Abstract
Lipopolysaccharide (LPS) from Porphyromonas gingivalis prevented apoptosis of HL60-derived neutrophils, which could not be restored upon the addition of interleukin-10. Signaling of P. gingivalis LPS through Toll-like receptor 2 (TLR2), not TLR4, may account for the inhibiting effect of P. gingivalis LPS on apoptosis and provide a mechanism for the development of destructive periodontal inflammation.
Porphyromonas gingivalis is a strongly implicated periodontal pathogen and is a predominant species in the gingival pockets of some individuals with advanced and severe periodontal disease. It is mostly found in deep periodontal pockets and active sites (13, 21), and elevated levels of opsonic activity to P. gingivalis have been found in the serum of patients with a history of destructive periodontal disease (42).
P. gingivalis has developed various mechanisms to evade the immune response. These include production of a trypsin-like protease that degrades many serum proteins including immunoglobulins, complement components, acute-phase proteins, and proteinase inhibitors (5, 13). Neutrophils are the predominant line of defense in the gingival crevice. P. gingivalis culture products have been reported to interfere with neutrophil elimination of the organism by causing inappropriate stimulation of the neutrophil, enhancing complement receptor 3 expression, and inhibiting phagocytosis (40, 41).
High levels of lipopolysaccharide (LPS) and lipid A from P. gingivalis have been reported to delay neutrophil apoptosis (10, 27) but induce lymphocyte apoptosis (7). P. gingivalis LPS has also been reported to increase the production of interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-8 by neutrophils (33, 43).
In this study, the effects of P. gingivalis LPS on the apoptosis of neutrophils derived from the human promyelocytic cell line, HL60, were determined. The use of HL60 cells allowed us to look at a homogenous neutrophil population over an extended time period. The results showed that P. gingivalis LPS prevented apoptosis of HL60-derived neutrophils and that apoptosis could not be restored upon the addition of IL-10.
HL60 cells (European Collection of Cell Cultures, Wiltshire, United Kingdom) are nonadherent and were grown in suspension culture containing RPMI 1640 medium plus l-glycerol (300 mg/liter) with 10% fetal bovine serum and 1% antibiotic-antimycotic solution (all from Life Technologies, Paisley, United Kingdom). Cells were maintained at 1 × 105 to 5 × 105 cells per ml at 37°C in a humidified environment with 5% CO2 in a vented tissue culture flask (Life Technologies) and replated when the density reached 1 × 106/ml. To obtain neutrophils, cells were stimulated with 10−7 M all-trans retinoic acid (Sigma-Aldrich Company Ltd., Dorset, United Kingdom). Cells were plated at a concentration of no more than 2.5 × 105/ml so that after differentiation the cell density obtained was no higher than 106/ml. The ability of differentiated HL60 cells to undergo apoptosis was determined by fluorescence microscopy with an Annexin V-FITC (fluorescein isothiocyanate) apoptosis detection kit (Oncogene Research Products, Calbiochem-Novabiochem Ltd., Nottingham, United Kingdom) following the manufacturer's RAPID annexin binding protocol. At 3-h intervals a 0.5-ml sample was taken from each flask and the percentage of apoptotic cells was determined. In each experiment three flasks were differentiated and three slides were prepared from each flask. The experiments were repeated twice, and the results were expressed as the means ± standard deviations. Total apoptosis was reached when 85% of the cells were apoptotic, as after this point cells began to undergo secondary necrosis and disintegrate.
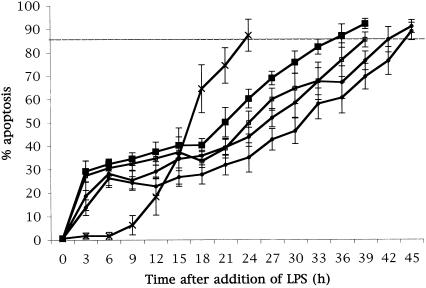
To ensure that HL60-derived neutrophils responded in a manner similar to peripheral blood neutrophils, LPS from Escherichia coli was used as a positive control. At differentiation E. coli 055:B5 LPS (catalogue number L2637; Sigma-Aldrich Company Ltd.) was added at concentrations of 1, 10, 100, or 1,000 ng/ml. E. coli LPS delayed apoptosis by 12 h at a concentration of 1 ng/ml, by 15 h at 10 ng/ml, by 18 h at 100 ng/ml, and by 21 h at 1,000 ng/ml, indicating a dose-dependent effect (Fig. 1). This result supports the findings of other in vitro (3, 8, 14, 16, 19) and in vivo (4) studies of humans. However, in about 30% of the cells, there was an induction of apoptosis at time points earlier than these.
