstream of NRAS because AG1478 (50 μm) had no effect on ERK activation by a constitutively active NRAS mutant (Fig. 5C). Therefore, treatment of HBL cells with NDP-MSH activated a Tyr kinase located upstream of NRAS and sensitive to relatively high concentrations of AG1478, thus most likely different from EGFR family members. Because transactivation of RTKs downstream of GPCRs is best established for the EGFR (30), we wanted to confirm lack of involvement of this receptor by means of a more specific inhibitor of the EGFR family. We used PD153035, a selective compound that inhibits EGFR with an IC50 as low as 25 pm (60). After preincubation of HBL cells with PD153035 at a 0.1 μm concentration, i.e. several orders of magnitude above its IC50 for the EGFR, we did not observe any inhibition of basal, NDP-MSH-stimulated, or mutant NRAS-dependent ERK phosphorylation in HBL cells (Fig. 5D). Thus, EGFR is not the RTK transactivated on MC1R stimulation in HBL cells.
Figure 5.
Activation of Tyr phosphorylation by NDP-MSH. A, Serum-deprived HBL cells were challenged with NDP-MSH for the times shown and Tyr phosphorylation was detected by Western blot. B, Failure of cAMP to induce Tyr phosphorylation in melanoma cells. HBL cells were incubated with NDP-MSH or FSK and probed for pTyr by Western blot. C, RTK inhibition completely blocks ERK activation by NDP-MSH. Control, untransfected HBL cells were preincubated with increasing concentrations of AG1478 for 45 min and then treated with NDP-MSH. Cell extracts were analyzed for ERK phosphorylation. As a control for specificity, cells transiently transfected with empty vector or the NRAS constitutively active mutant Q61R, as indicated, were incubated with a fixed 50 μm concentration of AG1478 for 1 h, and pERK was detected by Western blot. D, NDP-MSH-induced Tyr phosphorylation is not mediated by the EGFR. HBL cells transfected with empty vector or the Q61R NRAS mutant were pretreated or not with the specific EGFR inhibitor PD153035 (0.1 μm, 45 min) and then challenged with 10−7 m NDP-MSH for the times shown. pERK levels were estimated by Western blot. Identical results were obtained in two independent experiments.
Src and cKIT link MC1R signaling to ERK activation
The next series of experiments aimed at the characterization of the putative RTK transactivated by MC1R. We focused on cKIT, a RTK expressed in cells of the melanocytic lineage (19,61). cKIT is the receptor for STEEL/SCF, a cytokine that regulates melanoblast proliferation, differentiation, migration, and survival (19). Loss-of-function (LOF) dominant alleles of cKIT yield a white coat in mice (19), and LOF mutations in humans are causally associated with piebaldism and cochlear deafness (21,22). Moreover, oral administration of the specific inhibitor of cKIT RTK activity 4-t-butylphenyl-N-(4-imidazol-1-yl-phenyl)sulfonamide (ISCK03) causes reversible and dose-dependent hair depigmentation in C57BL/6 mice and topical application of this drug decreases UV-induced pigmentation and epidermal melanin in Brownish guinea pigs in vivo (62).
Activation of cKIT by SCF triggers autophosphorylation on several cytoplasmic Tyr residues, with phosphorylation at Tyr721 allowing binding and activation of phosphatidylinositol 3 kinase (61,63). NDP-MSH induced the transient phosphorylation of cKIT Tyr721 in HBL cells (Fig. 6A), indicative of rapid transactivation of cKIT. Similar results were obtained for HBL cells expressing the RHC mutants (not shown), but no significant increases in cKIT phosphorylation were observed in HBL cells treated with FSK (Fig. 6B). ISCK03 is a phenyl-imidazolosulfonamide compound that selectively inhibits cKIT activity at low micromolar concentrations without any effect on HGF-induced ERK phosphorylation in 501mel melanoma cells (62). Preincubation of HBL cells with ISCK03 (5 μm) effectively blocked NDP-MSH-induced ERK phosphorylation without effect on mutant NRAS-induced ERK activity (Fig. 6C). Given that the pharmacology of ISCK03 is relatively poorly established, two better characterized inhibitors of cKIT were used: GTP-14564 and Sunitinib (SU11248) (Fig. 6D). GTP-14564 has been reported to inhibit cKIT at low micromolar concentrations. At the working concentration used in Fig. 6D (1.0 μm), the compound is extremely selective because it has no effect on most Tyr and serine/threonine kinases such as EGFR, kinase insert domain-containing receptor, or human epidermal growth factor receptor 2 (IC50 ≥ 10 μm), Src, PKA, AKT, MEK, or ERK (64). Sunitinib is a highly potent inhibitor of cKIT (Ki ∼4 nm) used as a chemotherapeutic agent (65) and was used at a 10 nm concentration. Both compounds inhibited ERK activation by NDP-MSH in HBL cells (Fig. 6D) but had no effect on ERK phosphorylation in cells expressing a constitutively active NRAS mutant (not shown). Although Sunitinib is not fully specific for cKIT and also targets vascular EGFR and platelet-derived growth factor receptor with similar potency, taken together the results obtained with the three pharmacological inhibitors strongly suggested that cKIT is the RTK transactivated on stimulation of the MC1R.
Figure 6.
cAMP-independent transactivation of cKIT in NDP-MSH-stimulated HBL cells. A, Kinetics of cKIT activation by NDP-MSH. Serum-starved HBL cells were stimulated with NDP-MSH for the times shown and analyzed for cKIT phosphorylation at Tyr721 by Western blot. B, Lack of cKIT activation by FSK. Cells were treated with FSK (10−5 m, 30 min) or NDP-MSH (10−7 m, 4 min) as positive control and blotted for cKIT phosphorylation at Tyr721 (upper panel), ERK activation (middle panel), or total ERK (lower panel, loading control). C, The cKIT inhibitor ISCK03 blocks ERK activation by NDP-MSH. Cells were transiently transfected with the empty vector (pcDNA3) or with a constitutively active NRAS mutant (Q61R), preincubated with ISCK03 (1 μM, 1 h), and then challenged with NDP-MSH for 4 min. Cell extracts were analyzed by Western blot for pERK. D, Impaired ERK activation by NDP-MSH in HBL cells treated with the cKIT inhibitors GTP-14564 and Sunitinib. HBL cells were pretreated for 1 h with the indicated concentrations of the cKIT inhibitors and then challenged with NDP-MSH (10−7 m, 2 or 4 min) before estimation of pERK levels by Western blot.
