Abstract
One of the major pathogenic determinants of Vibrio cholerae, the cholera toxin, is encoded in the genome of a filamentous phage, CTXφ. CTXφ makes use of the chromosome dimer resolution system of V. cholerae to integrate its single stranded genome into one, the other, or both V. cholerae chromosomes. Here, we review current knowledge about this smart integration process.
Keywords: dif, site-specific recombination, XerC, XerD
Introduction
Most bacteriophages are detrimental to their host metabolism. However, phages also participate in the horizontal transfer of genes among bacteria because their genome can harbour other genes than those strictly required for their life cycle. This can be highly beneficial to the bacterial host. Indeed, many bacterial virulence factors are associated with phage-like DNA sequences. More strikingly, the exotoxins produced by many pathogenic bacteria are encoded in the genome of lysogenic phages. This is notably the case in Bordetella avium1, Clostridium botulinum2, Corynebacterium diphtheria3, Escherichia coli4, Pseudomonas aeruginosa5, Shigella dysenteriae6, Staphylococcus aureus7 and Streptococcus pyogenes8. The integrated prophages harboured by these bacteria profit from the multiplication of their host in the environment, which is in turn favoured by the virulence factors they bring to their host.
The study of Vibrio cholerae, the agent of the deadly diarrhoeal disease cholera, provides a fascinating case of such a bacterium-phage co-evolution. V. cholerae is the host for a variety of phages, commonly known as vibriophages, which can be lytic, non-lytic, virulent or temperate9. On the one hand, phage predation of V. cholerae has been reported to be a factor that influences seasonal epidemics of cholera10. On the other hand, one of the major virulence factors of V. cholerae, cholera toxin, is encoded in the genome of an integrated prophage CTXΦ11,12. Furthermore, different variants of the phage CTXΦ exist, which participate in the genetic diversity of epidemic causing cholera strains13–15. Two different attachment sites were found for this family of phages on the V. cholerae genome. They correspond to the dimer resolution sites of the two V. cholerae chromosomes, dif1 and dif216. Indeed, in contrast to most other lysogenic phages, such as bacteriophage λ17, CTXΦ does not encode its integrase, but makes use of XerC and XerD, the two host-encoded tyrosine recombinases that normally function to resolve chromosome dimmers18. This mode of integration is all the more intriguing since CTXΦ phages belong to the filamentous phage family, which are generally not lysogenic and which harbour a single stranded circular genome. Nevertheless, CTXΦ-like prophages were found integrated in the genome of several bacterial species, notably in pathogenic E. coli strains19 and in Yersinia pestis20. Finally, it is remarkable to observe that many filamentous phages and/or genetic elements other than CTXΦ seem to have hijacked the chromosome dimer resolution system of V. cholerae for integration. Thus, TLC21, VEJ22, VGJ23, VSK24, VSKK (AF452449), KSF-1F24, fs125, fs226, f23714, were all found to be integrated at dif1 and/or dif2. Such a diversity of elements has not been observed in any other genera than the vibrios. Together, these elements participate in the dissemination of virulence factors among V. cholerae strains11,28,29 and in the emergence of new genetic variants of epidemic strains of V. cholera13. We review current knowledge on the integration mechanism of filamentous vibriophages that hijack the XerCD recombinases, with a special focus on CTXΦ.
CTXΦintegration mechanism: exception or new paradigm?
CTXΦ has a ~7-kb ss(+)DNA genome arranged in two modular structures, the “RS” and“core”. The core region harbours seven genes, which are psh, cep, gIIICTX, ace, zot, ctxA and ctxB. While the psh, cep, gIIICTX, ace and zot encoded proteins are needed for phage morphogenesis, the products of the ctxAB genes are not strictly required for the life cycle of the phage but are responsible for the severe diarrhoea associated with cholera11. Three proteins, designated as RstR, RstA and RstB, are encoded in RS. Genetic analyses indicated that RstA is essential for phage replication and that RstB plays a crucial role in integration30. RstR acts as a transcriptional repressor by inhibiting the activity of PrstA, the only phage promoter required for CTXΦ replication and integration30. Several CTXΦ have been reported. These can be classified into four families based on the sequence of their rstR gene. These categories were designated as CTXΦET, CTXΦCl, CTXΦClc and CTXΦEnv according to the host cells in which they were originally isolated31–33.
