Abstract
Candida albicans is a commensal dimorphic yeast of the digestive tract that causes hematogenously disseminated infections in immunocompromised individuals. Endogenous invasive candidiasis develops from C. albicans adhering to the intestinal epithelium. Adherence is mediated by the cell wall surface, a domain composed essentially of mannopyranosyl residues bound to proteins, the N-linked moiety of which comprises sequences of α-1,2- and β-1,2-linked mannose residues. β-1,2-linked mannosides are also associated with a glycolipid, phospholipomannan, at the C. albicans surface. In order to determine the roles of β-1,2 and α-1,2 oligomannosides in the C. albicans-enterocyte interaction, we developed a model of adhesion of C. albicans VW32 blastospores to the apical regions of differentiated Caco-2 cells. Preincubation of yeasts with monoclonal antibodies (MAbs) specific for α-1,2 and β-1,2 mannan epitopes resulted in a dose-dependent decrease in adhesion (50% of the control with a 60-μg/ml MAb concentration). In competitive assays β-1,2 and α-1,2 tetramannosides were the most potent carbohydrate inhibitors, with 50% inhibitory concentrations of 2.58 and 6.99 mM, respectively. Immunolocalization on infected monolayers with MAbs specific for α-1,2 and β-1,2 oligomannosides showed that these epitopes were shed from the yeast to the enterocyte surface. Taken together, our data indicate that α-1,2 and β-1,2 oligomannosides are involved in the C. albicans-enterocyte interaction and participate in the adhesion of the yeasts to the mucosal surface.
Candida species are part of the commensal flora of the mucosa and skin in humans and other vertebrates. In immunocompromised or intensive-care patients, increased mucosal proliferation secondary to use of broad-spectrum antibiotics, together with reduced host defenses and physical alteration of the mucosal barriers, may result in bloodstream invasion. Altogether, candidemia accounts for ∼10% of nosocomial bloodstream infections, and C. albicans is the causative agent in 50 to 70% of disseminated candidiasis (13, 18, 20, 36, 48).
Molecular typing methods have shown an overall genetic similarity between C. albicans strains obtained from blood cultures and colonizing strains obtained from the gastrointestinal tracts of the same patients, confirming endogenous acquisition as the main source of invasive candidiasis (40, 47). On the basis of this model, adhesion of the yeasts to the epithelium of the digestive tract is a prerequisite for colonization and a critical step in the pathogenesis of invasive candidiasis. Characterization of the adhesins and ligands involved in the C. albicans-enterocyte interaction thus appears to be a necessary approach to developing strategies aimed at reducing mucosal colonization and preventing bloodstream invasion.
Interaction of C. albicans with host cell surfaces is mediated by the yeast cell wall, a complex and dynamic structure containing glucan, chitin and mannoproteins (reviewed in reference 6). The outermost layers of the C. albicans cell wall are made of phosphopeptidomannan (PPM), a polymer of mannose residues and proteins commonly referred to as mannan (3, 6). Mannan has been shown to play a role in adherence (27), immunomodulation (11), and antigenic variability (43). The PPM glycan moiety is composed of O-linked and N-linked oligomannosides. The N-linked part consists of a backbone of α-1,6-linked mannopyranose residues with branches composed of α-1,2- and α-1,3-linked mannopyranose units and terminal β-1,2 linkages in C. albicans serotype A (7). Short branches composed of β-1,2-linked mannopyranose residues are linked to PPM through phosphodiester bridges in C. albicans serotypes A and B. These side chains are referred to as the acid-labile fraction of PPM, since they are cleaved by mild acid treatment (45). β-1,2 oligomannosidic chains have also been identified on a 14- to 18-kDa glycolipid, referred to as phospholipomannan (PLM) (46), that is expressed at and shed from the C. albicans cell wall (25, 39).
β-1,2 mannosidic linkages are uncommon structures whose presence has been reported in only few bacterial and yeast species (30, 35). In C. albicans, their presence was first identified on PPM by Shibata et al. (41). These oligomannoside sequences are involved in the adhesion of C. albicans to the macrophage membrane, at least in part through binding to galectin 3, a member of a family of carbohydrate binding proteins implicated in a variety of biological functions (17, 25). β-1,2 oligomannosides also generate protective antibodies (22) and induce cytokine production (26). These unique carbohydrate sequences thus appear to play a key role in the C. albicans-host balance.
Despite the importance of the digestive tract as the main source of invasive candidiasis, few groups have reported analyses of the C. albicans-enterocyte interaction at the cellular and molecular levels (44, 49, 50). In the present paper, we describe a model of adhesion of C. albicans blastospores to the human enterocyte cell line Caco-2 and its use to analyze the role of oligomannosides with distinct anomere-type linkages, either α or β, in the attachment of C. albicans blastospores to Caco-2 cells. Indeed, galectin-3, which binds β-1,2 oligomannosides on the macrophage (17), is also expressed in intestinal epithelial cells (1, 8). Moreover, recent studies with a mouse model of candidiasis showed that oral administration of synthetic β-1,2 oligomannosides could reduce colonization of the gut, presumably by competing with the natural flora for binding to enterocytes (12). We were thus interested in understanding the basis of this phenomenon at the cellular and molecular levels.
MATERIALS AND METHODS
Growth and preparation of C. albicans strain for adherence assay.
C. albicans strain VW32 (serotype A) (5) was used throughout this study. This strain, which was originally isolated from a patient with human renal candidiasis, has been employed in prior studies of C. albicans-macrophage interactions (16, 17, 25) and in the chemical characterization of C. albicans β-mannosidic epitopes (15, 46). Stock cultures were maintained at −20°C on Sabouraud dextrose agar. For adhesion experiments, stock cultures were plated on Sabouraud dextrose agar and incubated at 37°C. After 18 to 20 h of culture, the yeasts were recovered, washed with phosphate-buffered saline (PBS), and resuspended in PBS. The yeast concentration was determined by hemocytometer and growth on Sabouraud dextrose agar. For experiments using heat-killed yeasts, blastospores were treated at 100°C for 15 min, washed in PBS by centrifugation, and resuspended in PBS.
Growth and differentiation of Caco-2 cells.
Caco-2 cells were obtained from the American Type Culture Collection (HTB 27) and were cultivated in the absence of antibiotics and antifungal agents. Cells (passages 4 to 15) were grown to confluence in 25-cm2 flasks at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (3 volumes) with Ham F12 (1 volume) containing 10% fetal calf serum, glutamine, and 1 g of glucose/liter. For adhesion experiments, ∼4 × 105 cells were seeded on each 11-mm-diameter glass coverslip in 24-well plastic dishes. The cultures were maintained at 37°C and 5% CO2, and the medium of each culture well was replaced every other day until it was used.