FIG. 1.
E. coli LPS delays HL60-derived neutrophil apoptosis. E. coli LPS was added to differentiated HL60 cells at concentrations of 1 ng/ml (▪), 10 ng/ml (□), 100 ng/ml (•), and 1,000 ng/ml (○). Differentiated cells that received no LPS were used as an internal control (X). The addition of E. coli LPS induced apoptosis of 35% of the population at earlier time points compared to time for the control but delayed apoptosis of the remaining population in a dose-dependent manner.
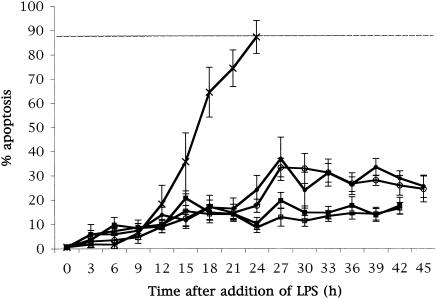
Purified P. gingivalis W50 LPS (prepared by Haroun Shah, Public Health Laboratory Service, Colindale, London [30, 31], and donated by Veronica Booth, London Hospital Dental School, London, United Kingdom) was added to the cell cultures at concentrations of 0.1, 1, 10, or 100 ng/ml. LPS from P. gingivalis prevented apoptosis of the majority of neutrophils, with a maximum of 35% of cells apoptotic after 45 h in culture (Fig. 2). Apoptosis in these cultures occurred at earlier time points and then remained at a stable level. Such a profound effect on neutrophil apoptosis by LPS has not previously been reported. Clearly, such a delay in apoptosis would prolong an acute inflammatory response with a consequently increased potential for tissue damage. These results confirm those of a previous study which also showed P. gingivalis LPS, albeit at a concentration 1 log higher, to delay neutrophil apoptosis (27). The experiments reported here extend those results as they were carried out over an extended time frame and were tested at concentrations more likely to be found in the subgingival plaque and gingival crevice/pocket (6 and clinical observation).
FIG. 2.
P. gingivalis LPS prevents HL60-derived neutrophil apoptosis. P. gingivalis LPS was added to differentiated HL60 cells at concentrations of 0.1 ng/ml (▪), 1 ng/ml (□), 10 ng/ml (•), and 100 ng/ml (○). Differentiated cells which received no LPS were plotted as a control (X). P. gingivalis LPS prevented apoptosis of the majority of the cell population, with a maximum of 35% of cells undergoing apoptosis after 45 h. Apoptosis occurred at earlier time points and remained at a stable level for the remaining culture period.
IL-10 was originally discovered as a Th2-secreted product that inhibited cytokine production from Th1 cells (20, 22). It has since been shown that IL-10 has effects on various cell types including T cells, B cells, macrophages, NK cells, mast cells, and neutrophils (2, 12, 20, 22). The effects of IL-10 on neutrophils include negative modulation of the production of IL-1, IL-8, IL-12, and TNF-α, up-regulation of IL-1 receptor antagonist production, inhibition of prostanoid and platelet-activating factor synthesis, and inhibition of phagocytosis. IL-10 is reported to counteract the delay in neutrophil apoptosis caused by LPS and proinflammatory cytokines such as granulocyte colony-stimulating factor, TNF-α, and interferon gamma but itself has no effect on basal survival rates (4, 14). It has been suggested that, as IL-10 can down-regulate proinflammatory cytokine production by macrophages and induce apoptosis in LPS- and cytokine-stimulated neutrophils, it may have a protective role in disorders such as endotoxic shock (17), pulmonary inflammation (4), and severe sepsis (14).