As mentioned earlier, the integration of CTXΦ into the V. cholerae genome depends on two host encoded tyrosine recombinases, XerC and XerD18. XerC and XerD normally serve to resolve circular bacterial chromosome dimers generated by RecA mediated homologous recombination by adding a crossover at a specific 28 bp site dif on the chromosome16. The dif sites consist of specific 11-bp binding sites for each of the two Xer recombinases, separated by a 6-bp central region34. These are generally located opposite to the origin of replication of bacterial chromosomes16. Two dif sites are present on the genome of V. cholerae, one for each of the two circular chromosomes of the bacterium35. Three different chromosome dimer resolution sites (dif1, dif2 and difG) have been identified among the different V. cholerae strains characterized to date36 (Table I).
Table I.
Sequences of the chromosome dimer resolution sites found in V. cholerae strains
| Site | Sequence |
|---|---|
| dif1 | AGTGCGTATTA TGTATG TTATGTTAAAT |
| dif2 | AATGCGTATTA CGTGCG TTATGTTAAAT |
| difG | AGTGCGTATTA GGTATA TTATGTTAAAT |
| Source: Ref. 36 |
The ssDNA (+) genome of CTXΦ harbours two dif like sites (attP1 and attP2). These are arranged in opposite orientation and are separated by ~90-bp DNA segment in the phage genome37. Integration of CTXΦ at the dif loci of V. cholerae depends on the formation of a forked hairpin structure of 150 bp in the region encompassing attP1 and attP2 in the (+) ssDNA genome38 (Fig. 1). The hybridization of attP1 and attP2 at the stem of this hairpin unmasks the phage attachment site, attP(+). Integration occurs, XerC and XerD recombine this site with one of the two dimer resolution sites harboured by the host cell. This process only requires the catalytic activity of XerC: a single pair of strands is exchanged, which results in the formation of a pseudo-Holliday junction.
Fig. 1.
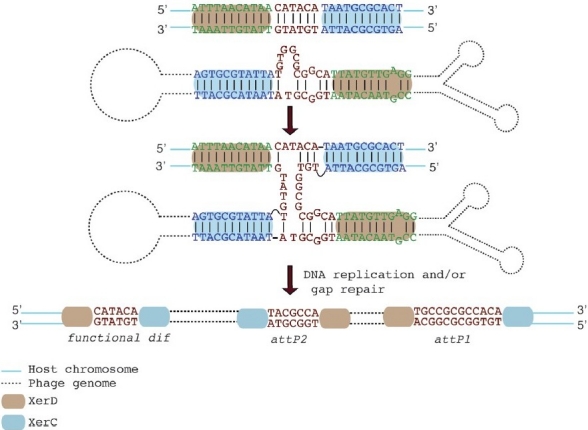
Schematic representation of the XerCD mediated site-specific recombination reaction between the single stranded (+) DNA genome of CTXΦ and V. cholerae dif1. Blue and green bases indicate XerC and XerD binding sites. Bases of the central region of these sites are shown in red. The recombination reaction stops after the exchange of a single pair of strands, which is catalyzed by XerC. Integration is completed when the resulting pseudo-Holliday junction needs to be processed by the host DNA replication and/or DNA repair machineries. Integration of the phage generates one new functional dif site and two non-functional dif like sequences, attP2 and attP1, on the host chromosome38.
A proof of principle for this mechanism of integration was originally obtained for the El Tor variant of CTXΦ and dif1 based on in vivo work performed in Escherichia coli and in vitro work performed with the E. coli Xer recombinases38. Later on, a sensitive and quantitative assay was developed to confirm the ssDNA(+) integration model of CTXΦET into the dif1 site of a V. cholerae El Tor strain36. This system was also used to define rules of compatibilities between the phage attachment sites harboured by the different CTXΦ variants characterized to date and their host dimer resolution sites36 : integration is solely determined by possibility to form Watson-Crick or w0 obble base pair interactions to stabilize the exchange of strands promoted by XerC-catalysis between the phage attachment site and its target dimer resolution site (Table II and Fig. 1). These rules explain how integration of CTXΦET is restricted to dif1, how CTXΦCl can target both dif1 and dif2, and how a third CTXΦ variant targets difG (Table II). This single stranded integration model is not restricted to CTXΦ. Analysis of the att P sites of CUS-1F and Ypf-F phages revealed features for direct ssDNA integration into the chromosome dimer resolution site harboured by their respective host cells38. Another family of mobile genetic element, the integrons, also integrates in the bacterial chromosome via a single stranded intermediate39.