Fluorescent probes and antibodies.
Monoclonal antibody (MAb) EBCA1 is a rat immunoglobulin (IgM) that reacts with α-linked mannose (24). MAb 5B2 is a mouse-rat chimeric IgM that reacts with β-linked mannose (23). The control MAb A255 is a mouse IgM that recognizes a protein of the respiratory syncytial virus. It was provided by P. Pothier (Dijon, France). Uvitex 2B was purchased from LD Bio Diagnostics (Lyon, France). For immunofluorescence experiments, the secondary antibodies Alexa Fluor 488 goat anti-mouse IgM (μ chain) and Alexa Fluor 488 goat anti-rat IgM (μ chain) (1/200 dilutions in PBS; Molecular Probes, Leiden, The Netherlands) were employed for MAbs 5B2 and EBCA1, respectively. Detection of actin in confocal-microscopy experiments was performed with Alexa Fluor 568-phalloidin (1 U of phalloidin per well; Molecular Probes). For double-labeling experiments, the secondary antibody Alexa Fluor 594 goat anti-rat IgM (1/200 dilution in PBS; Molecular Probes) was employed for MAb EBCA1.
Carbohydrates.
d-Glucose, d-galactose, galactosamine, N-acetylglucosamine, d-mannose, d-fucose, l-fucose, N-acetylgalactosamine, N-acetylneuraminic acid, d-xylose, d-lactose, N-acetyllactosamine, d-mannosamine, mannan from Saccharomyces cerevisiae, hyaluronic acid, fetuin, asialofetuin, laminarin, heparin, fucoidan, and chondroitin sulfate A and C were purchased from Sigma-Aldrich Chimie (Saint Quentin Fallavier, France) and stored according to the recommendations of the manufacturer. d-Rhamnose and α-methylmannoside were purchased from Acros Organics (Noisy le Grand, France) and stored at 4°C. α-1,2 tetramannosides and β-1,2 tetramannosides were synthesized according to a previously described protocol (12) and stored at 4°C until they were used. All carbohydrates were diluted extemporaneously in PBS for attachment inhibition assays.
Adherence of C. albicans blastospores to Caco-2 cells.
Monolayers for adhesion experiments were used 15 to 21 days after being seeded. The medium in each well was aspirated and replaced by 300 μl of PBS preincubated at 37°C and containing 103 blastospores. After 30 min at 37°C, the monolayers were washed three times to remove nonadhering blastospores, fixed in 2% glutaraldehyde for 10 min, and washed twice. For detection of adherent blastospores, Uvitex 2B (1/100 dilution in PBS) was added to each well for 5 min in the dark at room temperature. The coverslips were rinsed, counterstained with 0.2% Evans Blue in PBS for 30 s, rinsed three times, and mounted inverted on microscope slides. In all the experiments described, the Caco-2 monolayers were washed with PBS at 37°C. Attachment was quantified by immunofluorescence microscopy using a Zeiss Axioscop 2 microscope with an excitation filter at 360 nm and a suppression filter at 460 nm. The percentage of adhesion in each culture was determined as the ratio of the number of adherent yeasts on the entire surface of the coverslip to the inoculum evaluated by quantitative culture. Each condition was tested in triplicate, and three separate experiments were performed.
Attachment inhibition assays.
The effects of MAbs 5B2 and EBCA1 on the attachment of C. albicans blastospores to Caco2 cells were studied by using the adherence assay described above. Yeasts were suspended in PBS and preincubated with purified MAbs at 60, 6, or 3 μg/ml for 1 h at 37°C under mild agitation. Agglutinated blastospores were pelleted by a 3-min centrifugation at 200 × g. Supernatants containing nonagglutinated blastospores were diluted with PBS, and 103 yeast cells were transferred to Caco-2 monolayers. The adhesion experiment was then performed as described above. In parallel, 20-μl fractions of each supernatant were recovered, placed on immunofluorescence slides, air dried, reacted with secondary antibodies, and examined with a Zeiss axioscope 2 microscope to assess the labeling of the blastospores with primary MAbs. All experiments included two positive control wells (cocultures in PBS alone without inhibiting antibody) and duplicate testing of attachment with each antibody concentration. Three separate experiments were performed.
To determine the effect of synthetic β-1,2 and α-1,2 tetramannosides and control carbohydrates on adhesion, Caco-2 monolayers were incubated at 37°C in PBS containing the following concentrations of the carbohydrates tested. d-Glucose, d-galactose, galactosamine, N-acetylglucosamine, d-mannose, d-fucose, l-fucose, N-acetylgalactosamine, N-acetylneuraminic acid, d-xylose, d-lactose, N-acetyllactosamine, d-mannosamine, d-rhamnose, and α-methylmannoside were tested at 10, 50, and 250 mM. Mannan from S. cerevisiae, hyaluronic acid, fetuin, asialofetuin, laminarin, heparin, fucoidan, and chondroitin sulfate A and C were tested at 0.2, 1, and 5 mg/ml. For β-1,2 mannotetraoses and α-1,2 mannotetrasoses, the concentrations tested were 0.3, 1.5, and 7 mM. After 30 min, 103 yeast cells were added to each well and coincubated with Caco-2 cells for 30 min at 37°C. The adhesion experiment was then performed as described above. All experiments included a series of positive control wells (cocultures in PBS alone without inhibiting carbohydrates) and triplicate testing of attachment with each carbohydrate concentration. Three separate experiments were performed for each carbohydrate tested.
In both series of inhibition experiments, the results of adhesion without inhibiting antibodies or competing carbohydrates were set to 100%, and adhesion in the presence of antibodies or carbohydrates was expressed as the ratio of the percentage of adhesive yeasts in the presence of a given concentration of antibody or carbohydrate to the percentage of adhesive yeasts in positive control wells.
Immunofluorescence confocal microscopy.