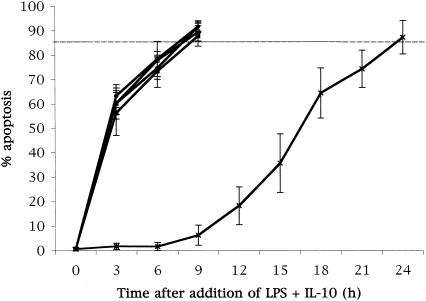
At differentiation, E. coli LPS was added to the cell cultures at a concentration of 1 ng/ml. IL-10 was then added at concentrations of 0.001, 0.01, 0.1, or 1 ng/ml. The combination of IL-10 and E. coli LPS induced apoptosis after 3 h in culture (Fig. 3). This effect was shown at all doses of IL-10 tested. In addition, the IL-10-LPS combination induced apoptosis in 85% of the total cell population after 9 h compared with the 24 h needed for the spontaneous, unstimulated apoptosis to reach the same level. When compared to the delayed apoptosis with LPS alone, total apoptosis with the IL-10-LPS combination was induced 27 h earlier. In our system, as in previous reports, IL-10 alone had no effect on basal survival rates (data not shown).
FIG. 3.
IL-10 counteracts delay in apoptosis by E. coli LPS. E. coli LPS (1 ng/ml) was added to differentiated HL60 cells along with IL-10 at concentrations of 0.001 ng/ml (▪), 0.01 ng/ml (□), 0.1 ng/ml (•), or 1 ng/ml (○). Cells which received neither LPS nor IL-10 were used as a control (X). The addition of IL-10 with E. coli LPS induced apoptosis of the total cell population by 6 h in culture, compared to 24 h for unstimulated cells and 36 h for E. coli LPS without IL-10.
As IL-10 induced apoptosis when it was added to cultures containing E. coli LPS, we tested the effect of IL-10 on the delay in apoptosis caused by P. gingivalis LPS by adding 0.1 and 10 ng of LPS/ml to cell cultures together with 0.1 ng of IL-10/ml. IL-10 did not affect the kinetics of the profoundly delayed apoptosis induced by P. gingivalis LPS (Fig. 4). This result would indicate that the down-regulatory effects of IL-10 previously reported do not extend to P. gingivalis LPS. This result also demonstrates that LPS from different bacterial species, while showing an array of biological effects, also exhibits substantial differences for a given effect.
FIG. 4.
No effect of IL-10 on prevention of apoptosis by P. gingivalis LPS. IL-10 (0.1 ng/ml) was added to differentiated HL60 cells with P. gingivalis LPS at a concentration of either 0.1 ng/ml (▪) or 10 ng/ml (□). Differentiated cells which received no additional stimulus were used as a control (X). The addition of IL-10 did not alter the effect of P. gingivalis LPS in preventing neutrophil apoptosis.
Differential effects of P. gingivalis and E. coli LPS have been reported in various cell types, including monocytes and epithelial cells, both in vitro (1, 18, 26, 29) and in vivo (28). These differences are most likely due to distinct signaling pathways through the Toll-like receptors (TLRs) as LPS from P. gingivalis was recently shown to bind to TLR2, not the common LPS receptor TLR4 (9, 11, 15, 25, 32, 35). LPS from P. gingivalis has also been reported as antagonistic for TLR4 (44). It has been suggested that the binding of different LPS moieties to TLRs may be, at least in part, due to the secondary structure of lipid A (23). However, studies with fibroblasts have shown P. gingivalis LPS to signal through TLR4 (24, 36, 37). This finding may indicate a difference in signaling in this particular cell type or reflect a difference between parenchymal and lymphoid-derived cells. IL-10 has been found to inhibit the effect of P. gingivalis LPS-induced IL-6 production in fibroblasts (39). As this study showed no effect of IL-10, it further suggests use of a different signaling pathway in these two cell types.
The ability of P. gingivalis LPS to stimulate cells through TLR2 may have skewed the immune response, allowing the bacteria to persist and invade the stratified epithelium. The ability of P. gingivalis to prolong the life span of neutrophils while causing inappropriate activation could possibly result in increased and prolonged inflammation and damage to surrounding tissue. This ability is further highlighted by the finding that P. gingivalis can induce expression of cell adhesion molecules as well as chemokine and cytokine release from gingival epithelium, thereby recruiting neutrophils (34, 38). These findings support the role of P. gingivalis in the initiation and exacerbation of destructive periodontal disease.