Table II.
Sequences of the dif-like sites harboured by CTXΦ variant
| CTXΦ variant | attP sequence | Integration site | Accession number |
|---|---|---|---|
| El Tor | AGTGCGTATTA TGTGGCGCGGCA TTATGTTGAGG (attP1) AATGCGTATTA TACGCCA TTATGTTACGG (attP2) | dif1 | VCU83796 |
| Classical | AGTGCGTATTA TGTGGCGCGGCA TTATGTTGAGG (attP1) AATGCGTATTA CTCGCCA TTATGTTACGG (attP2) | dif1 dif2 | AY349175 |
| Calcutta | AGTGCGTATTA TGTGGCGCGGCA TTATGTTGAGG (attP1) AATGCGTATTA TACGCCA TTATGTTACGG (attP2) | dif1 | AF110029 |
| G | AGTGCGTATTA GGTGGTGCGGCA TTATGTTGAGG (attP1) AATGCGTATTA GGGGCA TTATGTTACGG (attP2) | difG | AF416590 |
| Source: Ref. 40 |
Integration mechanism of CTXΦ-associated genetics elements
Several filamentous phages other than CTXΦ are found to be integrated at the dif loci of V. cholera13,22,23. To date, there is no report about their particular integration mechanism. Like CTXΦ, they do not encode a dedicated recombinase. In addition, a 29-bp dif like sequence can be identified in many of them (Table III). It is, therefore, very likely that these phages take control of the host XerC and XerD recombinases to integrate into the genome of their host. However, the presence of a single putative XerCD binding site on their genome makes it unlikely that the ssDNA form of their genome is directly used as a substrate for integration. We rather favour a model in which the double stranded replicative form of these phages is used for integration (Fig. 2). We are currently investigating this model using the tools we have developed for the study of CTXΦ40.
Table III.
Sequences of the dif-ured by other vibriophages
| Phage | Genome size (kb) | attP sequence | Host | Integration site | Accession number |
|---|---|---|---|---|---|
| VEJ | 6.8 | ACTTCGCATTA TGTCGGC TTATGGTAAAA | V. cholerae | dif1 | NC012757 |
| VGJ | 7.5 | ACTTCGCATTA TGTCGGC TTATGGTAAAA | V. cholerae | dif1 | AY242528.1 |
| VSK | 6.9 | ACTTCGCAGTA TGTCGGC TTATGGTAAAA | V. cholerae | dif1 | NC003327 |
| VSKK | 6.8 | ACTTCGCATTA TGTCGGC TTATGGTAAAA | V. cholerae | dif1 | AF452449 |
| KSF1 | 7.1 | UK | V. cholerae | UK | AY714348 |
| fs1 | 6.3 | UK | V. cholerae | UK | NC004306.1 |
| fs2 | 8.6 | AGTGCGTATTA TGTCGGC TTATGGTAAAA | V. cholerae | dif1 | AB002632 |
| f237 | 8.7 | AGTGCGCATTA TGGGCGC TTATGTTGAAT | V. cholerae V. parahemolyticus | dif1 | NC002362 |
| UK, unknow; Source: Ref. 40 |
Fig. 2.
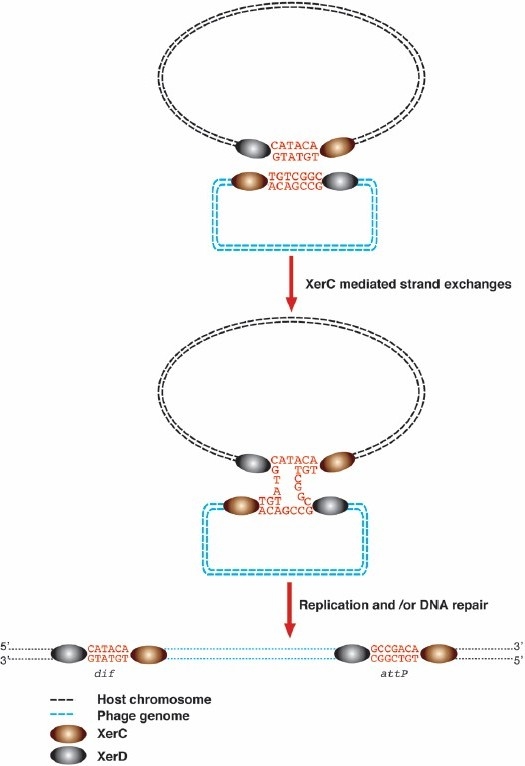
Putative mechanism of lysogenic conversion by the second type of filamentous phages that are found integrated into the chromosomal dimer resolution sites of V. cholerae40.
