Abstract
There has been a prevailing perception that Th1 and Th2 immune responses induce antagonistic immune effector mechanisms during an infection. We investigated the role of the Th1 cytokine gamma interferon (IFN-γ) and the Th2 cytokine interleukin-5 (IL-5) in murine filariasis infections with the rodent filarial nematode Litomosoides sigmodontis with regard to immune responses to the parasite. Earlier data showed an important role for IL-5 and IFN-γ in effective immune responses to filarial infection. Therefore, in this study it was asked whether IL-5 and IFN-γ act synergistically or antagonistically. Indeed, IL-5 as well as IFN-γ knockout (KO) mice show a higher worm load than the wild-type controls. IFN-γ/IL-5 double-KO mice had a significantly higher worm load than any of the single-KO mice, suggesting a synergism between IFN-γ and IL-5 in controlling worm infection. Neutrophils are known to play an important role for the containment and encapsulation process of the worms. In infected IFN-γ KO, IL-5 KO, and IFN-γ/IL-5 double-KO mice, neutrophils were significantly reduced in chemotactic activity levels compared to controls. In addition, the level of phagocytosis activity of neutrophils from IFN-γ/IL-5 double-KO mice was further decreased in comparison to that of the single-KO mice. Levels of tumor necrosis factor alpha, which is an important factor for neutrophil activation, were found to be reduced in macrophages from KO mice. In conclusion, these results argue for immune effector mechanisms in murine filarial infection that are dependent on both IFN-γ and IL-5. Synergistic effects of the two cytokines may be mediated, at least in part, by neutrophils for the control of adult worms.
There has been a common view that Th1 and Th2 immune responses act antagonistically toward each other. For example, a strong Th1 immune response is necessary for an effective immune reaction to Mycobacterium leprae. A Th2 response led to high level of susceptibility during this infection, and animals as well as humans were unable to control the infection (23, 29, 44). In C57BL/6 mice Leishmania major induces an effective Th1 immune response, while in BALB/c mice a nonprotective Th2 immune response is induced (1, 14, 38). Moreover, in this model it was shown that gamma interferon (IFN-γ) as a typical Th1 cytokine and interleukin-5 (IL-5) as a typical Th2 cytokine have antagonistic effects (38). In contrast, a strong Th2 immune response was reported to be important for survival of an infection with Schistosoma mansoni. Survival was attributed to the ability of Th2 cells to secrete cytokines that were anti-inflammatory. A failure to produce anti-inflammatory cytokines (because of IL-4 deficiency) led to elevated production of inflammatory cytokines such as IFN-γ and the death of the animals (8, 9, 15).
Human filariasis puts at risk more than a 1 billion people and affects over 180 million people. Analysis of the role of the Th1 cytokine IFN-γ and the Th2 cytokine IL-4 in a non-fully-permissive filarial model using Brugia malayi demonstrated that lack of either IFN-γ or IL-4 prolongs the time required to achieve sterile immunity (5). In this filarial model no clear antagonism between Th1 and Th2 immune response was detected. Both Th1 and Th2 immune mechanisms play important roles for an effective immune response in this non-fully-permissive filarial model.
In our study we used Litomosoides sigmodontis as a model for human filarial disease. The use of the rodent filarial species L. sigmodontis has the advantage of allowing the observation of the complete life cycle of the parasite, including the development of infective stage 3 larvae (L3) into adult worms and the adult worm persistence during patency (30). The full permissivity of this filarial model allows the examination of immune mechanisms against all stages of the parasite during the course of infection. The important role of the Th2 cytokine IL-4 for resistance or susceptibility has been shown in C57BL/6 mice, which usually rejected an L. sigmodontis infection. Only C57BL/6 IL-4 knockout (KO) mice acquire a patent infection (26).