Cultures for immunofluorescence confocal microscopy were performed in 24-well culture dishes containing removable porous inserts (0.1-μm pore diameter; BD Falcon). Approximately 0.5 × 105 cells were seeded in the upper compartment. The cultures were maintained at 37°C and 5% CO2, and the medium in each well was replaced every other day. The monolayers were used 15 to 21 days postseeding. The adhesion assays were performed as described above, with an inoculum of 105 yeast cells per well for cocultures of 30 to 60 min. When longer interactions were analyzed, an inoculum of 103 yeast cells per well was used. Following incubation, the monolayers were washed three times to remove nonadhering blastospores. They were then fixed for 10 min at room temperature in a 3.7% formaldehyde solution in PBS. After two more washes, the monolayers were extracted with 0.1% Triton X-100 in PBS for 3 to 5 min. Each well was then washed twice, and the monolayers were reacted with Alexa Fluor 568-phalloidin for 20 min at room temperature for F-actin labeling in Caco-2 cells. After two washes, the monolayers were incubated with MAb 5B2 or EBCA1 (30 μg/ml; 1 h at 37°C). The monolayers were washed twice and reacted with Alexa Fluor-labeled secondary antibodies. The preparations were examined by confocal laser scanning microscopy on a Leica TCS 4000 microscope. The cell monolayers were optically sectioned in horizontal (x-y) or vertical (x-z) planes every 0.8 μm. Five separate experiments were performed to verify the absence of internalization of reactive yeasts at 30 or 60 min postinfection, and 20 microscopic fields were examined for each culture.
Double-labeling experiments on infected monolayers.
Caco-2 monolayers grown on glass coverslips were infected with 103 blastospores per well 15 to 21 days postseeding. At 12 h postinfection, the monolayers were washed to remove nonadherent blastospores. They were then fixed for 10 min at room temperature in a 3.7% formaldehyde solution in PBS. After two washes, the monolayers were reacted sequentially with MAb EBCA1, Alexa Fluor 594 goat anti-rat IgM, MAb 5B2, and Alexa Fluor 488 goat anti-mouse IgM at the concentrations given above. All incubations were performed for 1 h at 37°C and were followed by two washes. The monolayers were examined on a Nikon E600 Eclipse microscope.
Statistical analysis.
The within-day and between-day repeatabilities were studied by one-way analysis of variance. In attachment inhibition assays, the differences among 5B2, A255, and EBCA1 antibodies, as well as those between α-1,2 and β-1,2 tetramannosides, were studied, along with the concentrations, by two-way analysis of variance. The concentration associated with a 50% inhibition (IC50) was estimated with its standard error by modeling the inhibition curve according to the following equation: y = a/(a + x), where y is the percentage of inhibition, x is the concentration, and a is the value of x associated with a y of 50%; the minimization algorithm used for this nonlinear regression was the Gauss-Newton type.
RESULTS
Characterization of a model of adhesion of C. albicans blastospores to Caco-2 cells.
In order to identify the molecular partners involved at the initial stage of the C. albicans-enterocyte interaction, we developed a model of adhesion of C. albicans blastospores to Caco-2 cells. The binding of blastospores to monolayers increased from 10 to 60 min of incubation. After 60 min, clusters of blastospores indicating multiplication of the inoculum were observed, which precluded accurate evaluation of the initial attachment process. Moreover, germ tubes, hyphae, and pseudohyphae were detected, and these fungal elements express surface molecules, i.e., putative adhesins, distinct from blastospores (31). Our model was thus optimized to analyze C. albicans attachment after a 30-min interaction. Since the degree of maturation of Caco-2 cells increases until the cells are differentiated 14 to 21 days after being seeded (37), we evaluated the attachment of C. albicans to Caco-2 monolayers from 5 to 40 days postseeding. The binding of blastospores increased from 5 to 15 days and reached a plateau after 15 days. When the monolayers were infected with 103 blastospores 15 to 21 days postseeding, an average of 120 to 250 adherent yeast cells were detected after a 30-min interaction. Altogether, the mean within-day coefficient of variation of the model was 9%, whereas the between-day variance gave a coefficient of variation of 29%.
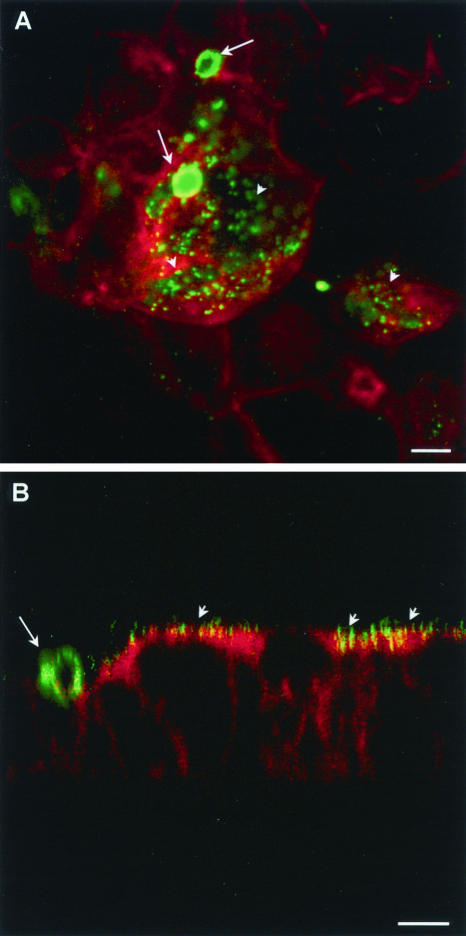
A key problem in setting up the model was to verify that yeasts counted after washings were adherent yeasts, i.e., yeasts that were attached to the surfaces of the cells as opposed to blastospores that could have been internalized by enterocytes. The Uvitex 2B dye met this requirement. This marker is excluded from live phagocytes (29) and has been employed to identify nonphagocytized yeasts in a model of Candida-endothelial-cell interaction (19). Moreover, fluorescence confocal microscopy analysis of permeabilized monolayers at 30 and 60 min of coculture confirmed that C. albicans blastospores were present at the apical surfaces of the cells only (Fig. 1). No internalized yeasts were detected in these experiments.
FIG. 1.