Interestingly, the two TLC elements integrated in strain N16961 are flanked by the half of the dif sequence (TGTGCGCATTA TGTATG for one and AGTGCATATTA TGTATG for the other). It is, therefore, reasonable to argue that their integration might be linked to the activity of the Xer recombinases.
Future prospects
The particular mode of integration of CTXΦ raises several questions. First, the efficiency of integration of a circular single stranded DNA molecule harbouring the sole attachment site of CTXΦ is very low38. However, it becomes extremely efficient when the RS region of the phage is included36. One likely explanation is that constant production and/or stabilization of the phage single stranded circular genome compensate for the instability of single stranded DNA in bacterial cells. RstB, which has been shown to be a single stranded DNA binding protein41, could play a role in the stabilization of the integration substrate. Accordingly, its biochemical properties and sequence differ from those of the single stranded DNA binding protein encoded in the genome of VGJF, a phage that seems to rely on double stranded DNA integration40. Second, only one pair of strands is exchanged between the single stranded DNA genome of CTXΦand the double stranded DNA genome of its host, which leaves open the question of how the resulting pseudo-Holliday junction intermediate is processed. Is it stably maintained until the next round of bacterial DNA replication or processed by some host DNA repair machinery? What occurs when the replication fork collides against this unusual structure? Finally, it is intriguing that so many phages take advantage of the Xer recombination system of vibrios as compared to other bacterial species. We wonder if it could be related to the particular life style and environment of the vibrios and/or their particular genome structure and management.
References
- 1.Shelton CB, Crosslin DR, Casey JL, Ng S, Temple LM, Orndorff PE. Discovery, purification, and characterization of a temperate transducing bacteriophage for Bordetella avium. J Bacteriol. 2000;182:6130–6. doi: 10.1128/jb.182.21.6130-6136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujii N, Oguma K, Yokosawa N, Kimura K, Tsuzuki K. Characterization of bacteriophage nucleic acids obtained from Clostridium botulinum types C and D. Appl Environ Microbiol. 1988;54:69–73. doi: 10.1128/aem.54.1.69-73.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes RK, Barksdale L. Genetic analysis of tox+ and tox-bacteriophages of Corynebacterium diphtheriae. J Virol. 1969;3:586–98. doi: 10.1128/jvi.3.6.586-598.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newland JW, Strockbine NA, Miller SF, O’Brien AD, Holmes RK. Cloning of Shiga-like toxin structural genes from a toxin converting phage of Escherichia coli. Science. 1985;230:179–81. doi: 10.1126/science.2994228. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi T, Baba T, Matsumoto H, Terawaki Y. Phage-conversion of cytotoxin production in Pseudomonas aeruginosa. Mol Microbiol. 1990;4:1703–9. doi: 10.1111/j.1365-2958.1990.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 6.McDonough MA, Butterton JR. Spontaneous tandem amplification and deletion of the shiga toxin operon in Shigella dysenteriae1. Mol Microbiol. 1999;34:1058–69. doi: 10.1046/j.1365-2958.1999.01669.x. [DOI] [PubMed] [Google Scholar]
- 7.Betley MJ, Mekalanos JJ. Staphylococcal enterotoxin A is encoded by phage. Science. 1985;229:185–7. doi: 10.1126/science.3160112. [DOI] [PubMed] [Google Scholar]