Acknowledgments
We thank Smithkline-Beecham for a research award.
We thank V. Booth and H. Shah for the supply of P. gingivalis LPS.
Editor: A. D. O'Brien
REFERENCES
- 1.Bainbridge, B., and R. Darveau. 2001. Porphyromonas gingivalis lipopolysaccharide: an unusual pattern recognition receptor ligand for the innate host defense system. Acta Ondontol. Scand. 59:131-138. [DOI] [PubMed] [Google Scholar]
- 2.Cassatella, M. 1998. The neutrophil: one of the cellular targets of interleukin-10. Int. J. Clin. Lab Res. 28:148-161. [DOI] [PubMed] [Google Scholar]
- 3.Colotta, F., F. Re, N. Polentarutti, S. Sozzani, and A. Mantovani. 1992. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80:2012-2020. [PubMed] [Google Scholar]
- 4.Cox, G. 1996. IL-10 enhances resolution of pulmonary inflammation in vivo by promoting apoptosis of neutrophils. Am. J. Physiol. 271:L566-L571. [DOI] [PubMed] [Google Scholar]
- 5.Ebersole, J., and D. Cappelli. 2000. Acute-phase reactants in infections and inflammatory diseases. Periodontol. 2000. 23:19-49. [DOI] [PubMed] [Google Scholar]
- 6.Fine, D., C. Mendieta, M. Barnett, D. Furgang, A. Naini, and J. Vincent. 1992. Endotoxin levels in periodontally healthy and diseased sites: correlation with levels of gram-negative bacteria. J. Periodontol. 63:897-901. [DOI] [PubMed] [Google Scholar]
- 7.Geatch, D., J. Harris, P. Heasman, and J. Taylor. 1999. In vitro studies of lymphocyte apoptosis induced by the periodontal pathogen Porphyromonas gingivalis. J. Periodontol. Res. 34:70-78. [DOI] [PubMed] [Google Scholar]
- 8.Hachiya, O., Y. Takeda, H. Miyata, H. Watanabe, T. Yamashita, and F. Sendo. 1995. Inhibition by bacterial lipopolysaccharide of spontaneous and TNF-alpha-induced human neutrophil apoptosis in vitro. Microbiol. Immunol. 39:715-723. [DOI] [PubMed] [Google Scholar]
- 9.Hanazawa, S., K. Nakada, Y. Ohmori, T. Miyoshi, S. Amano, and S. Kitano. 1985. Functional role of interleukin 1 in periodontal disease: induction of interleukin 1 production by Bacteroides gingivalis lipopolysaccharide in peritoneal macrophages from C3H/HeN and C3H/HeJ mice. Infect. Immun. 50:262-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiroi, M., T. Shimojima, M. Kashimata, T. Miyata, H. Takano, M. Takahama, and H. Sakagami. 1998. Inhibition by Porphyromonas gingivalis LPS of apoptosis induction in human peripheral blood polymorphonuclear leukocytes. Anticancer Res. 18:3475-3480. [PubMed] [Google Scholar]
- 11.Hirschfeld, M., J. Weis, V. Toshchakov, C. Salkowski, M. Cody, D. Ward, N. Qureshi, S. Michalek, and S. Vogel. 2001. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard, M., and A. O'Garra. 1992. Biological properties of interleukin 10. Immunol. Today 13:198-200. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa, I., K. Nakashima, T. Koseki, T. Nagasawa, H. Watanabe, S. Arakawa, H. Nitta, and T. Nishihara. 1997. Induction of the immune response to periodontopathic bacteria and its role in the pathogenesis of periodontitis. Periodontol. 2000. 14:79-111. [DOI] [PubMed] [Google Scholar]
- 14.Keel, M., U. Ungethum, U. Steckholzer, E. Niederer, T. Hartung, O. Trentz, and W. Ertel. 1997. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood 90:3356-3363. [PubMed] [Google Scholar]
- 15.Kirikae, T., H. Nitta, F. Kirikae, Y. Suda, S. Kusumoto, N. Qureshi, and M. Nakano. 1999. Lipopolysaccharides (LPS) of oral black-pigmented bacteria induce tumor necrosis factor production by LPS-refractory C3H/HeJ macrophages in a way different from that of Salmonella LPS. Infect. Immun. 67:1736-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, A., M. Whyte, and C. Haslett. 1993. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 54:283-288. [PubMed] [Google Scholar]