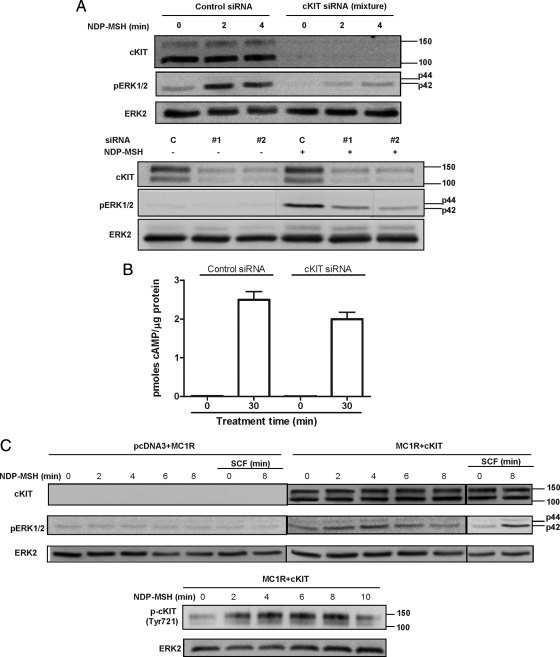
We confirmed these pharmacological evidences of cKIT involvement in ERK activation downstream of MC1R by a series of more conclusive molecular approaches. Depletion of cKIT with a mixture of four predesigned small interfering RNA (siRNA) oligonucleotides abolished MC-dependent ERK activation (Fig. 7A). This result was confirmed with two independent siRNA oligonucleotides used individually. Moreover, the specificity of this inhibitory action and the absence of off-target effects were suggested by comparable stimulation of cAMP synthesis by NDP-MSH in the presence of cKIT-directed or control siRNA (Fig. 7B). To further demonstrate that cKIT is required for positive functional coupling of MC1R to the ERKs, we performed functional reconstitution experiments in human embryonic kidney (HEK) 293 cells transfected with MC1R or cKIT, alone or in combination. cKIT expression was analyzed by Western blot that showed two major bands of approximately 110 and 145 kDa, most likely corresponding to the de novo incompletely processed form and the mature protein, respectively. Treatment with NDP-MSH failed to achieve ERK stimulation in cells expressing MC1R alone, but the MC caused a reproducible increase in ERK phosphorylation in cells expressing both MC1R and cKIT, of similar intensity as the one produced by stimulation with the cKIT ligand SCF (Fig. 7C). As a further control, cells transfected to express cKIT alone showed increased pERK levels after stimulation with SCF but not with NDP-MSH (not shown). Of note, a NDP-MSH-induced time-dependent phosphorylation of cKIT was also detected in cells expressing MC1R and cKIT (Fig. 7C, lower blot).
Figure 7.
Relationship of cKIT expression and MC-dependent ERK phosphorylation. A, Effect of cKIT silencing on ERK phosphorylation induced by NDP-MSH. In the upper series of blots, cells were transfected with a mixture of four cKIT-directed siRNA or a scrambled sequence as a negative control and stimulated with NDP-MSH for the times shown. Efficient silencing was verified by analyzing total cKIT levels (upper blot), and ERK activation was assessed by estimation of pERK levels (middle blot). Total ERK2 was used as loading control (lower blot). In the lower series, cells transfected with control siRNA (C) or with two unrelated individual cKIT-directed siRNAs (no. 1 and no. 2) were stimulated with NDP-MSH (10−7 m, 4 min) and analyzed for cKIT, pERK, and total ERK as above. B, Lack of effect of cKIT silencing by siRNA on MC-dependent cAMP production. Cells treated with the same siRNA mix as in the upper blot were analyzed for intracellular cAMP by RIA (results are the mean ± sem, n ≥ 4). C, Reconstitution of cKIT and ERK activation by NDP-MSH in HEK293 cells transfected with MC1R and cKIT. HEK293 cells were transfected to express MC1R alone (blot labeled pcDNA + MC1R) or MC1R and cKIT simultaneously. In the upper series of blots, cells were serum deprived and stimulated with NDP-MSH (10−7 m) or SCF (50 ng/ml) for the times shown. ERK phosphorylation, total cKIT expression (labeled cKIT), cKIT phosphorylation at Tyr721 (labeled pcKIT), and total ERK2 were detected by Western blot.
Melanoma progression is often associated with loss of cKIT expression (24,25). If ERK activation by MC1R is indeed dependent on transactivation of cKIT, then loss of cKIT expression in melanoma cells should uncouple MC1R from the ERKs. We analyzed a panel of human melanoma cell lines for cKIT expression. Only cell lines WT for NRAS or BRAF were considered to avoid interferences due to mutations in these upstream components of the ERK pathway. Four such cKIT-positive and 5 cKIT-negative cell lines were identified (Fig. 8A), according to the presence or absence of a majority band of approximately 145 kDa in Western blots, consistent with the electrophoretic mobility reported for the mature, glycosylated form of the protein (66). These cell lines were tested for MC-induced ERK activation. The ERKs were activated by NDP-MSH in cKIT-positive cell lines, whereas cKIT-negative cells were unresponsive (Fig. 8, B and C). Of note, neither cKIT-positive nor cKIT-negative cells increased ERK phosphorylation when stimulated with FSK (not shown).
Figure 8.
Functional coupling of MC1R to the ERK pathway in human melanoma cell lines is dependent on cKIT expression. A, Expression of cKIT in human melanoma cell lines. Cell extracts from LOCE-MM28, 61, 79, 94, 98, 104, 117, 118, and 119 human melanoma cells were analyzed for cKIT expression by Western blot. B, Effects of NDP-MSH on pERK levels in cKIT positive and negative human melanoma cells. After serum deprivation, cultures of the indicated cell lines were stimulated with NDP-MSH, and ERK phosphorylation was compared by Western blot. C, Quantification of ERK activation in cKIT positive and negative human melanoma cells. Two independent blots as shown in B were quantified, and the intensity of the pERK signal was normalized to the intensity of the band in control cells, after correction for loading. Results are given as fold increase in pERK (mean ± range).
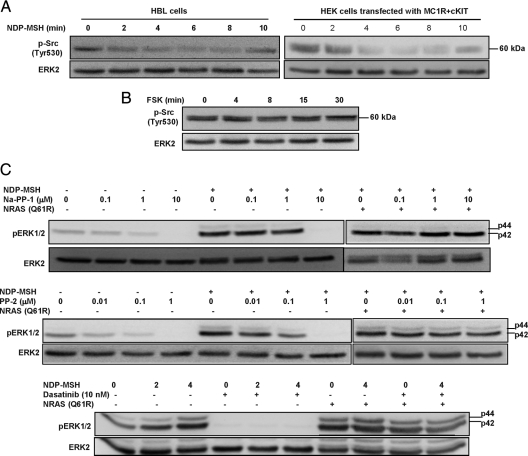
Overall, the results presented thus far prove that MC1R stimulation triggers activation of the ERKs through the cAMP-independent transactivation of cKIT. Moreover, our data formally exclude cAMP- and PKC-related events as the mechanisms relaying MC binding to the MC1R to ERK activation. In an attempt to identify components of this signaling pathway downstream of MC1R and upstream of cKIT, we focused on Src, a non-RTK that has been shown to play key roles in RTK transactivation (30,56,67,68,69). Src kinase activity is controlled by phosphorylation/dephosphorylation, with a major role for changes in the phosphorylation status of a specific Tyr residue located near the C terminus (Tyr530 in human Src). When phosphorylated, this residue binds intramolecularly to a Src homology 2 domain, leading to the stabilization of a compact and inactive conformation. Regulated dephosphorylation of Tyr530 disrupts this intramolecular interaction and is sufficient to convert Src to an open and catalytically active state (68,69,70), which can be further activated by phosphorylation of other residues, mainly Tyr416. Accordingly, the functional status of Src can be estimated from the degree of phosphorylation of the C-terminal Tyr530 by means of phosphospecific antibodies. Stimulation with NDP-MSH of HBL melanoma cells or heterologous HEK293 cells transfected to express both MC1R and cKIT caused a rapid and transient dephosphorylation of Src Tyr530 (Fig. 9A). Thus, MC signaling resulted in activation of Src. Importantly, FSK failed to attenuate the phosphorylation of Tyr530 in Src (Fig. 9B), even though SCF triggered ERK activation (see below). These data suggest that Src is activated downstream of MC1R in HBL melanoma cells and that its activation by NDP-MSH is cAMP independent.