- 8.Weeks CR, Ferretti JJ. The gene for type A streptococcal exotoxin (erythrogenic toxin) is located in bacteriophage T12. Infect Immun. 1984;46:531–6. doi: 10.1128/iai.46.2.531-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidolin A, Manning PA. Genetics of Vibrio cholerae and its bacteriophages. Microbiol Rev. 1987;51:285–98. doi: 10.1128/mr.51.2.285-298.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faruque SM, Islam MJ, Ahmad QS, Faruque AS, Sack DA, Nair GB, et al. Self-limiting nature of seasonal cholera epidemics: role of host-mediated amplification of phage. Proc Natl Acad Sci USA. 2005;102:6119–24. doi: 10.1073/pnas.0502069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waldor MK, Mekalanos JJ. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–4. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 12.De SN. Enterotoxicity of bacteria-free culture-filtrate of Vibrio cholerae. Nature. 1959;183:1533–4. doi: 10.1038/1831533a0. [DOI] [PubMed] [Google Scholar]
- 13.Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ, et al. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc Natl Acad Sci USA. 2009;106:15442–7. doi: 10.1073/pnas.0907787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faruque SM, Tam VC, Chowdhury N, Diraphat P, Dziejman M, Heidelberg JF, et al. Genomic analysis of the Mozambique strain of Vibrio cholerae O1 reveals the origin of El Tor strains carrying classical CTX prophage. Proc Natl Acad Sci USA. 2007;104:5151–6. doi: 10.1073/pnas.0700365104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimsey HH, Nair GB, Ghosh A, Waldor MK. Diverse CTXphis and evolution of new pathogenic Vibrio cholerae. Lancet. 1998;352:457–8. doi: 10.1016/S0140-6736(05)79193-5. [DOI] [PubMed] [Google Scholar]
- 16.Val ME, El Kennedy SP, Karoui M, Bonné L, Chevalier F, Barre FX. FtsK-dependent dimer resolution on multiple chromosomes in the pathogen Vibrio cholerae. PLoS Genet. 2008;4:e1000201. doi: 10.1371/journal.pgen.1000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azaro MA, Landy A. λ Integrase and the λ Int Family. In: Craig NL, Craigie R, Gellert M, Lambowitz AM, editors. Mobile DNA II. Washington, D.C: American Society of Microbiology; 2002. pp. 118–48. [Google Scholar]
- 18.Huber KE, Waldor MK. Filamentous phage integration requires the host recombinases XerC and XerD. Nature. 2002;417:656–9. doi: 10.1038/nature00782. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez MD, Lichtensteiger CA, Caughlan R, Vimr ER. Conserved filamentous prophage in Escherichia coli O18:K1:H7 and Yersinia pestis biovar orientalis. J Bacteriol. 2002;184:6050–5. doi: 10.1128/JB.184.21.6050-6055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derbise A, Chenal-Francisque V, Pouillot F, Fayolle C, Prévost MC, Médigue C, et al. A horizontally acquired filamentous phage contributes to the pathogenicity of the plague bacillus. Mol Microbiol. 2007;63:1145–57. doi: 10.1111/j.1365-2958.2006.05570.x. [DOI] [PubMed] [Google Scholar]
- 21.Rubin EJ, Lin W, Mekalanos JJ, Waldor MK. Replication and integration of a Vibrio cholerae cryptic plasmid linked to the CTX prophage. Mol Microbiol. 1998;28:1247–54. doi: 10.1046/j.1365-2958.1998.00889.x. [DOI] [PubMed] [Google Scholar]
- 22.Campos J, Martinez E, Izquierdo Y, Fando R. VEJφ, a novel filamentous phage of Vibrio cholerae able to transduce the cholera toxin genes. Microbiology. 2010;156:108–15. doi: 10.1099/mic.0.032235-0. [DOI] [PubMed] [Google Scholar]
- 23.Campos J, Martínez E, Suzarte E, Rodríguez BL, Marrero K, Silva Y, et al. VGJφ, a novel filamentous phage of Vibrio cholerae0, integrates into the same chromosomal site as CTXφ. J Bacteriol. 2003;185:5685–96. doi: 10.1128/JB.185.19.5685-5696.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kar S, Ghosh RK, Ghosh AN, Ghosh A. Integration of the DNA of a novel filamentous bacteriophage VSK from Vibrio cholerae 0139 into the host chromosomal DNA. FEMS Microbiol Lett. 1996;145:17–22. doi: 10.1111/j.1574-6968.1996.tb08550.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakasone N, Honma Y, Toma C, Yamashiro T, Iwanaga M. Filamentous phage fs1 of Vibrio cholerae O139. Microbiol Immunol. 1998;42:237–9. doi: 10.1111/j.1348-0421.1998.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen DT, Nguyen BM, Tran HH, Ngo TC, Le TH, Nguyen HT, et al. Filamentous vibriophage fs2 encoding the rstC gene integrates into the same chromosomal region as the CTX phage [corrected] FEMS Microbiol Lett. 2008;284:225–30. doi: 10.1111/j.1574-6968.2008.01200.x. [DOI] [PubMed] [Google Scholar]