Previous analyses have shown that Th1 and Th2 immune responses are necessary for an effective immune response to filarial infections. BALB/c IFN-γ KO mice had a higher worm survival rate due to impaired worm encapsulation of the adult filariae (35). Neutrophils, which are known to be important for the encapsulation process (3), were reduced in numbers in IFN-γ KO mice. Moreover, neutrophils of IFN-γ KO mice showed reduced chemotaxis and phagocytosis levels compared to those of wild-type mice (35). A depletion of IL-5 or the use of IL-5 KO (5-KO) mice leads to a reduced level of accumulation of neutrophils in the thoracic cavity and a higher worm load in the late stage of the infection (3, 46). Furthermore, these KO mice showed a higher level of microfilaremia than wild-type mice. Both cytokines IL-5 and IFN-γ were involved in the production of tumor necrosis factor alpha (TNF-α) (35, 45), which is important for the recruitment of neutrophils (3).
In this study we analyzed L. sigmodontis infections in BALB/c, 5-KO, IFN-γ KO (γ-KO), and IFN-γ/IL-5 double-KO (γ/5-KO) mice. While IFN-γ deficiency results in a higher worm load, IL-5 deficiency leads to higher survival rates at all stages of the filariae in the host. The main focus of this study was to examine the interaction of IL-5 and IFN-γ deficiency in γ/5-KO mice and to answer the question of whether the cytokines IFN-γ and IL-5 act in a synergistic or antagonistic manner during the course of an infection with L. sigmodontis.
MATERIALS AND METHODS
Animal maintenance and infection of mice with L. sigmodontis.
BALB/c γ-KO-backcrossed mice were obtained from The Jackson Laboratory (Bar Harbor, Maine). BALB/c 5-KO mice (24) were supplied by K. I. Matthaei (Australian National University, Canberra, Australia). BALB/c γ/5-KO mice were crossed from γ-KO and 5-KO mice, and the success of this breeding was analyzed by PCR using primers for IL-5/5-KO and IFN-γ/IFN-γ KO mice as follows: for IFN-γ wild-type mice, primers 5′AGA AGT AAG TGG AAG GGC CCA GAA G 3′ and 5′AGG GAA ACT GGG AGA GGA GAA ATA T 3′; for IFN-γ KO mice, primers 5′TCA GCG CAG GGG CGC CCG GTT CTT T 3′ and 5′ATC GAC AAG ACC GGC TTC CAT CCG A 3′; and for IL-5 wild-type/KO mice, primers 5′CTT CCA TTG CCC ACT CTG TAC T 3′ and 5′CTG GCC TTC ACC TCC TGA TCC TC 3′. Detailed protocols have been described previously for γ-KO mice (12) and for 5-KO mice (24). At least five mice for each group were used per experiment.
The KO strains were housed under specific-pathogen-free conditions in microisolator cages. KO offspring as well as BALB/c wild-type mice (originally from Charles River, Sulzfeld, Germany) and cotton rats were bred at the animal facilities of the Bernhard Nocht Institute. Natural infections of mice with L. sigmodontis were performed as described previously (2, 4). L. sigmodontis lives naturally in cotton rats (Sigmodon hispidus), which develop very high levels of microfilariae in blood (up to 10,000 microfilariae/μl of blood). During a blood meal on an infected rat, the arthropod intermediate host (Ornithonyssus bacoti mite) ingests the microfilariae, which molt twice and develop to L3 within 10 days. In a following blood meal the mites transmit the L3 onto cotton rats. Similarly, in the case of murine infection, the L3-containing mites are allowed to take blood from mice and thereby transmit the L3. In the rodent host the L3 migrate to the thoracic cavity and reach sexual maturity within 25 to 33 days. Viviparous female worms start producing microfilariae, which are detected in the blood after day 50 postinfection (p.i.).
Parasite recovery.
The number of adult worms was counted at day 80 p.i. by using Dumont forceps. To do this, the thoracic cavity, the representative site for assessment of worm numbers (4, 28), was flushed with 10 ml of phosphate-buffered saline (PBS)-1% fetal calf serum (FCS) and worms were allowed to sediment. The sediment was also used to determine the number of inflammatory nodules. The presence of microfilaremia was determined in EDTA-treated peripheral blood after staining with Hinkelmann's solution (0.5% [wt/vol] eosin Y, 0.5% [wt/vol] phenol, 0.185 [vol/vol] formaldehyde in distilled water), as described previously (4).