Figure 9.
Activation of Src by MC1R signaling and possible role in downstream activation of the ERK pathway. A, Kinetics of Src activation in HBL cells (left panel) or HEK293 cells transfected with MC1R and cKIT (right panel) after stimulation with 10−7 m NDP-MSH (left panel). Src activation was assessed by decreased inhibitory Tyr530 phosphorylation. B, Lack of Src activation by FSK. HBL cells were treated with 10−5 m FSK for the times shown and tested for Src activation as in A. C, Effect of the Src inhibitors Na-PP1 (upper panel), PP-2 (middle panel), and Dasatinib (lower panel) on ERK activation mediated by NDP-MSH or by the constitutively active Q61R mutant of NRAS. HBL cells transfected with empty pcDNA3 or a construct corresponding to the Q61R NRAS mutant were preincubated with the Src inhibitors at the concentrations shown and then challenged with NDP-MSH (10−7 m, 5 min). ERK activation was estimated by Western blot.
A role for Src in transmitting the MC signal to the ERKs was next analyzed with a set of three different pharmacological inhibitors. The pyrazolopyrimidine derivatives 4-amino-1-tert-butyl-3-(1′-naphthyl)pyrazolo[3,4-d]pyrimidine (Na-PP1) and 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP-2) are potent and selective inhibitors of the Src family of non-RTKs (71) with a submicromolar IC50 for Src inhibition and potencies about 1 order of magnitude lower for a few other kinases such as p38 MAPK (72). These compounds were used at final concentrations ranging from 0.1 to 10 μm. The clinically relevant Dasatinib was described as a potent pan-Src inhibitor with IC50 values near 1 nm (73), but it has also been shown to inhibit several RTKs including cKIT at higher concentrations, with IC50 around 100 nm (74). Accordingly, Dasatinib was used at a fixed concentration of 10 nm, expected to block Src-dependent responses with minor effects on the activity of cKIT and other RTKs. Preincubation of HBL cells with Na-PP1 or PP-2 before stimulation with NDP-MSH dramatically inhibited MC-induced ERK phosphorylation in a dose-dependent manner (Fig. 9C), with a higher potency for PP-2 consistent with the reported pharmacological data for these compounds (72). ERK activation was completely blocked by 1 μm PP-2 but was already attenuated at nanomolar concentrations of the inhibitor. Although the specificity of PP-2 is not absolute, those doses should be selective for Src (71). Moreover, Dasatinib at a 10 nm concentration completely abolished ERK activation by NDP-MSH (Fig. 9C). This concentration is most likely sufficient to completely inhibit Src activity with marginal effects on cKIT, according to the reported IC50. Failure of Na-PP-1, PP-2, or Dasatinib to block mutant RAS-induced ERK phosphorylation at comparable doses placed their targets upstream of NRAS and further suggested selectivity of the compound (Fig. 9C).
To locate the position of Src in the signaling pathway linking MC1R to the ERKs and to further test a model of MC1R-dependent ERK activation based on cKIT transactivation mediated by Src, we analyzed the effects of cKIT stimulation with SCF on Src activity. SCF did not induce the dephosphorylation of the inhibitory phospho Tyr530, even though the cKIT ligand triggered ERK activation (Fig. 10A). Finally, we confirmed the previous results obtained with an antibody specific for the phosphorylated form of cKIT Tyr721 by means of a different antibody that recognizes phosphoTyr703. Phosphorylation of Tyr703 has been shown to provide a docking site for the adaptor protein Grb2 that may link cKIT activation to the RAS/RAF/MEK/ERK pathway (61,75). As shown in Fig. 10B, NDP-MSH triggered the phosphorylation of cKIT Tyr703. In the presence of the Src inhibitor PP-2 at a 1 μm concentration, NDP-MSH-induced Tyr703 phosphorylation was blocked, with a concomitant inhibition of ERK phosphorylation. Conversely, the SCF-dependent phosphorylation of Tyr703 was much less sensitive to PP-2, suggesting that the inhibitor targets a component of the signaling pathway located upstream of cKIT. Moreover, PP-2 had no effect on SCF-dependent ERK phosphorylation, even at high concentrations previously found to completely block MC1R signaling to the ERKs (Fig. 10B).
Figure 10.
Src is most likely located upstream of cKIT. A, Inability of the cKIT ligand SCF to attenuate inhibitory Tyr530 phosphorylation in HBL melanoma cells. Serum-starved cells were stimulated with SCF (50 ng/ml) for the times shown and analyzed for Tyr530 phosphorylation by Western blot. B, Effect of PP-2 on cKIT phosphorylation and ERK activation following stimulation with NDP-MSH or SCF. Control HBL cells or cells pretreated with the concentrations of PP-2 shown were challenged with 10−7 m NDP-MSH or 50 ng/ml SCF (4 min) and then lysed and tested for activatory cKIT phosphorylation at Tyr703 and pERK levels. C, A model for signaling from MC1R. Two different signaling pathways originate from the MC1R, the cAMP (left branch) and the ERK pathways (right branch). Both pathways converge at the level of MITF. cAMP increases MITF gene expression via PKA and CREB, and ERK-dependent phosphorylation of MITF increases its transcriptional activity but targets the protein for degradation. Whereas functional coupling to the cAMP cascade is strongly impaired by the RHC mutations, ERK activation is triggered by transactivation of cKIT via Src and is comparably efficient for variant and WT MC1R.
Overall, the data presented above showed that Src was activated by NDP-MSH independently of cAMP and suggest that pharmacological inhibition of Src kinase activity blocked MC1R-mediated activation of cKIT and the ERKs. Moreover, given that Src is not activated by SCF and that PP-2 has a small or negligible effect on SCF-mediated cKIT and ERK activation, it would appear that the non-RTK is located upstream of cKIT in the pathway linking MC1R and the ERKs (Fig. 10C).
Discussion
It has been reported that treatment of mouse melanoma cells with MC1R ligands or with cAMP-elevating agents such as FSK and the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine leads to stimulation of the ERKs, and accordingly it has been assumed that human MC1R signaling to the ERKs is also dependent on cAMP (32). However, we have presented here extensive and conclusive evidence disproving a positive role for cAMP in MC1R-mediated activation of the ERKs in human melanocytic cells. This evidence includes the following: 1) efficient activation of the ERKs by MC1R mutant alleles with impaired or absent cAMP signaling in NHM and human melanoma cells at physiological levels of receptor expression and hormone concentration, 2) different dose-response curves for ERK activation and cAMP production at physiological MC1R levels, 3) failure of the adenylyl cyclase activator FSK to activate the ERKs, 4) inability of the cAMP antagonist Rp-cAMP to block ERK activation in human melanoma cells expressing WT MC1R, and 5) lack of effect of the adenylyl cyclase inhibitor DDA on MC1R-dependent ERK activation despite complete inhibition of MC-stimulated cAMP production. This combination of complementary genetic and pharmacological approaches unequivocally showed that cAMP is neither sufficient nor necessary for positive functional coupling of MC1R to the ERK pathway. Moreover, we found a reproducible inhibition of basal levels of phosphorylated ERK after treatment of HBL cells with FSK, extending previous reports of cAMP-dependent suppression of ERK activity in RAS-mutated human melanoma cells (55) to at least one cell line WT for NRAS and BRAF.