- 27.Faruque AS, Alam K, Malek MA, Khan MG, Ahmed S, Saha D, et al. Emergence of multidrug-resistant strain of Vibrio cholerae O1 in Bangladesh and reversal of their susceptibility to tetracycline after two years. J Health Popul Nutr. 2007;25:241–3. [PMC free article] [PubMed] [Google Scholar]
- 28.Campos J, Martínez E, Marrero K, Silva Y, Rodríguez BL, Suzarte E, et al. Novel type of specialized transduction for CTX phi or its satellite phage RS1 mediated by filamentous phage VGJ phi in Vibrio cholerae. J Bacteriol. 2003;185:7231–40. doi: 10.1128/JB.185.24.7231-7240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis BM, Waldor MK. Filamentous phages linked to virulence of Vibrio cholerae. Curr Opin Microbiol. 2003;6:35–42. doi: 10.1016/s1369-5274(02)00005-x. [DOI] [PubMed] [Google Scholar]
- 30.Waldor MK, Rubin EJ, Pearson GD, Kimsey H, Mekalanos JJ. Regulation, replication, and integration functions of the Vibrio cholerae CTXphi are encoded by region RS2. Mol Microbiol. 1997;24:917–26. doi: 10.1046/j.1365-2958.1997.3911758.x. [DOI] [PubMed] [Google Scholar]
- 31.Davis BM, Kimsey HH, Chang W, Waldor MK. The Vibrio cholerae O139 Calcutta bacteriophage CTXphi is infectious and encodes a novel repressor. J Bacteriol. 1999;181:6779–87. doi: 10.1128/jb.181.21.6779-6787.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maiti D, Das B, Saha A, Nandy RK, Nair GB, Bhadra RK. Genetic organization of pre-CTX and CTX prophages in the genome of an environmental Vibrio cholerae non-O1, non-O139 strain. Microbiology. 2006;152:3633–41. doi: 10.1099/mic.0.2006/000117-0. [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay AK, Chakraborty S, Takeda Y, Nair GB, Berg DE. Characterization of VPI pathogenicity island and CTXphi prophage in environmental strains of Vibrio cholerae. J Bacteriol. 2001;183:4737–46. doi: 10.1128/JB.183.16.4737-4746.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barre F-X, Sherratt DJS. Xer Site-Specific Recombination: Promoting Chromosome Segregation. In: Craig NL, Craigie R, Gellert M, Lambowitz A, editors. Mobile DNA II. vol.1. Washington, D.C: American Society of Microbiology; 2002. pp. 149–61. [Google Scholar]
- 35.Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–83. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das B, Bischerour J, Val ME, Barre FX. Molecular keys of the tropism of integration of the cholera toxin phage. Proc Natl Acad Sci USA. 2010;107:4377–82. doi: 10.1073/pnas.0910212107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLeod SM, Waldor MK. Characterization of XerC- and XerD-dependent CTX phage integration in Vibrio cholerae. Mol Microbiol. 2004;54:935–47. doi: 10.1111/j.1365-2958.2004.04309.x. [DOI] [PubMed] [Google Scholar]
- 38.Val ME, Bouvier M, Campos J, Sherratt D, Cornet F, Mazel D, et al. The single-stranded genome of phage CTX is the form used for integration into the genome of Vibrio cholerae. Mol Cell. 2005;19:559–66. doi: 10.1016/j.molcel.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Bouvier M, Demarre G, Mazel D. Integron cassette insertion: a recombination process involving a folded single strand substrate. Embo J. 0 J;24:4356–67. doi: 10.1038/sj.emboj.7600898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das B, Bischerour J, Barre FX. VGJφ-integration and excision mechanisms contribute to the genetic diversity of Vibrio cholerae epidemic strains. PNAS. 2011 doi: 10.1073/pnas.1017061108. doi: 10.1073/pnas.1017061108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falero A, Caballero A, Ferrán B, Izquierdo Y, Fando R, Campos J. DNA binding proteins of the filamentous phages CTXphi and VGJphi of Vibrio cholerae. J Bacteriol. 2009;191:5873–6. doi: 10.1128/JB.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