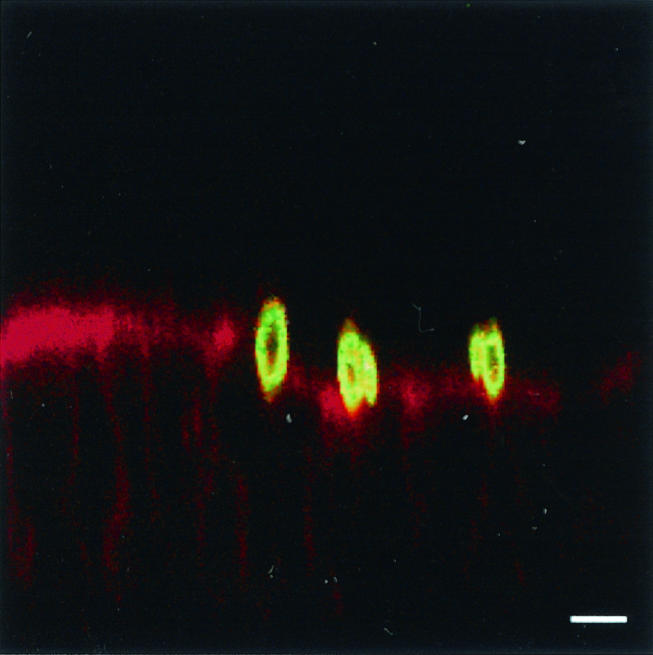
Confocal laser scanning microscopy of Caco-2 monolayers at 30 min postinfection with C. albicans blastospores. Monolayers sectioned in vertical planes (x-z) were processed for double labeling using Alexa fluor 568-phalloidin (red) and MAb 5B2 (green), and a merged image is presented. The apical region of the cell is identified by strong reactivity of phalloidin with the actin network of the brush border. Yeast cells are present at the surfaces of the cells. Bar, 5 μm.
Inhibition of C. albicans adhesion to Caco-2 cells by MAbs specific for α-linked or β-1,2-linked oligomannosides.
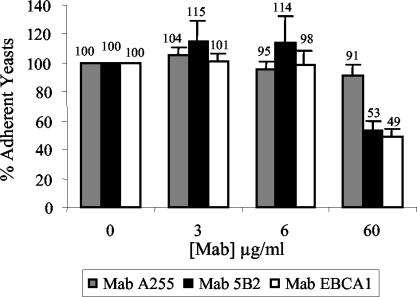
To establish the role of α- and β-mannosidic sequences in the C. albicans-Caco-2 interactions, we first performed neutralizing experiments with 5B2 and EBCA1, two MAbs that bind β-1,2 and α-1,2 mannoside epitopes, respectively (23, 24). An isotype-matched irrelevant MAb was used as a control. Since 5B2 and EBCA1 agglutinated C. albicans blastospores, yeasts treated with the MAbs were centrifuged and adhesion experiments were performed with the nonagglutinated blastospores present in the supernatants. Fractions of these nonagglutinated yeasts were assayed in parallel by immunofluorescence to verify that they bound the MAbs (Table 1). In experiments performed with 60 μg of MAbs/ml, the percentages of adhesion of C. albicans to Caco-2 cells were 91.4 ± 7.17, 53 ± 6.21, and 49 ± 5.56% for A255 (control), 5B2 (anti-β-1,2 mannoside), and EBCA1 (anti-α-1,2 mannoside) antibodies, respectively (Fig. 2), and statistical analysis showed that both anti-mannoside antibodies differed significantly from the A255 control antibody (P < 0.05). The increased adherence of blastospores treated with MAb 5B2 at 6 μg/ml (114 ± 18.2%) was significant (P < 0.05) compared to that of A255 (95.0 ± 6.08%) (Fig. 2). These results reflect a significant interaction between antibody and concentration, as the difference between antibodies is much higher at 60 than at 6 μg/ml. Other attachment percentages did not differ significantly.
TABLE 1.
Immunofluorescence reactivities of fractions of yeast suspensions exposed to MAbs 5B2, EBCA1, and A255 in attachment inhibition assaysa
| MAb | Reactivityb
|
||
|---|---|---|---|
| 3 μg/ml | 6 μg/ml | 60 μg/ml | |
| 5B2 | − | ± | ++ |
| EBCA1 | − | ± | ++ |
| A255 | − | − | − |
See Fig. 2.
−, no reactivity; ±, weak reactivity; ++, strong reactivity.
FIG. 2.
Effects of MAbs 5B2 (anti-β-1,2 mannose), EBCA1 (anti-α-1,2 mannose), and A255 (control antibody) on attachment of C. albicans blastospores to Caco-2 monolayers. Yeast cells were incubated with 3, 6, and 60 μg of antibodies/ml or with PBS alone prior to and during adhesion. Attachment is expressed as a percentage of that of the controls to which no antibody was added. The values are the means plus standard deviations of three independent experiments, including testing in duplicate wells.
Competitive inhibition of C. albicans adhesion to Caco-2 cells by glycans.
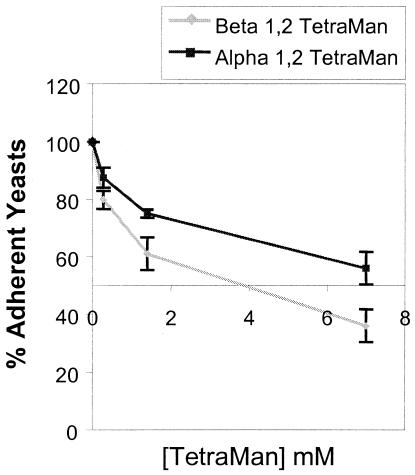
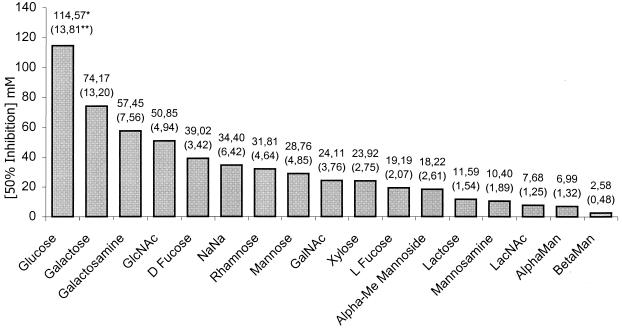
We next performed competitive assays using synthetic β-1,2 and α-1,2 tetramannosides with antigenic profiles that mimic the antigenicities of their natural counterparts in the C. albicans cell wall (12). A panel of mono- and disaccharides and complex glycans was also evaluated for the ability to inhibit C. albicans binding to Caco-2 cells. In these experiments, the concentration associated with a 50% inhibition was correctly estimated, since its coefficient of variation (the standard error divided by the estimate) ranged from 9 to 19% between products. The concentrations at which synthetic β-1,2 and α-1,2 tetramannosides inhibited 50% of C. albicans attachment to Caco-2 monolayers (IC50s) were 2.58 (standard error, 0.481) and 6.99 (standard error, 1.32) mM, respectively (Fig. 3), and these two glycans were the most potent inhibitors of C. albicans adhesion to Caco-2 cells (Fig. 4). Moreover, when the analysis took into account the IC50 range (IC50 ± 2 standard errors), the results for β-1,2 oligomannosides ranged from 1.62 to 3.54 mM and differed significantly from those for all other glycans tested, including α-1,2 oligomannosides (IC50 range, 4.36 to 9.64 mM). None of the complex glycans reduced adherence at 1 mg/ml or below.