Overall, these data called for a reevaluation of current paradigms on the cross talk between the cAMP and ERK pathways in human melanocytic cells (53,54,76), and, accordingly we analyzed possible cAMP-independent mechanisms accounting for ERK activation in MC-stimulated human melanocytic cells. We found that in HBL cells, signaling from MC1R to the ERKs is mediated by the transactivation of cKIT. Again, this conclusion was reached on the basis of both pharmacological and molecular approaches that showed: 1) a rapid increase of tyrosine phosphorylation and cKIT activation in cells treated with NDP-MSH but not FSK; 2) efficient blockade of ERK activation in MC-stimulated cells by three cKIT inhibitors (ISCK03, GTP-14564, and Sunitinib) but not by EGFR-specific inhibitors (PD 1153035 and low concentrations of AG 1478) under conditions that fail to block downstream signaling initiated at the level of NRAS; 3) ablation of MC-induced ERK activation after silencing of cKIT expression with siRNA; 4) ERK activation after MC stimulation of cKIT-positive human melanoma cells but not cell lines lacking cKIT expression; and 5) reconstitution of cKIT activation by MCs and a positive pERK response to the MC1R ligand in HEK cells simultaneously transfected with MC1R and cKIT constructs but not with either one of the individual constructs alone.
The precise mechanisms coupling MC1R activation by MC hormones and cKIT transactivation are currently under study in our laboratory. Our data strongly suggest that the non-RTK Src plays a key role upstream of cKIT (Figs. 9 and 10). Indeed, we found that Src was rapidly activated by treatment of HBL cells with NDP-MSH but not with FSK or the cKIT ligand SCF. Similar results were obtained in HEK cells transfected to express MC1R and cKIT. Moreover, the Src inhibitors Na-PP1, Dasatinib, and PP-2 blocked ERK activation in MC-stimulated cells. Because concerns have been raised about the selectivity of pharmacological Src inhibitors (67,71,77), we took special care to ascertain sufficient specificity and found that under our experimental conditions, Na-PP1 and PP-2 had no effect on either cKIT or downstream kinases as shown by inability to block ERK phosphorylation in cells stimulated with the cKIT ligand SCF or expressing a constitutively active NRAS mutant. Dasatinib was equally unable to block mutant NRAS-driven ERK phosphorylation. Moreover, our data clearly located Src upstream of cKIT and NRAS because of the following: 1) Src was not activated by direct stimulation of cKIT with SCF, 2) incubation of HBL cells with the Src inhibitor PP-2 under conditions that completely block the pERK response to NDP-MSH had no effect on either SCF-induced cKIT autophosphorylation at Tyr703 or ERK activation, and 3) under identical conditions, PP-2 abolished NDP-MSH-mediated phosphorylation of cKIT Tyr703. Overall, these results strongly support the involvement of Src in MC signaling to the ERKs, although this point should be confirmed by complementary molecular approaches.
The molecular events linking MC1R to Src on the one hand and Src to cKIT on the other remain unknown. Concerning the latter, we have found expression of the cKIT ligand SCF in HBL and other human melanoma cells (Herraiz Serrano, C., C. Jiménez-Cervantes, and J. C. García-Borrón, unpublished observations), and accordingly one attractive possibility would be the MC-induced proteolytic activation of a latent form of this growth factor, with release of the active form on the extracellular side of the cell membrane. Such a proteolytic ectodomain shedding mechanism has been demonstrated for the transactivation of several RTKs by GPCR signaling in various cell types (28,30). However, preliminary experiments showed that the broad spectrum matrix metalloprotease inhibitor GM1001 had no effect on MC1R-dependent ERK activation (results not shown). Accordingly, other transactivation mechanisms appear more likely, particularly in the light of the extremely complex interplay of Src family kinases and RTKs (67) and reports of direct and selective phosphorylation of cKIT and other RTKs by Src family kinases (78,79,80).
Since the first report of EGFR activation by a GPCR (81), transactivation has been shown for various RTKs such as fibroblast growth factor-1, human epidermal growth factor receptor 2, IGF-I receptor, tyrosine kinase receptor, platelet-derived growth factor receptor, and vascular endothelial growth factor receptor. Therefore, transactivation seems a rather general phenomenon that may underlie the mitogenic effects of signaling from certain GPCRs (30). However, transactivation of cKIT by MC1R signaling offers several unique aspects. On one hand, it is the first report of activation of cKIT by signaling from a GPCR. On the other, we have found compelling evidence that whereas cKIT transactivation and hence ERK activation are comparable for WT-MC1R and the RHC mutants, the RHC forms display a clear LOF phenotype in signaling to the cAMP cascade (41,42,43,44,45). Accordingly, the RHC alleles correspond to imbalanced signaling variants rather than to bona fide hypomorphic proteins. This observation can be interpreted in terms of the different dose-response curves for stimulation of the ERK and cAMP pathways, which show that the former is fully activated at much smaller concentrations of active hormone-receptor complexes. Thus, the MC1R/cKIT system described here (Fig. 10C) shows that different signaling pathways originating from a single GPCR can be differentially altered by naturally occurring mutations, with phenotypic consequences.
Another unique aspect of cKIT transactivation by MC1R signaling consists of the convergence of the cAMP and the ERK pathways triggered by the MC1R at the level of MITF, a master regulator of melanocyte biology (3,4,7). cAMP stimulates MITF gene expression, and the ERKs phosphorylate the MITF protein, resulting in increased transcriptional activity but decreased intracellular stability (82). Accordingly, the cellular responses to the MCs might be fine-tuned by an atypical feedback loop whereby the cAMP-dependent transcriptional induction of MITF would be counterbalanced by ERK-dependent down-regulation of MITF protein after its activation. Some evidence in support of a regulatory loop involving cAMP and the ERKs has been reported for mouse melanocytic cells (83). In human melanocytes harboring RHC mutant MC1R alleles, the function of this feedback loop would be altered due to the differential impact of the mutations on signaling to cAMP production or ERK activation (48). It is tempting to speculate that loss of positive cAMP-dependent effects and retention of negative ERK-dependent regulatory influences would cooperate to maintain low levels of MITF in variant human melanocytes, thus decreasing their melanogenic activity.
Our finding that MC1R recruits cKIT to activate the ERKs independently on cAMP also accounts for the involvement of NRAS in MC-mediated ERK signaling previously reported by others (32). Moreover, it might provide a molecular basis for the observation that treatment of melanocytes with αMSH activates the phosphatidylinositol 3-kinase-AKT pathway (84), a process that may participate in the protective action of the MCs against UV radiation-induced cellular damage. Indeed, by transactivating a RTK, the MC1R would trigger antiapoptotic and survival pathways.
Human MC1R is a well-established melanoma susceptibility gene (85) and the RHC alleles are strongly associated with increased risk for melanoma and other skin cancers. It will be interesting to analyze whether imbalanced signaling to the cAMP and ERK pathways, with impairment of cAMP and photoprotection-related activity but retention of proliferation-promoting signaling contributes to the association of variant RHC alleles such as R151C, R160W, and D294H with skin cancer. In any case, the identification of the cAMP-independent, cKIT-dependent transactivation of the ERK pathway by the MCs has unexpected and wide implications for our understanding of the functional connections of pathways critical for the regulation of melanocyte proliferation and differentiation.