Determination of proportions of inflammatory cells in thoracic cavity fluid.
Proportions of inflammatory, pleural exudate cells were determined using cytospin preparations; 200 μl of thoracic cavity cells (2 × 105/ml in PBS-1% FCS) were centrifuged using a Shandon cytocentrifuge with a glass slide and absorptive filter paper. Cytospins were then stained using Wright-Giemsa stain (Sigma, Munich, Germany). Neutrophils and eosinophils were differentially enumerated, and the total number of migrated cells in the thoracic cavity was calculated.
Flow cytometric analysis.
Proportions of B cells in the thoracic cavity were determined using flow cytometric analysis. A total of 100 μl of thoracic cavity cells (2 × 106/ml in RPMI 1640-1% FCS) was incubated for 30 min on ice with a combination of a 1:100 dilution of phycoerythrin (PE)-conjugated anti-B220 antibody (RA3-6B2) and fluorescein isothiocyanate (FITC)-conjugated anti-Mac-1 antibody (M1/170.15). To determine CD4 and CD8 T-cell levels, 100 μl of thoracic cavity cells was incubated with a 1:100 dilution of a FITC-conjugated rat monoclonal antibody specific for CD4 (YTS 191.1) and an R-PE-conjugated anti-CD8 antibody (YTS 169.4) (Medac, Hamburg, Germany). A double staining was performed with a 1:100 dilution of an anti-DX5 R-PE-conjugated antibody (DX5) and an anti-CD3e FITC-conjugated antibody (145-2C11) (both from Becton Dickinson/Pharmingen, Heidelberg, Germany) to detect DX5+/CD3− NK cells and DX5+/CD3+ T cells.
The cells were washed with PBS-1% bovine serum albumin and fixed in 1% (vol/vol) formaldehyde. The samples were measured by a FACScan (Becton Dickinson) and analyzed using Cell Quest software (Becton Dickinson). Viable lymphocytes were gated with the forward and side scatter, and 5,000 gated events were collected.
Cell culture.
The growth of thoracic cavity macrophage cultures was carried out after adhesion on 96-well culture plates (Greiner, Frickenkausen, Germany) in RPMI 1640-5% FCS at 37°C and 5% CO2 for 2 h. First, a fluorescence-activated cell sorter (FACS) analysis was performed to determine the relative proportion of macrophages in the thoracic cavity cells. According to these data, the cell input was calculated such that 40,000 macrophages/well (as determined by FACS) were allowed to adhere. The nonadherent cells were removed by washing three times with PBS. Purity was >95% (data not shown). Macrophages were grown for 48 h in the presence of RPMI 1640-5% FCS alone or 100 μg of adult worm antigen/ml or 20 ng of IFN-γ (Becton Dickinson)/ml and 20 ng of lipopolysaccharide (LPS) (Escherichia coli O55; Sigma)/ml. Supernatants were then removed for cytokine determination.
Cytokine assays.
Concentrations of cytokines IL-5, IL-10, and TNF-α in cell culture supernatant were determined by specific two-site enzyme-linked immunosorbent assays using standard protocols. The (biotinylated) antibody pairs for capture and detection were purchased from Becton Dickinson in the combination recommended. Recombinant cytokines (Becton Dickinson) were used as standards. All enzyme-linked immunosorbent assays were developed after incubation with streptavidin-peroxidase complex (1:5,000 dilution; Boehringer, Mannheim, Germany) and with 3,5,3′,5′-tetramethylbenzidine (Roth, Karlsruhe, Germany) (dissolved in dimethyl sulfoxide at 6 mg/ml) as the substrate. Sensitivity was 10 pg/ml.
Neutrophil chemotaxis assay.