FIG. 3.
Effects of synthetic mannotetraoses (TetraMan) on attachment of C. albicans blastospores to Caco-2 monolayers. The monolayers were incubated with β-1,2 mannotetraoses and α-1,2 mannotetraoses at 0.3, 1.5, and 7 mM prior to and during adhesion. Attachment is expressed as a percentage of that of the control cultures to which no competing carbohydrate was added. The values are the means ± standard deviations of three independent experiments, including testing in triplicate wells.
FIG. 4.
Inhibition of adherence of C. albicans blastospores to Caco-2 monolayers by carbohydrates. GlcNac, N-acetylglucosamine; NaNa, N-acetylneuraminic acid; GalNac, N-acetylgalactosamine; Alpha-Me, α-methylmannoside; LacNac, N-acetyllactosamine; AlphaMan, α-1,2 tetramannoside; BetaMan, β-1,2 tetramannoside. The concentration of carbohydrate at which adhesion is 50% of adhesion in the absence of carbohydrate (IC50) (*) was obtained by modeling the inhibition curves (as shown in Fig. 3) following the method described in Materials and Methods. **, standard error of the IC50.
Shedding of antigens containing α-1,2- and β-1,2-linked mannose residues during coculture of C. albicans with Caco-2 cells.
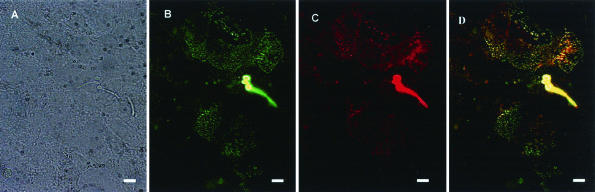
As antigens containing β-1,2 mannose residues are shed during the interaction of C. albicans with macrophages (25), we were next interested in determining whether shedding of α-1,2 or β-1,2 mannosides occurred during the C. albicans-Caco-2 interaction. To this end, cocultures of C. albicans with Caco-2 cells were subjected to indirect immunofluorescence detection of β-1,2 and α-1,2 mannosides with MAbs 5B2 and EBCA1, respectively. At 30 min postinfection, examination of the monolayers showed that MAb 5B2 detected islands of reactive material close to the yeasts (Fig. 5A). A more diffuse localization of the labeling was observed after 12 h of coculture (data not shown). Upon examination in the x-z orientation, the 5B2-reactive material was localized at the surfaces of the monolayers (Fig. 5B). When heat-killed blastospores were used, the percentage of adhesion was low (∼2.5% of the inoculum), and no reactive material was detected at the surfaces of the monolayers with MAb 5B2 (data not shown). Similar experiments were performed with MAb EBCA1, and clusters of reactive material were detected at the surfaces of the monolayers in the vicinity of the yeasts (data not shown). Similarly, no reactivity of MAb EBCA1 was detected upon incubation of the monolayers with heat-killed blastospores (data not shown). Double labeling of infected monolayers with 5B2 and EBCA1 MAbs (Fig. 6) showed that both antibodies essentially bound to the same domains of the monolayers (Fig. 6D). However, within a reactive domain, not all spots of 5B2-reactive material colocalized with spots of EBCA1-reactive material(Fig. 6D).
FIG. 5.
Immunofluorescence confocal microscopy analysis of β-1,2 oligomannosidic epitopes after a 30-min (A) or 12-h (B) culture of Caco-2 cells with C. albicans. The monolayers were processed for double labeling using Alexa Fluor 568-phalloidin (red) and MAb 5B2 (green), and merged images are presented. (A) Islands of material reactive with MAb 5B2 (arrowheads) in the vicinity of C. albicans (arrows). (B) Analysis in the vertical plane shows that β-1,2 oligomannosidic epitopes shed by C. albicans (long arrow) are present at the apical surfaces of the cells (short arrows). Bars, 5 μm.
FIG. 6.
Double-labeling experiment with MAbs 5B2 and EBCA1 on Caco-2 monolayers incubated for 12 h with C. albicans. (A to C) The same microscopic field was examined under phase-contrast microscopy (A), immunofluorescence microscopy for reactivity of MAb 5B2 with β-1,2 oligomannoside (green) (B), and immunofluorescence microscopy for reactivity of MAb EBCA1 with α-1,2 oligomannoside (red) (C). Both MAbs react with C. albicans blastospore and germ tube and with clusters of antigenic material at the surface of the cells. (D) Merged image of panels B and C. The secondary antibodies were Alexa Fluor 488 goat anti-mouse IgM (B) and Alexa Fluor 594 goat anti-rat IgM (C). Bars, 5 μm.
DISCUSSION
Most studies of the adhesion of C. albicans to human cells were performed with buccal epithelial cells (4, 10, 33), esophageal epithelial cells (14), endothelial cells (19), fibroblasts (32), keratinocytes (34), HeLa cells (21), or macrophages (16), and although intestinal colonization is accepted as the main source of bloodstream dissemination (38, 40, 47), little is known about the molecular basis of attachment of C. albicans to enterocytes. Three recent studies have addressed the C. albicans-enterocyte interaction. Weide and Ernst showed that transcellular migration of C. albicans occurred during a 12- to 24-h interaction (49). Wiesner et al. demonstrated that the absence of a functional INT1 gene, a gene involved in filamentous growth, attachment to HeLa cells, and virulence, was associated with decreased adherence of C. albicans blastospores to enterocytes (50). In a model of undifferentiated Caco-2 cells, Timpel et al. showed that a C. albicans strain defective in PMT1, a gene encoding a mannosyltransferase involved in the O-glycosylation pathway, showed decreased adherence (44). We chose to study the attachment of C. albicans to Caco-2 cells. This cell line derives from a human colorectal adenocarcinoma, expresses characteristics of enterocytic differentiation upon reaching confluence (37), and has been used to study interactions of human intestinal cells with bacteria (51), virus (2), protozoa (28), and C. albicans (44, 49, 50). The limitation of this model is that Caco-2 cells do not produce mucins. Therefore, our system is not suitable to investigate to what extent mucins may modulate the C. albicans-Caco-2 interaction (10).