Materials and Methods
Materials
A RIA kit for cAMP was from Amersham Pharmacia Biotech (Little Chalfont, UK). The transfection reagent Lipofectamine 2000 and competent DH5α cells were from Invitrogen, (Carlsbad, CA). αMSH, endothelin 1, DDA, PMA, nifedipine, PD98059, (±)-Bay K8644, Igepal CA-630, BSA, EDTA, phenylmethylsulfonyl fluoride, bicinchoninic acid, ampicillin, β-mercaptoethanol, and sodium dodecyl sulfate were from Sigma Chemical Company (St. Louis, MO). The synthetic αMSH analog NDP-MSH, FSK, Ro31–8425, AG1478, ISCK03, GTP-14564, PD153035, NA-PP-1, and PP-2 were from Calbiochem (Darmstadt, Germany). The cKIT inhibitor Sunitinib malate was from Tocris Bioscience (Bristol, UK), and the pan-Src inhibitor Dasatinib from Biotang Inc. (Waltham, MA). The cAMP analog Rp-cAMP was from Biolog (Bremen, Germany). Recombinant human SCF was from Genscript (Piscataway, NJ). The anti-pERK1/2 (pERK1/2) rabbit polyclonal IgG, the anti-ERK2 rabbit polyclonal IgG, the anti-pTyr (PY99) mouse monoclonal antibody, the anti-phospho-cKIT (Tyr 721) rabbit polyclonal antibody, the anti-p-c-Src (Tyr 530) rabbit polyclonal antibody, and the anti-c-Src mouse monoclonal antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-cKIT (D13A2) rabbit monoclonal antibody and the anti-phospho-cKIT (Tyr703) rabbit monoclonal antibody were from Cell Signaling (Beverly, MA). Reagents for SDS-PAGE and Western blot were from Bio-Rad (Richmond, CA). Other reagents were from Merck (Darmstadt, Germany) or Prolabo (Barcelona, Spain).
Cell culture
Cell culture reagents were from GIBCO-Life Technologies (Gaithersburg, MD). PC12 cells were grown in 12-well dishes using DMEM, supplemented with 15% fetal calf serum (FCS), 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate. HEK293 cells were grown in RPMI 1640, with 10% FCS, and the same antibiotics. HBL (LOCE-MM1) human melanoma cells were grown in MEM with antibiotics and 10% FCS. LOCE-MM61, 79, 94, 98, 104, and 117 human melanoma cells (established in the LOCE, Université Libre de Bruxelles, Brussels, Belgium) were cultured in HAM F-10 medium supplemented with 10% FCS and antibiotics (100 U/ml penicillin, 100 μg/ml kanamycin sulfate, 100 μg /ml streptomycin). Primary NHM cultures were obtained from neonatal foreskins as described (86,87), with the approval of the University of Cincinnati Medical Center Institutional Review Board. Cultures were grown in melanocyte growth medium containing bovine pituitary extract. This was removed 2–3 d before, and for the duration of, each experiment.
Expression constructs and transfection
All expression constructs were prepared in pcDNA3 (Invitrogen). The following expression constructs have been previously described: WT-MC1R, the Flag-tagged RHC variants R151C, R160W, and D294H (44,45,88). NRAS Q61R was amplified by PCR and cloned into pcDNA3. cDNA encoding for human cKIT was obtained from Open Biosystems (Huntsville, AL) and was subcloned into pcDNA3. All constructs were verified by double-strand automated sequencing as described (50).
Cells grown to approximately 80% confluence were transfected with 0.3 μg plasmid DNA/well, using Opti-MEM to dilute DNA and Lipofectamine (Invitrogen). Stable transfectants were obtained as described (45,52) and were cultured in the presence of 800 μg/ml G418 sulfate. Transfections with siRNA were performed as previously described using a pool of four target-specific 20–25 mer siRNAs (Santa Cruz Biotechnology) and two individual cKIT-specific siRNA (Applied Biosystems/Ambion, Foster City, CA) to knock down cKIT expression. As a negative control, we used a scrambled sequence that will not lead to the specific degradation of any mRNA. Cells were incubated with the transfection mixture for 6 h, and then normal growth medium was replaced and cells were further grown for 48 h.
Functional assays
Cell surface expression of WT and variant MC1R was determined by radioligand binding analysis as described previously (46,47). For cAMP measurements, cells grown in 12-well plates were transfected, serum deprived for 12–24 h, and stimulated as required. The medium was aspirated and the cells quickly washed with 800 μl ice-cold PBS. Cells were lysed with 200 μl/well 0.1 n HCl preheated at 70 C and scrapped. The mix was freeze dried, washed with 100 μl H2O, and freeze dried again. cAMP was measured with a commercial RIA, as per the manufacturer’s instructions. All cAMP assays were repeated at least twice, and for each independent experiment, duplicate or triplicate dishes were analyzed. Parallel dishes were used for protein determination with the bicinchoninic acid method.
Western blot
Cells were washed twice with PBS and solubilized in 75 μl solubilization buffer (100 ng/ml phenylmethylsulfonyl fluoride; 1% Igepal; and 1% phosphatase inhibitor mix containing 200 mm imidazole, 100 mm NaF, 115 mm sodium molibdate, 100 mm sodium o-vanadate, and 400 mm sodium tartrate). Samples were centrifuged (105,000 × g, 30 min) and a volume of supernatant containing 30 μg protein was mixed (2:1 ratio) with electrophoresis sample buffer (180 mm Tris-HCl, pH 6.8; 15% glycerol; 9% sodium dodecyl sulfate; 0.075% bromophenol blue; and 7.5% β-mercaptoethanol). Electrophoresis and Western blotting were performed as described (44,45,47). Blots were probed with the required antibodies and stained with a chemiluminescent substrate (Amersham, Little Chalfont, UK). Comparable loading was ascertained by stripping and reprobing the membranes with an anti-ERK2 antibody. Stripping was performed by washing the membranes with PBS, followed by treatment with 0.5 n NaOH, 10 min at room temperature, and a final 10-min wash with PBS.
Acknowledgments
We thank A. L. Kadekaro and R. Starner for invaluable help in the experiments with NHMs.