Casein-elicited peritoneal cells (27) from uninfected mice and thoracic cavity cells from L. sigmodontis-infected mice at day 80 p.i. were collected and resuspended in PBS-0.5 mM MgCl2-0.5 mM CaCl2-0.1% bovine serum albumin. The relative proportion of neutrophils was determined by a cytospin method. Neutrophil chemotactic activity was assayed as described previously (33, 35). Briefly, in the lower compartment of blind-well Boyden chambers (Bio-Rad, Munich, Germany), either medium or platelet-activating factor as a chemotaxin was introduced in a 100-μl solution at a concentration of 10−6 M; this dilution had been found in earlier studies to give optimal results (35). The chambers were covered with a polyvinylpyrrolidone-free polycarbonate filter (Nuclepore, Tübingen, Germany) (pore size, 3 μm). Cells (105) were layered on this filter in a 100-μl solution in the upper part of the chamber, and neutrophils were allowed to migrate for 1 h at 37°C and 5% CO2. Thereafter, the remaining cells were removed. The migrated neutrophils present in the lower chamber were lysed with Triton X-100 (0.1% vol/vol) to quantify them by assessment of neutrophil-specific β-glucuronidase levels. To do this, the lysates were incubated for 18 h with 100 μl of p-nitrophenyl-β-d-glucuronide (Sigma) (0.01 M in 0.1 M sodium acetate buffer [pH 4.0]) as the substrate. The enzymatic reaction was stopped by the addition of 100 μl of 0.4 M glycine buffer (pH 10), and the yellow color was measured photometrically at 405 nm. For the calculation of the number of migrated neutrophils, a calibration curve was generated with 103 to 105 cells lysed by 0.1% (vol/vol) Triton X-100 and incubated with p-nitrophenyl-β-d-glucuronide. All chemotaxis experiments were performed in duplicate. In parallel with the enzyme testing before the cell lysis, the migrated cells in the lower compartment of the Boyden chamber were checked for neutrophil purity by Giemsa-Wright staining.
Phagocytosis assay.
The phagocytosis capacity of neutrophils was measured using an assay described elsewhere (27), with modifications to accommodate casein-elicited neutrophils and L. sigmodontis infection (35). Zymosan (Sigma) was used as a marker for phagocytosis capacity. At 11 h after injection of 1 mg of casein in PBS, 0.5, 1.0, or 1.5 mg of zymosan in PBS was injected in the peritoneal cavity. After 1 h of incubation in the peritoneal cavity of the mouse, the cells were washed out of the peritoneal cavity and a Shandon centrifuge with a glass slide covered with absorptive filter paper was used at 800 rpm for 5 min for the centrifugation of 2 × 104 cells. The filter papers allowed the adsorption of the solution and, therefore, the adherence of the flattened cells to the surface of the glass slide. Afterwards, cells were dried overnight at room temperature. The cells were stained with Giemsa, and the neutrophils with ingested zymosan particles were counted.
Reconstitution of KO mice with IFN-γ and/or IL-5.
Neutrophils were induced by the injection of 1 mg of casein in PBS into the peritoneal cavity of KO mice. At 11 h later, 0.5 mg of zymosan and 0.25 μg of IL-5 in PBS were injected into the peritoneal cavity of 5-KO mice. γ-KO mice received 0.5 mg of zymosan and 0.25 μg of IFN-γ in PBS, while 0.25 μg of IL-5 and/or 0.25 μg of IFN-γ was injected into the peritoneal cavity of γ/5-KO mice. After 1 h of incubation, cells were harvested from the peritoneal cavity and stained with Giemsa as described for the phagocytosis assay.
Statistical analyses.
The parametric Student's t test was used to calculate filarial recovery rates. The nonparametric Mann-Whitney U test was used to compare numbers of microfilariae, inflammatory cells, and cytokines. P values of <0.05 were considered to represent significant differences.
RESULTS
IL-5 and IFN-γ deficiencies of double-KO mice increase susceptibility to L. sigmodontis compared to the susceptibility of single-KO mice.
First, we confirmed earlier results indicating that IFN-γ and IL-5 single-KO mice have a higher level of susceptibility than wild-type mice (Table 1) (35, 45), possibly due to the involvement of both IFN-γ and IL-5 in the immune defense against the worms (in part, by attracting and activating neutrophils in the thoracic cavity) (35, 45).