C. albicans blastospores that bound anti-α-1,2 or anti β-1,2 mannoside MAbs showed a 50% decrease in adhesion to Caco-2 monolayers when exposed to higher antibody concentrations (Fig. 2). Despite the moderate (but statistically significant) increase in adhesion observed with MAb 5B2 at 6 μg/ml (Fig. 2), for which we have no explanation, the major attachment inhibition occurring upon exposure of blastospores to both anti-mannoside antibodies at 60 μg/ml suggests that α-1,2- and β-1,2-linked oligomannoside sequences present at the yeast cell surface are involved in attachment to Caco-2 cells. One limitation of these experiments is the use of nonagglutinated blastospores present in supernatant fractions after mild centrifugation of the yeast preparations. Indeed, despite a dose-dependent reactivity with anti-mannoside antibodies by immunofluorescence assay (Table 1), we cannot rule out the possibility that this blastospore population has adhesive properties distinct from their agglutinated counterparts. Moreover, immunoglobulins bound to the yeast surface could mask adhesive epitopes other than α-1,2 and β-1,2 oligomannosides. We therefore performed competitive experiments with synthetic α-1,2 and β-1,2 tetramannosides. Previous studies of macrophages showed that similar inhibition was achieved by incubating cells with native or synthetic β-1,2 mannotetraoses (16), demonstrating that synthetic molecules with a degree of polymerization of four mannose residues showed normal biological activity. Furthermore, α-1,2 and β-1,2 tetramannosides reacted normally with antibodies specific for α- and β-mannosidic epitopes in an enzyme immunoassay, indicating that the synthetic molecules retained the antigenicities of their native counterparts (12). The competing effect obtained with α-1,2 and β-1,2 tetramannosides is a striking result of the present investigation. The inhibitions obtained with both molecular species are dose dependent (Fig. 3). The specificity of this effect was established by comparing a panel of carbohydrates. None of the complex glycans tested reduced attachment at 1 mg/ml, a finding similar to that reported in a model of the adhesion of Candida glabrata to Hep2 cells (9). Other mono- and disaccharides inhibited C. albicans attachment to Caco-2 cells, with IC50s ranging from 7.68 mM for N-acetyllactosamine to 114.57 mM for glucose (Fig. 4). Thus, among all carbohydrates tested, β-1,2 tetramannosides exhibited the strongest inhibitory effects on the adhesion of C. albicans to Caco-2 cells. This finding is consistent with the dramatic reduction in gastrointestinal colonization reported in the infant mouse model after oral administration of synthetic β-1,2 tetramannosides (12). Conversely, the inhibitory effect observed in our study with synthetic α-1,2 tetramannosides contrasts with their lack of activity on gastrointestinal colonization in the mouse model (12). The reason why in vivo and in vitro data obtained with β-1,2 tetramannosides are correlated whereas those obtained with α-1,2 oligomannosides are not is unclear. In macrophages, oligomannosides with distinct anomere types of linkage have distinct receptors, since β-1,2 mannosides bind galectin 3 (17) and α-1,2 mannosides react with the macrophage mannose receptor (42). If α-1,2 and β-1,2 mannosides also bind distinct receptors at the enterocyte surface, one possible explanation is that Caco-2 cells express both receptors, whereas infant mouse enterocytes express receptors for β-1,2 mannosides only. An alternative hypothesis could be that enterocyte receptors for α-1,2 mannosides were saturated in the infant mouse model by ubiquitous α-mannosidic sequences expressed by the bacterial flora of the gastrointestinal tract in vivo.
We next examined by immunofluorescence the fate of C. albicans α-1,2 and β-1,2 mannosidic epitopes during coculture with Caco-2 cells. The reactivity of MAbs 5B2 and EBCA1 with antigenic material localized at the surface of the cells (Fig. 5 and 6) suggests that α-1,2 and β-1,2 oligomannosides are released by C. albicans during interaction with Caco-2 monolayers and bind to the surfaces of the cells. No such labeling was observed upon incubation of monolayers with heat-killed blastospores. The distribution of α-1,2 and β-1,2 mannoside labeling on Caco-2 monolayers was heterogeneous and was localized close to C. albicans at 30 min postinfection (Fig. 5A). Low levels of antigen shedding relative to enterocyte surfaces may account for this observation, a hypothesis supported by the increased reactivity of monolayers upon prolonged incubation with C. albicans. Double-labeling experiments with EBCA1 and 5B2 antibodies identified domains of the monolayers that reacted with both antibodies. In such domains, merged images showed that clusters of α-1,2 and β-1,2 mannoside reactivity did not necessarily colocalize (Fig. 6D), an observation consistent with the existence of a cell-specific or nonsynchronous expression of receptors for α-1,2 and β-1,2 oligomannosides. The shedding of β-1,2 mannoside epitopes in association with C. albicans PLM and adhesion to the host cell membrane were previously reported during cocultures with macrophages (25). At present, we cannot decide whether β-1,2 mannosidic epitopes shed by C. albicans during coculture with enterocytes correspond to PLM or to PPM. However, the fact that some of the shed material reacted with 5B2 but not with EBCA1 suggests that PLM is involved, since the carbohydrate moiety of PLM is composed of β-1,2 oligomannosides only. Altogether, this body of informations highlights the need to characterize Caco-2 receptors for α-1,2 and β-1,2 oligomannosides of C. albicans and to determine the biological significance of this shedding process in the Candida-Caco-2 interaction.
Finally, Timpel et al. demonstrated recently that a homozygous C. albicans mutant defective in PMT1, a gene encoding a mannosyltransferase involved in the O-glycosylation pathway, showed reduced adherence to Caco-2 cells. This observation pointed to the role of O-linked carbohydrates in the adherence of C. albicans to enterocytes (44). Interestingly, the present paper indicates that β-1,2- and α-1,2-linked oligomannosides, two components of the N-glycan moiety expressed at the surface of C. albicans, participate in the adhesion of blastospores to Caco-2 cells. Therefore, and despite the fact that the monolayers employed in the two studies were presumably not in the same state of differentiation, both O-linked and N-linked carbohydrates seem to represent putative adhesins in the host-fungus interplay that takes place at the intestinal mucosal surface. The characterization of the glycosylation pathways of C. albicans, including their regulation and response to the yeast environment, will thus be pivotal to understanding the commensal-pathogen transition and the physiopathological events occurring in the early stages of invasive candidiasis.