Footnotes
This work was supported by Grant SAF2009-10942 (to J.C.G.-B.) from the Comisión Interministerial de Ciencia y Tecnología, Spain; Fondo Europeo de Desarrollo Regional funds (European Community) and Grant 464/2008 from the Comunidad Autónoma de la Región de Murcia, Plan de Ciencia y Tecnología 2007/10 (to J.C.G.-B.); National Institutes of Health Grants R01 ES009110 and R01 ES017561 (to Z.A.-M.). The work also received financial support from Lambeau-Marteaux, MEDIC foundations, and the “Amis de l’Institut Bordet” Association in Belgium. C.H.S. holds a fellowship from the Fundación Séneca, Comunidad Autónoma de la Región de Murcia, Spain.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 17, 2010
Abbreviations: cKIT, Stem cell factor receptor; CREB, cAMP response element binding protein; DDA, 2′, 5′-dideoxyadenosine; EGFR, epidermal growth factor receptor; FCS, fetal calf serum; FSK, forskolin; GPCR, G protein-coupled receptor; HEK, human embryonic kidney; ISCK03, 4-t-butylphenyl-N-(4-imidazol-1-yl-phenyl)sulfonamide; LOF, loss-of-function; MARCKS, myristoylated alanine-rich PKC substrate; MC, melanocortin; MC1R, melanocortin 1 receptor; MEK, MAPK kinase; MITF, microphthalmia-associated transcription factor; Na-PP1, 4-amino-1-tert-butyl-3-(1′-naphthyl)pyrazolo[3,4-d]pyrimidine; NDP, [Nle4, d-Phe7]; NHM, normal human melanocyte; pERK, phosphorylated ERK; PKA, protein kinase A; PKC, protein kinase C; PMA, phorbol-12-myristate-13-acetate; PP-2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; RAF, rapid accelerated fibrosarcoma; RAS, RAt sarcoma; Rp, isomer; RHC, red hair color; RTK, receptor tyrosine kinase; SCF, stem cell factor; siRNA, small interfering RNA; Tyr, tyrosine; WT, wild type.
References
- Slominski A, Tobin DJ, Shibahara S, Wortsman J 2004 Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84:1155–1228 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Brenner M, Hearing VJ 2007 The regulation of skin pigmentation. J Biol Chem 282:27557–27561 [DOI] [PubMed] [Google Scholar]
- Buscà R, Ballotti R 2000 Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res 13:60–69 [DOI] [PubMed] [Google Scholar]
- D'Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, Igras V, Kunisada T, Granter SR, Nishimura EK, Ito S, Fisher DE 2006 Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature 443:340–344 [DOI] [PubMed] [Google Scholar]
- Cohen C, Zavala-Pompa A, Sequeira JH, Shoji M, Sexton DG, Cotsonis G, Cerimele F, Govindarajan B, Macaron N, Arbiser JL 2002 Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin Cancer Res 8:3728–3733 [PubMed] [Google Scholar]
- Böhm M, Moellmann G, Cheng E, Alvarez-Franco M, Wagner S, Sassone-Corsi P, Halaban R 1995 Identification of p90RSK as the probable CREB-Ser133 kinase in human melanocytes. Cell Growth Differ 6:291–302 [PubMed] [Google Scholar]
- Levy C, Khaled M, Fisher DE 2006 MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med 12:406–414 [DOI] [PubMed] [Google Scholar]
- Rees JL 2004 The genetics of sun sensitivity in humans. Am J Hum Genet 75:739–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Wakamatsu K 2003 Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Res 16:523–531 [DOI] [PubMed] [Google Scholar]
- Katz M, Amit I, Yarden Y 2007 Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim Biophys Acta 1773:1161–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, App H, Zhang XF, Banerjee P, Brautigan DL, Rapp UR, Avruch J 1992 Raf-1 activates MAP kinase-kinase. Nature 358:417–421 [DOI] [PubMed] [Google Scholar]
- Wellbrock C, Karasarides M, Marais R 2004 The RAF proteins take centre stage. Nat Rev Mol Cell Biol 5:875–885 [DOI] [PubMed] [Google Scholar]
- Murphy LO, Blenis J 2006 MAPK signal specificity: the right place at the right time. Trends Biochem Sci 31:268–275 [DOI] [PubMed] [Google Scholar]
- Alsina J, Gorsk DH, Germino FJ, Shih W, Lu SE, Zhang ZG, Yang JM, Hait WN, Goydos JS 2003 Detection of mutations in the mitogen-activated protein kinase pathway in human melanoma. Clin Cancer Res 9:6419–6425 [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA 2002 Mutations of the BRAF gene in human cancer. Nature 417:949–954 [DOI] [PubMed] [Google Scholar]
- Gray-Schopfer V, Wellbrock C, Marais R 2007 Melanoma biology and new targeted therapy. Nature 445:851–857 [DOI] [PubMed] [Google Scholar]
- Omholt K, Karsberg S, Platz A, Kanter L, Ringborg U, Hansson J 2002 Screening of N-ras codon 61 mutations in paired primary and metastatic cutaneous melanomas: mutations occur early and persist throughout tumor progression. Clin Cancer Res 8:3468–3474 [PubMed] [Google Scholar]
- Sensi M, Nicolini G, Petti C, Bersani I, Lozupone F, Molla A, Vegetti C, Nonaka D, Mortarini R, Parmiani G, Fais S, Anichini A 2006 Mutually exclusive NRASQ61R and BRAFV600E mutations at the single-cell level in the same human melanoma. Oncogene 25:3357–3364 [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B 2003 The role of Kit-ligand in melanocyte development and epidermal homeostasis. Pigment Cell Res 16:287–296 [DOI] [PubMed] [Google Scholar]
- Bennett DC, Lamoreux ML 2003 The color loci of mice—a genetic century. Pigment Cell Res 16:333–344 [DOI] [PubMed] [Google Scholar]
- Spritz RA, Beighton P 1998 Piebaldism with deafness: molecular evidence for an expanded syndrome. Am J Med Genet 75:101–103 [DOI] [PubMed] [Google Scholar]
- Syrris P, Heathcote K, Carrozzo R, Devriendt K, Elcioglu N, Garrett C, McEntagart M, Carter ND 2002 Human piebaldism: six novel mutations of the proto-oncogene KIT. Hum Mutat 20:234 [DOI] [PubMed] [Google Scholar]
- Antonescu CR, Busam KJ, Francone TD, Wong GC, Guo T, Agaram NP, Besmer P, Jungbluth A, Gimbel M, Chen CT, Veach D, Clarkson BD, Paty PB, Weiser MR 2007 L576P KIT mutation in anal melanomas correlates with KIT protein expression and is sensitive to specific kinase inhibition. Int J Cancer 121:257–264 [DOI] [PubMed] [Google Scholar]
- Huang S, Jean D, Luca M, Tainsky MA, Bar-Eli M 1998 Loss of AP-2 results in downregulation of c-KIT and enhancement of melanoma tumorigenicity and metastasis. EMBO J 17:4358–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali PG, Nicotra MR, Winkler AB, Cavaliere R, Bigotti A, Ullrich A 1992 Progression of human cutaneous melanoma is associated with loss of expression of c-kit proto-oncogene receptor. Int J Cancer 52:197–201 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK 2005 Transduction of receptor signals by β-arrestins. Science 308:512–517 [DOI] [PubMed] [Google Scholar]
- Marinissen MJ, Gutkind JS 2001 G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci 22:368–376 [DOI] [PubMed] [Google Scholar]