TABLE 1.
Number of worms and nodules at 80 days p.i.
| Mouse strain | No. of worms | No. of nodules |
|---|---|---|
| BALB/c | 51 ± 10 | 2.2 ± 0.83 |
| γ-KO | 83 ± 18b | 1.4 ± 0.89b |
| 5-KO | 159 ± 68b,c | 0b,c |
| γ/5-KO | 354 ± 25b,c,d | 0b,c |
Data for mouse strains other than BALB/c represent the results of Mann-Whitney U tests.
Significantly different in comparison to results for BALB/c mice (P < 0.05).
Significantly different in comparison to results for γ-KO mice (P < 0.05).
Significantly different in comparison to results for 5-KO mice (P < 0.05).
The data are consistent with a decreased encapsulation process of adult worms between days 50 and 80 p.i., leading to higher worm loads in γ-ΚΟ and 5-KO mice (Table 1). However, since the possibility of antagonistic mechanisms of parasite containment between IL-5 and IFN-γ cannot be excluded, it was of interest to investigate whether the combination of IL-5 and IFN-γ deficiencies would lead to the reduction or enhancement of parasite loads. Table 1 shows clearly that the latter was the case. These data suggest that IL-5 and IFN-γ together have a synergistic effect.
The higher worm load in the double-KO mice does not lead to a higher level of microfilaremia.
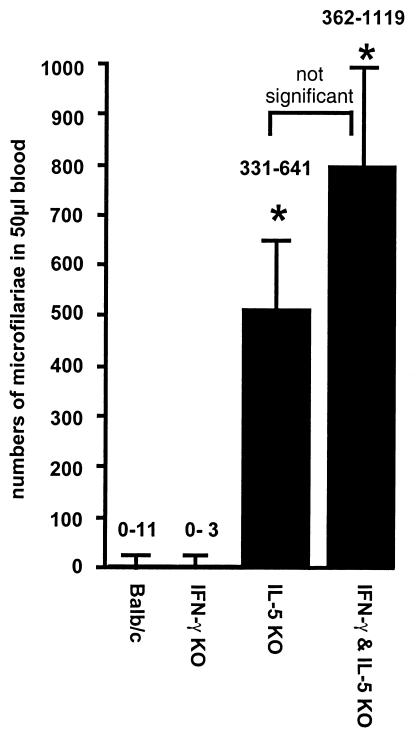
The 5-KO mutation had a very strong effect on the survival of microfilariae, as analyzed at day 80 p.i. (Fig. 1). The IFN-γ deficiency did not result in a level of microfilaria burden at day 80 p.i. higher than that seen with the wild type. Consistently, the higher worm burden in the γ/5 KO mice did not lead to an increase of microfilaria burden compared to that seen with 5-KO mice (Fig. 1).
FIG. 1.
Numbers of microfilariae in infected 5-KO and γ/5-KO mice were significantly increased in comparison to those seen with the γ-KO and BALB/c control mice at day 70 p.i. No significant differences were detectable between 5-KO and γ/5-KO mice. The graph shows the medians, and the numbers represent the range of microfilaria counts in each group. The data are representative of one of two experiments (*, P < 0.05 [Mann-Whitney U test with Bonferroni correction]), with five animals per group.
Lack of IL-5 and/or IFN-γ results in reduced neutrophil accumulation in the thoracic cavity of infected mice.
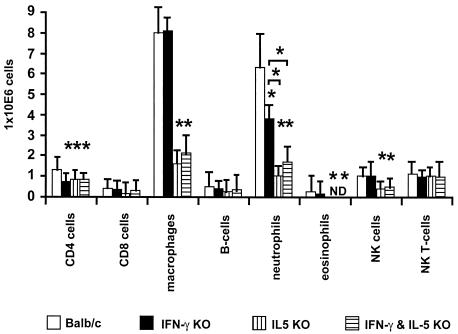
Previous data concerning the formation of inflammatory nodules and the containment of adult worms showed that the presence of neutrophils was essential for an effective immune response (3). Therefore, it was important to investigate whether, in the absence of IFN-γ and/or IL-5 in KO mice, there were reduced numbers of neutrophils in the thoracic cavity. The analyses of different populations from thoracic cavity immune cells were done by FACS and cytospin techniques. The results were equivalent, with both methods showing a decreased number of neutrophils in all KO strains (Fig. 2). Furthermore, a deficiency of IL-5 or IL-5 plus IFN-γ resulted in significantly lower numbers of neutrophils in the thoracic cavity in comparison to the results seen with the γ-KO mice (Fig. 2).