- 17.Marchant, A., C. Bruyns, P. Vandenabeele, D. Abramowicz, C. Gerard, A. Delvaux, P. Ghezzi, T. Velu, and M. Goldman. 1994. The protective role of interleukin-10 in endotoxin shock. Prog. Clin. Biol. Res. 388:417-423. [PubMed] [Google Scholar]
- 18.Martin, M., J. Katz, S. Vogel, and S. Michalek. 2001. Differential induction of endotoxin tolerance by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. J. Immunol. 167:5278-5285. [DOI] [PubMed] [Google Scholar]
- 19.Moore, F., S. Socher, and C. Davis. 1991. Tumor necrosis factor and endotoxin can cause neutrophil activation through separate pathways. Arch. Surg. 126:70-73. [DOI] [PubMed] [Google Scholar]
- 20.Moore, K., A. O'Garra, R. d. Waal, P. Vieira, and T. Mosmann. 1993. Interleukin-10. Annu. Rev. Immunol. 11:165-190. [DOI] [PubMed] [Google Scholar]
- 21.Moore, W., and L. Moore. 1994. The bacteria of periodontal diseases. Periodontol. 2000. 5:66-77. [DOI] [PubMed] [Google Scholar]
- 22.Mosmann, T. 1994. Properties and functions of interleukin-10. Adv. Immunol. 56:1-26. [PubMed] [Google Scholar]
- 23.Netea, M., M. van Deuren, B. Kullberg, J.-M. Cavaillon, and J. Van der Meer. 2002. Does the shape of lipid A determine the interaction of LPS with toll-like receptors? Trends Immunol. 23:135-139. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa, T., Y. Asai, M. Hashimoto, O. Takeuchi, T. Kurita, Y. Yoshikai, K. Miyake, and S. Akira. 2002. Cell activation by Porphyromonas gingivalis lipid A molecule through toll-like receptor 4- and myeloid differentiation factor 88-dependent signaling pathway. Int. Immunol. 14:1325-1332. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa, T., H. Shimauchi, H. Uchida, and Y. Mori. 1996. Stimulation of splenocytes in C3H/HeJ mice with Porphyromonas gingivalis lipid A in comparison with enterobacterial lipid A. Immunobiology 196:399-414. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa, T., and H. Uchida. 1996. Differential induction of IL-1beta and IL-6 production by the nontoxic lipid A from Porphyromonas gingivalis in comparison with synthetic Escherichia coli lipid A in human peripheral blood mononuclear cells. FEMS Immunol. Med. Microbiol. 14:1-13. [DOI] [PubMed] [Google Scholar]
- 27.Preshaw, P., R. Schifferle, and J. Walters. 1999. Porphyromonas gingivalis lipopolysaccharide delays human polymorphonuclear leukocyte apoptosis in vitro. J. Periodontal Res. 34:197-202. [DOI] [PubMed] [Google Scholar]
- 28.Pulendran, B., P. Kumar, C. Cutler, M. Mohamadzadeh, T. Van Dyke, and J. Banchereau. 2001. lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 167:5067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts, F., G. Richardson, and S. Michalek. 1997. Effects of Porphyromonas gingivalis and Escherichia coli lipopolysaccharides on mononuclear phagocytes. Infect. Immun. 65:3248-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah, H., S. Gharbia, and C. O'Toole. 1992. Assessment of the relative cytotoxicity of Porphyromonas gingivalis cells, products, and components on human epithelial cell lines. J. Periodontol. 63:44-51. [DOI] [PubMed] [Google Scholar]
- 31.Shah, H., S. Seddon, and S. Gharbia. 1989. Studies on the virulence properties and metabolism of pleiotropic mutants of Porphyromonas gingivalis (Bacteroides gingivalis) W50. Oral Microbiol. Immunol. 4:19-23. [DOI] [PubMed] [Google Scholar]
- 32.Shimauchi, H., T. Ogawa, H. Uchida, J. Yoshida, H. Ogoh, T. Nozaki, and H. Okada. 1996. Splenic B-cell activation in lipopolysaccharide-non-responsive C3H/HeJ mice by lipopolysaccharide of Porphyromonas gingivalis. Experientia 52:909-917. [DOI] [PubMed] [Google Scholar]