- Werry TD, Sexton PM, Christopoulos A 2005 “Ins and outs” of seven-transmembrane receptor signalling to ERK. Trends Endocrinol Metab 16:26–33 [DOI] [PubMed] [Google Scholar]
- Borrell-Pagès M, Rojo F, Albanell J, Baselga J, Arribas J 2003 TACE is required for the activation of the EGFR by TGF-α in tumors. EMBO J 22:1114–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzker R, Böhmer FD 2003 Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol 4:651–657 [DOI] [PubMed] [Google Scholar]
- Amsen EM, Pham N, Pak Y, Rotin D 2006 The guanine nucleotide exchange factor CNrasGEF regulates melanogenesis and cell survival in melanoma cells. J Biol Chem 281:121–128 [DOI] [PubMed] [Google Scholar]
- Buscà R, Abbe P, Mantoux F, Aberdam E, Peyssonnaux C, Eychène A, Ortonne JP, Ballotti R 2000 Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J 19:2900–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englaro W, Rezzonico R, Durand-Clément M, Lallemand D, Ortonne JP, Ballotti R 1995 Mitogen-activated protein kinase pathway and AP-1 are activated during cAMP-induced melanogenesis in B-16 melanoma cells. J Biol Chem 270:24315–24320 [DOI] [PubMed] [Google Scholar]
- Garcia-Borrón JC, Sánchez-Laorden BL, Jiménez-Cervantes C 2005 Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res 18:393–410 [DOI] [PubMed] [Google Scholar]
- Box NF, Wyeth JR, O'Gorman LE, Martin NG, Sturm RA 1997 Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum Mol Genet 6:1891–1897 [DOI] [PubMed] [Google Scholar]
- Duffy DL, Box NF, Chen W, Palmer JS, Montgomery GW, James MR, Hayward NK, Martin NG, Sturm RA 2004 Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Hum Mol Genet 13:447–461 [DOI] [PubMed] [Google Scholar]
- Healy E, Flannagan N, Ray A, Todd C, Jackson IJ, Matthews JN, Birch-Machin MA, Rees JL 2000 Melanocortin-1-receptor gene and sun sensitivity in individuals without red hair. Lancet 355:1072–1073 [DOI] [PubMed] [Google Scholar]
- Sturm RA 2002 Skin colour and skin cancer—MC1R, the genetic link. Melanoma Res 12:405–416 [DOI] [PubMed] [Google Scholar]
- Sturm RA, Duffy DL, Box NF, Chen W, Smit DJ, Brown DL, Stow JL, Leonard JH, Martin NG 2003 The role of melanocortin-1 receptor polymorphism in skin cancer risk phenotypes. Pigment Cell Res 16:266–272 [DOI] [PubMed] [Google Scholar]
- Sturm RA, Duffy DL, Box NF, Newton RA, Shepherd AG, Chen W, Marks LH, Leonard JH, Martin NG 2003 Genetic association and cellular function of MC1R variant alleles in human pigmentation. Ann NY Acad Sci 994:348–358 [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Shekar SN, Shekar SL, Newton RA, James MR, Stow JL, Duffy DL, Sturm RA 2007 Receptor function, dominant negative activity and phenotype correlations for MC1R variant alleles. Hum Mol Genet 16:2249–2260 [DOI] [PubMed] [Google Scholar]
- Healy E, Jordan SA, Budd PS, Suffolk R, Rees JL, Jackson IJ 2001 Functional variation of MC1R alleles from red-haired individuals. Hum Mol Genet 10:2397–2402 [DOI] [PubMed] [Google Scholar]
- Newton RA, Smit SE, Barnes CC, Pedley J, Parsons PG, Sturm RA 2005 Activation of the cAMP pathway by variant human MC1R alleles expressed in HEK and in melanoma cells. Peptides 26:1818–1824 [DOI] [PubMed] [Google Scholar]
- Sánchez-Laorden BL, Sánchez-Más J, Martínez-Alonso E, Martínez-Menárguez JA, García-Borrón JC, Jiménez-Cervantes C 2006 Dimerization of the human melanocortin 1 receptor: functional consequences and dominant-negative effects. J Invest Dermatol 126:172–181 [DOI] [PubMed] [Google Scholar]
- Sánchez-Laorden BL, Jiménez-Cervantes C, García-Borrón JC 2007 Regulation of human melanocortin 1 receptor signaling and trafficking by Thr-308 and Ser-316 and its alteration in variant alleles associated with red hair and skin cancer. J Biol Chem 282:3241–3251 [DOI] [PubMed] [Google Scholar]
- Sánchez-Laorden BL, Herraiz C, Valencia JC, Hearing VJ, Jiménez-Cervantes C, García-Borrón JC 2009 Aberrant trafficking of human melanocortin 1 receptor variants associated with red hair and skin cancer: steady-state retention of mutant forms in the proximal golgi. J Cell Physiol 220:640–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Más J, Olivares Sánchez C, Ghanem G, Haycock J, Lozano Teruel JA, García-Borrón JC, Jiménez-Cervantes C 2002 Loss-of-function variants of the human melanocortin-1 receptor gene in melanoma cells define structural determinants of receptor function. Eur J Biochem 269:6133–6141 [DOI] [PubMed] [Google Scholar]
- Herraiz C, Jiménez-Cervantes C, Zanna P, García-Borrón JC 2009 Melanocortin 1 receptor mutations impact differentially on signalling to the cAMP and the ERK mitogen-activated protein kinase pathways. FEBS Lett 583:3269–3274 [DOI] [PubMed] [Google Scholar]
- Tada A, Pereira E, Beitner-Johnson D, Kavanagh R, Abdel-Malek ZA 2002 Mitogen- and ultraviolet-B-induced signaling pathways in normal human melanocytes. J Invest Dermatol 118:316–322 [DOI] [PubMed] [Google Scholar]
- Scott MC, Wakamatsu K, Ito S, Kadekaro AL, Kobayashi N, Groden J, Kavanagh R, Takakuwa T, Virador V, Hearing VJ, Abdel-Malek ZA 2002 Human melanocortin 1 receptor variants, receptor function and melanocyte response to UV radiation. J Cell Sci 115:2349–2355 [DOI] [PubMed] [Google Scholar]
- Eves P, Haycock J, Layton C, Wagner M, Kemp H, Szabo M, Morandini R, Ghanem G, García-Borrón JC, Jiménez-Cervantes C, MacNeil S 2003 Anti-inflammatory and anti-invasive effects of α-melanocyte-stimulating hormone in human melanoma cells. Br J Cancer 89:2004–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Más JS, Gerritsen I, Hahmann C, Jiménez-Cervantes C, García-Borrón JC 2003 Rate limiting factors in melanocortin 1 receptor signalling through the cAMP pathway. Pigment Cell Res 16:540–547 [DOI] [PubMed] [Google Scholar]
- Dumaz N, Marais R 2005 Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft fur Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. FEBS J 272:3491–3504 [DOI] [PubMed] [Google Scholar]
- Stork PJ, Schmitt JM 2002 Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12:258–266 [DOI] [PubMed] [Google Scholar]
- Dumaz N, Hayward R, Martin J, Ogilvie L, Hedley D, Curtin JA, Bastian BC, Springer C, Marais R 2006 In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res 66:9483–9491 [DOI] [PubMed] [Google Scholar]
- Sun Y, McGarrigle D, Huang XY 2007 When a G protein-coupled receptor does not couple to a G protein. Mol Biosyst 3:849–854 [DOI] [PubMed] [Google Scholar]
- Ramsden JJ 2000 MARCKS: a case of molecular exaptation? Int J Biochem Cell Biol 32:475–479 [DOI] [PubMed] [Google Scholar]
- Kim CS, Lee SH, Kim RY, Kim BJ, Li SZ, Lee IH, Lee EJ, Lim SK, Bae YS, Lee W, Baik JH 2002 Identification of domains directing specificity of coupling to G-proteins for the melanocortin MC3 and MC4 receptors. J Biol Chem 277:31310–31317 [DOI] [PubMed] [Google Scholar]