Acknowledgments
This work was supported by the “Réseau Infections Fongiques” from the Ministère de l'Education Nationale, de la Recherche et de la Technologie (MNERT); by a grant from the University Hospital of Dijon (Appel d'Offre Interne 1999); by “Programme Hospitalier de Recherche Clinique,” appel d'offre régional 2001; and by “Conseil Régional de Bourgogne” (grant 02/513/CP/02/S276).
Editor: T. R. Kozel
REFERENCES
- 1.Barondes, S. H., D. N. Cooper, M. A. Gitt, and H. Leffler. 1994. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 269:20807-20810. [PubMed] [Google Scholar]
- 2.Brunet, J. P., N. Jourdan, J. Cotte-Laffitte, C. Linxe, M. Geniteau-Legendre, A. Servin, and A. M. Quero. 2000. Rotavirus infection induces cytoskeleton disorganization in human intestinal epithelial cells: implication of an increase in intracellular calcium concentration. J. Virol. 74:10801-10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calderone, R. A. 1993. Recognition between Candida albicans and host cells. Trends Microbiol. 1:55-58. [DOI] [PubMed] [Google Scholar]
- 4.Cameron, B. J., and L. J. Douglas. 1996. Blood group glycolipids as epithelial cell receptors for Candida albicans. Infect. Immun. 64:891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantelli, C., P. A. Trinel, A. Bernigaud, T. Jouault, L. Polonelli, and D. Poulain. 1995. Mapping of beta-1,2-linked oligomannosidic epitopes among glycoconjugates of Candida species. Microbiology 141:2693-2697. [DOI] [PubMed] [Google Scholar]
- 6.Chaffin, W. L., J. L. Lopez-Ribot, M. Casanova, D. Gozalbo, and J. P. Martinez. 1998. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol. Mol. Biol. Rev. 62:130-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan, N., D. Li, P. Singh, R. Calderone, and M. Kruppa. 2002. The cell wall of Candida spp., p. 159-175. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.
- 8.Cooper, D. N., and S. H. Barondes. 1999. God must love galectins; he made so many of them. Glycobiology 9:979-984. [DOI] [PubMed] [Google Scholar]
- 9.Cormack, B. P., N. Ghori, and S. Falkow. 1999. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 285:578-582. [DOI] [PubMed] [Google Scholar]
- 10.de Repentigny, L., F. Aumont, K. Bernard, and P. Belhumeur. 2000. Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect. Immun. 68:3172-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domer, J. E. 1989. Candida cell wall mannan: a polysaccharide with diverse immunologic properties. Crit. Rev. Microbiol. 17:33-51. [DOI] [PubMed] [Google Scholar]
- 12.Dromer, F., R. Chevalier, B. Sendid, L. Improvisi, T. Jouault, R. Robert, J. M. Mallet, and D. Poulain. 2002. Synthetic analogues of beta-1,2 oligomannosides prevent intestinal colonization by the pathogenic yeast Candida albicans. Antimicrob. Agents Chemother. 46:3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 14.Enache, E., T. Eskandari, L. Borja, E. Wadsworth, B. Hoxter, and R. Calderone. 1996. Candida albicans adherence to a human oesophageal cell line. Microbiology 142:2741-2746. [DOI] [PubMed] [Google Scholar]
- 15.Faille, C., J. M. Wieruszeski, G. Lepage, J. C. Michalski, D. Poulain, and G. Strecker. 1991. 1H-NMR spectroscopy of manno-oligosaccharides of the beta-1,2-linked series released from the phosphopeptidomannan of Candida albicans VW-32 (serotype A). Biochem. Biophys. Res. Commun. 181:1251-1258. [DOI] [PubMed] [Google Scholar]
- 16.Fradin, C., T. Jouault, A. Mallet, J. M. Mallet, D. Camus, P. Sinay, and D. Poulain. 1996. Beta-1,2-linked oligomannosides inhibit Candida albicans binding to murine macrophage. J. Leukoc. Biol. 60:81-87. [DOI] [PubMed] [Google Scholar]
- 17.Fradin, C., D. Poulain, and T. Jouault. 2000. β-1,2-linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect. Immun. 68:4391-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser, V. J., M. Jones, J. Dunkel, S. Storfer, G. Medoff, and W. C. Dunagan. 1992. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin. Infect. Dis. 15:414-421. [DOI] [PubMed] [Google Scholar]
- 19.Fratti, R. A., M. A. Ghannoum, J. E. Edwards, Jr., and S. G. Filler. 1996. Gamma interferon protects endothelial cells from damage by Candida albicans by inhibiting endothelial cell phagocytosis. Infect. Immun. 64:4714-4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fridkin, S. K., and W. R. Jarvis. 1996. Epidemiology of nosocomial fungal infections. Clin. Microbiol. Rev. 9:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gale, C. A., C. M. Bendel, M. McClellan, M. Hauser, J. M. Becker, J. Berman, and M. K. Hostetter. 1998. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science 279:1355-1358. [DOI] [PubMed] [Google Scholar]
- 22.Han, Y., T. Kanbe, R. Cherniak, and J. E. Cutler. 1997. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect. Immun. 65:4100-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hopwood, V., D. Poulain, B. Fortier, G. Evans, and A. Vernes. 1986. A monoclonal antibody to a cell wall component of Candida albicans. Infect. Immun. 54:222-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacquinot, P. M., Y. Plancke, B. Sendid, G. Strecker, and D. Poulain. 1998. Nature of Candida albicans-derived carbohydrate antigen recognized by a monoclonal antibody in patient sera and distribution over Candida species. FEMS Microbiol. Lett. 169:131-138. [DOI] [PubMed] [Google Scholar]
- 25.Jouault, T., C. Fradin, P. A. Trinel, A. Bernigaud, and D. Poulain. 1998. Early signal transduction induced by Candida albicans in macrophages through shedding of a glycolipid. J. Infect. Dis. 178:792-802. [DOI] [PubMed] [Google Scholar]