- 33.Sugita, N., A. Kimura, Y. Matsuki, T. Yamamoto, H. Yoshie, and K. Hara. 1998. Activation of transcription factors and IL-8 expression in neutrophils stimulated with lipopolysaccharide from Porphyromonas gingivalis. Inflammation 22:253-267. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama, A., A. Uehara, K. Iki, K. Matsushita, R. Nakamura, T. Ogawa, S. Sugawara, and H. Takada. 2002. Activation of human gingival epithelial cells by cell surface components of black-pigmented bacteria: augmentation of production of interelukin-8, granulocyte colony-stimulating factor and expression of intercellular adhesion molecule 1. J. Med. Microbiol. 51:27-33. [DOI] [PubMed] [Google Scholar]
- 35.Tanamoto, K.-I., S. Azumi, Y. Haishima, H. Kumada, and T. Umemoto. 1997. The lipid A moiety of Porphyromonas gingivalis lipopolysaccharide specifically mediates the activation of C3H/HeJ mice. J. Immunol. 158:4430-4436. [PubMed] [Google Scholar]
- 36.Wang, P.-L., Y. Azuma, M. Shinohara, and K. Ohura. 2000. Toll-like receptor 4-mediated signal pathway induced by Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Biochem. Biophys. Res. Commun. 273:1161-1167. [DOI] [PubMed] [Google Scholar]
- 37.Wang, P.-L., M. Oido-Mori, T. Fujii, Y. Kowashi, M. Kikuchi, Y. Suetsugu, J. Tanaka, Y. Azuma, M. Shinohara, and K. Ohura. 2001. Heterogenous expression of toll-like receptor 4 and downregulation of toll-like receptor 4 expression on human gingival fibroblasts by Porphyromonas gingivalis. Biochem. Biophys. Res. Commun. 288:863-867. [DOI] [PubMed] [Google Scholar]
- 38.Wang, P.-L., K. Sato, M. Oido, T. Fujii, Y. Kowashi, M. Shinohara, K. Ohura, H. Tani, and Y. Kuboki. 1998. Involvement of CD14 on human gingival fibroblasts in Porphyromonas gingivalis lipopolysaccharide-mediated interleukin-6 secretion. Arch. Oral Biol. 43:687-694. [DOI] [PubMed] [Google Scholar]
- 39.Wang, P.-L., S. Shirasu, M. Shinohar, Y. Azuma, M. Daito, H. Yasuda, and K. Ohura. 1999. IL-10 inhibits Porphyromonas gingivalis LPS-stimulated human gingival fibroblasts production of IL-6. Biochem. Biophys. Res. Commun. 263:372-377. [DOI] [PubMed] [Google Scholar]
- 40.Wilton, J., T. Hurst, R. Carman, and M. Macey. 1990. Effects of Porphyromonas gingivalis culture products on human polymorphonuclear leukocyte function. FEMS Immunol. Med. Microbiol. 2:285-293. [DOI] [PubMed] [Google Scholar]
- 41.Wilton, J., T. Hurst, and E. Scott. 1993. Inhibition of polymorphonuclear leucocyte phagocytosis by Porphyromonas gingivalis culture products in patients with adult periodontitis. Arch. Oral Biol. 38:285-289. [DOI] [PubMed] [Google Scholar]
- 42.Wilton, J., T. Hurst, and J. Sterne. 1993. Elevated opsonic activity for Porphyromonas (Bacteroides) gingivalis in serum from patients with a history of destructive periodontal disease. A case: control study. J. Clin. Periodontol. 20:563-569. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimura, A., Y. Hara, T. Kaneko, and I. Kato. 1997. Secretion of IL-1 beta, TNF-alpha, IL-8 and IL-1ra by human polymorphonuclear leukocytes in response to lipopolysaccharides from periodontopathic bacteria. J. Periodontal Res. 32:279-286. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimura, A., T. Kaneko, Y. Kato, D. Golenbrock, and Y. Hara. 2002. Lipopolysaccharides from periodontopathic bacteria Porphyromonas gingivalis and Capnocytophaga ochracea are antagonists for human toll-like receptor 4. Infect. Immun. 70:218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]