- Wachira SJ, Hughes-Darden CA, Taylor CV, Ochillo R, Robinson TJ 2003 Evidence for the interaction of protein kinase C and melanocortin 3-receptor signaling pathways. Neuropeptides 37:201–210 [DOI] [PubMed] [Google Scholar]
- Bridges AJ, Zhou H, Cody DR, Rewcastle GW, McMichael A, Showalter HD, Fry DW, Kraker AJ, Denny WA 1996 Tyrosine kinase inhibitors. 8. An unusually steep structure-activity relationship for analogues of 4-(3-bromoanilino)-6,7-dimethoxyquinazoline (PD 153035), a potent inhibitor of the epidermal growth factor receptor. J Med Chem 39:267–276 [DOI] [PubMed] [Google Scholar]
- Roskoski Jr R 2005 Structure and regulation of Kit protein-tyrosine kinase—the stem cell factor receptor. Biochem Biophys Res Commun 338:1307–1315 [DOI] [PubMed] [Google Scholar]
- Na YJ, Baek HS, Ahn SM, Shin HJ, Chang IS, Hwang JS 2007 [4-t-butylphenyl]-N-(4-imidazol-1-yl phenyl)sulfonamide (ISCK03) inhibits SCF/c-kit signaling in 501mel human melanoma cells and abolishes melanin production in mice and brownish guinea pigs. Biochem Pharmacol 74:780–786 [DOI] [PubMed] [Google Scholar]
- Lev S, Givol D, Yarden Y 1992 Interkinase domain of kit contains the binding site for phosphatidylinositol 3′ kinase. Proc Natl Acad Sci USA 89:678–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Kumagai H, Kawashima T, Tamitsu K, Irie M, Nakajima H, Suzu S, Shibuya M, Kamihira S, Nosaka T, Asano S, Kitamura T 2003 Selective cytotoxic mechanism of GTP-14564, a novel tyrosine kinase inhibitor in leukemia cells expressing a constitutively active Fms-like tyrosine kinase 3 (FLT3). J Biol Chem 278:32892–32898 [DOI] [PubMed] [Google Scholar]
- Patyna S, Laird AD, Mendel DB, O'farrell AM, Liang C, Guan H, Vojkovsky T, Vasile S, Wang X, Chen J, Grazzini M, Yang CY, Haznedar JO, Sukbuntherng J, Zhong WZ, Cherrington JM, Hu-Lowe D 2006 SU14813: a novel multiple receptor tyrosine kinase inhibitor with potent antiangiogenic and antitumor activity. Mol Cancer Ther 5:1774–1782 [DOI] [PubMed] [Google Scholar]
- Kasamatsu S, Hachiya A, Higuchi K, Ohuchi A, Kitahara T, Boissy RE 2008 Production of the soluble form of KIT, s-KIT, abolishes stem cell factor-induced melanogenesis in human melanocytes. J Invest Dermatol 128:1763–1772 [DOI] [PubMed] [Google Scholar]
- Bromann PA, Korkaya H, Courtneidge SA 2004 The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23:7957–7968 [DOI] [PubMed] [Google Scholar]
- Ingley E 2008 Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta 1784:56–65 [DOI] [PubMed] [Google Scholar]
- Roskoski Jr R 2004 Src protein-tyrosine kinase structure and regulation. Biochem Biophys Res Commun 324:1155–1164 [DOI] [PubMed] [Google Scholar]
- Xu W, Doshi A, Lei M, Eck MJ, Harrison SC 1999 Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell 3:629–638 [DOI] [PubMed] [Google Scholar]
- Karni R, Mizrachi S, Reiss-Sklan E, Gazit A, Livnah O, Levitzki A 2003 The pp60c-Src inhibitor PP1 is non-competitive against ATP. FEBS Lett 537:47–52 [DOI] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P 2007 The selectivity of protein kinase inhibitors: a further update. Biochem J 408:297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin RV, Shen Z, Cook LS, Doweyko AM, Pitt S, Pang S, Shen DR, Fang Q, de Fex HF, McIntyre KW, Shuster DJ, Gillooly KM, Behnia K, Schieven GL, Wityak J, Barrish JC 2006 2-Aminothiazole as a novel kinase inhibitor template. Structure-activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide (dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor. J Med Chem 49:6819–6832 [DOI] [PubMed] [Google Scholar]
- Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C 2006 Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood 108:286–291 [DOI] [PubMed] [Google Scholar]
- Thommes K, Lennartsson J, Carlberg M, Ronnstrand L 1999 Identification of Tyr-703 and Tyr-936 as the primary association sites for Grb2 and Grb7 in the c-Kit/stem cell factor receptor. Biochem J 341(Pt 1):211–216 [PMC free article] [PubMed] [Google Scholar]
- Dhomen N, Marais R 2007 New insight into BRAF mutations in cancer. Curr Opin Genet Dev 17:31–39 [DOI] [PubMed] [Google Scholar]
- Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA 2000 SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol 20:9018–9027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ 1999 c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 274:8335–8343 [DOI] [PubMed] [Google Scholar]
- Hansen K, Johnell M, Siegbahn A, Rorsman C, Engström U, Wernstedt C, Heldin CH, Rönnstrand L 1996 Mutation of a Src phosphorylation site in the PDGF β-receptor leads to increased PDGF-stimulated chemotaxis but decreased mitogenesis. EMBO J 15:5299–5313 [PMC free article] [PubMed] [Google Scholar]
- Lennartsson J, Wernstedt C, Engström U, Hellman U, Rönnstrand L 2003 Identification of Tyr900 in the kinase domain of c-Kit as a Src-dependent phosphorylation site mediating interaction with c-Crk. Exp Cell Res 288:110–118 [DOI] [PubMed] [Google Scholar]
- Daub H, Weiss FU, Wallasch C, Ullrich A 1996 Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 379:557–560 [DOI] [PubMed] [Google Scholar]
- Hemesath TJ, Price ER, Takemoto C, Badalian T, Fisher DE 1998 MAP kinase links the transcription factor microphthalmia to c-Kit signalling in melanocytes. Nature 391:298–301 [DOI] [PubMed] [Google Scholar]
- Englaro W, Bertolotto C, Buscà R, Brunet A, Pagès G, Ortonne JP, Ballotti R 1998 Inhibition of the mitogen-activated protein kinase pathway triggers B16 melanoma cell differentiation. J Biol Chem 273:9966–9970 [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G, Shertzer HG, Scott G, Abdel-Malek ZA 2005 α-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res 65:4292–4299 [DOI] [PubMed] [Google Scholar]
- Hayward NK 2003 Genetics of melanoma predisposition. Oncogene 22:3053–3062 [DOI] [PubMed] [Google Scholar]
- Abdel-Malek Z, Swope V, Collins C, Boissy R, Zhao H, Nordlund J 1993 Contribution of melanogenic proteins to the heterogeneous pigmentation of human melanocytes. J Cell Sci 106(Pt 4):1323–1331 [DOI] [PubMed] [Google Scholar]
- Abdel-Malek Z, Swope VB, Suzuki I, Akcali C, Harriger MD, Boyce ST, Urabe K, Hearing VJ 1995 Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc Natl Acad Sci USA 92:1789–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Más J, Guillo LA, Zanna P, Jiménez-Cervantes C, García-Borrón JC 2005 Role of G protein-coupled receptor kinases in the homologous desensitization of the human and mouse melanocortin 1 receptors. Mol Endocrinol 19:1035–1048 [DOI] [PubMed] [Google Scholar]