- 26.Jouault, T., G. Lepage, A. Bernigaud, P. A. Trinel, C. Fradin, J. M. Wieruszeski, G. Strecker, and D. Poulain. 1995. Beta-1,2-linked oligomannosides from Candida albicans act as signals for tumor necrosis factor alpha production. Infect. Immun. 63:2378-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanbe, T., and J. E. Cutler. 1998. Minimum chemical requirements for adhesin activity of the acid-stable part of Candida albicans cell wall phosphomannoprotein complex. Infect. Immun. 66:5812-5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurent, F., L. Eckmann, T. C. Savidge, G. Morgan, C. Theodos, M. Naciri, and M. F. Kagnoff. 1997. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect. Immun. 65:5067-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levitz, S. M., D. J. DiBenedetto, and R. D. Diamond. 1987. A rapid fluorescent assay to distinguish attached from phagocytized yeast particles. J. Immunol. Methods 101:37-42. [DOI] [PubMed] [Google Scholar]
- 30.Lindberg, B., K. Leontein, U. Lindquist, S. B. Svenson, G. Wrangsell, A. Dell, and M. Rogers. 1988. Structural studies of the O-antigen polysaccharide of Salmonella thompson, serogroup C1 (6,7). Carbohydr. Res. 174:313-322. [DOI] [PubMed] [Google Scholar]
- 31.Marot-Leblond, A., L. Grimaud, S. Nail, S. Bouterige, V. Apaire-Marchais, D. J. Sullivan, and R. Robert. 2000. New monoclonal antibody specific for Candida albicans germ tube. J. Clin. Microbiol. 38:61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merkel, G. J., and C. L. Phelps. 1988. Factors influencing the interaction of Candida albicans with fibroblast cell cultures. Infect. Immun. 56:792-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyakawa, Y., T. Kuribayashi, K. Kagaya, M. Suzuki, T. Nakase, and Y. Fukazawa. 1992. Role of specific determinants in mannan of Candida albicans serotype A in adherence to human buccal epithelial cells. Infect. Immun. 60:2493-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ollert, M. W., R. Sohnchen, H. C. Korting, U. Ollert, S. Brautigam, and W. Brautigam. 1993. Mechanisms of adherence of Candida albicans to cultured human epidermal keratinocytes. Infect. Immun. 61:4560-4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oxley, D., and S. G. Wilkinson. 1991. Structure of a mannan isolated from the lipopolysaccharide of the reference strain (S3255) for a new serogroup of Serratia marcescens. Carbohydr. Res. 212:213-217. [DOI] [PubMed] [Google Scholar]
- 36.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, G. V. Doern, M. E. Brandt, and R. A. Hajjeh. 1999. Trends in species distribution and susceptibility to fluconazole among bloodstream isolates of Candida species in the United States. Diagn. Microbiol. Infect. Dis. 33:217-222. [DOI] [PubMed] [Google Scholar]
- 37.Pinto, M., S. Robine-Leon, M. D. Appay, M. Kedinger, N. Triadou, E. Dussaulx, B. Lacroix, P. Simon-Assmann, K. Haffen, J. Fogh, and A. Zweibaum. 1983. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell 47:323-330. [Google Scholar]
- 38.Pittet, D., M. Monod, P. M. Suter, E. Frenk, and R. Auckenthaler. 1994. Candida colonization and subsequent infections in critically ill surgical patients. Ann. Surg. 220:751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poulain, D., C. Slomianny, T. Jouault, J. M. Gomez, and P. A. Trinel. 2002. Contribution of phospholipomannan to the surface expression of beta-1,2-oligomannosides in Candida albicans and its presence in cell wall extracts. Infect. Immun. 70:4323-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reagan, D. R., M. A. Pfaller, R. J. Hollis, and R. P. Wenzel. 1990. Characterization of the sequence of colonization and nosocomial candidemia using DNA fingerprinting and a DNA probe. J. Clin. Microbiol. 28:2733-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibata, N., T. Ichikawa, M. Tojo, M. Takahashi, N. Ito, Y. Okubo, and S. Suzuki. 1985. Immunochemical study on the mannans of Candida albicans NIH A-207, NIH B-792, and J-1012 strains prepared by fractional precipitation with cetyltrimethylammonium bromide. Arch. Biochem. Biophys. 243:338-348. [DOI] [PubMed] [Google Scholar]
- 42.Stahl, P. D., and R. A. Ezekowitz. 1998. The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 10:50-55. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki, S. 2002. Serological differences among the pathogenic Candida spp., p. 29-36. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.
- 44.Timpel, C., S. Strahl-Bolsinger, K. Ziegelbauer, and J. F. Ernst. 1998. Multiple functions of Pmt1p-mediated protein O-mannosylation in the fungal pathogen Candida albicans. J. Biol. Chem. 273:20837-20846. [DOI] [PubMed] [Google Scholar]
- 45.Trinel, P. A., G. Lepage, T. Jouault, G. Strecker, and D. Poulain. 1997. Definitive chemical evidence for the constitutive ability of Candida albicans serotype A strains to synthesize beta-1,2 linked oligomannosides containing up to 14 mannose residues. FEBS Lett. 416:203-206. [DOI] [PubMed] [Google Scholar]
- 46.Trinel, P. A., E. Maes, J. P. Zanetta, F. Delplace, B. Coddeville, T. Jouault, G. Strecker, and D. Poulain. 2002. Candida albicans phospholipomannan, a new member of the fungal mannose inositol phosphoceramide family. J. Biol. Chem. 277:37260-37271. [DOI] [PubMed] [Google Scholar]
- 47.Voss, A., R. J. Hollis, M. A. Pfaller, R. P. Wenzel, and B. N. Doebbeling. 1994. Investigation of the sequence of colonization and candidemia in nonneutropenic patients. J. Clin. Microbiol. 32:975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voss, A., J. A. Kluytmans, J. G. Koeleman, L. Spanjaard, C. M. Vandenbroucke-Grauls, H. A. Verbrugh, M. C. Vos, A. Y. Weersink, J. A. Hoogkamp-Korstanje, and J. F. Meis. 1996. Occurrence of yeast bloodstream infections between 1987 and 1995 in five Dutch university hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 15:909-912. [DOI] [PubMed] [Google Scholar]
- 49.Weide, M. R., and J. F. Ernst. 1999. Caco-2 monolayer as a model for transepithelial migration of the fungal pathogen Candida albicans. Mycoses 42(Suppl. 2):61-67. [PubMed] [Google Scholar]
- 50.Wiesner, S. M., C. M. Bendel, D. J. Hess, S. L. Erlandsen, and C. L. Wells. 2002. Adherence of yeast and filamentous forms of Candida albicans to cultured enterocytes. Crit. Care Med. 30:677-683. [DOI] [PubMed] [Google Scholar]
- 51.Witthoft, T., L. Eckmann, J. M. Kim, and M. F. Kagnoff. 1998. Enteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. Am. J. Physiol. 275:G564-G571. [DOI] [PubMed] [Google Scholar]