FIG. 2.
Numbers of accumulated cells in the thoracic cavity at day 80 p.i. All KO mice showed a significant reduction in levels of neutrophils and CD4+ T cells. Only the 5-KO and γ/5-KO mice showed a significant reduction in the numbers of macrophages, eosinophils, and NK cells. Each group contained five animals. The data are representative of one of two experiments (*, P < 0.05 [Mann-Whitney U test]). ND, not detectable. Bars show means ± standard deviations. B cells, macrophages, and CD4+ and CD8+ T cells were enumerated by FACS. Eosinophils and neutrophils were enumerated by a cytospin technique.
IL-5 and/or IFN-γ deficiency leads to a reduction in levels of the neutrophil-activating cytokine TNF-α.
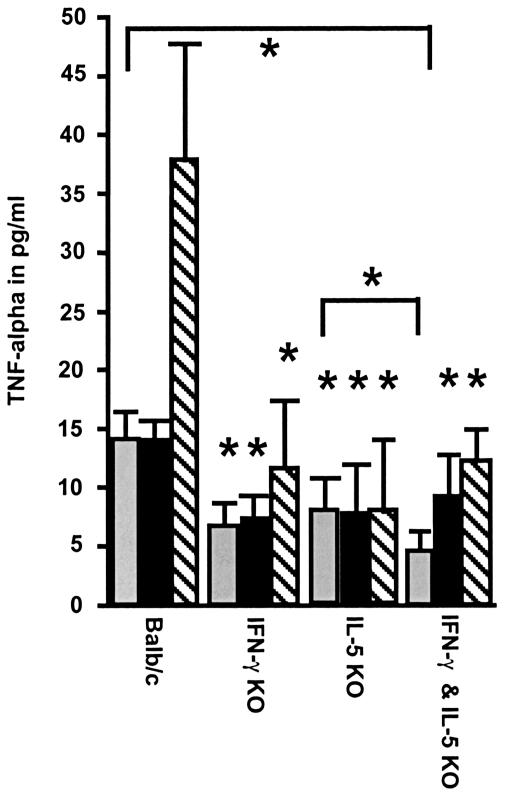
Compared to macrophages from wild-type mice, purified thoracic cavity macrophages (which were demonstrated to be the major source of TNF-α in the thoracic cavity) (3) taken from infected KO mice after stimulation by L. sigmodontis antigen or LPS produced less TNF-α (Fig. 3). However, after stimulation with medium the γ/5 double-KO mice showed an even lower concentration of TNF-α in the macrophage culture than the single 5-KO mice (Fig. 3).
FIG. 3.
TNF-α production by thoracic cavity macrophages after stimulation with medium (grey bars), L. sigmodontis antigen (black bars), or LPS (hatched bars). Thoracic cavity macrophages were taken after 80 days p.i. The data show that the addition of S. sigmodontis antigen or LPS to macrophage cultures from single-KO and γ/5-KO mice does not restore the defective production of TNF-α. Furthermore, γ/5-KO mice had the lowest level of production of TNF-α in the macrophage culture of the medium control cell sample. Each group contained five animals. The data are representative of one of two experiments (*, P < 0.05 [Mann-Whitney U test with Bonferroni correction]). Bars show means ± standard deviations. *, significant difference in comparison to the results for the BALB/c group.
We also examined IL-10 concentrations but found no significant differences between the KO and wild-type groups (data not shown).
Reduced chemotaxis from neutrophils of IL-5, IFN-γ, and IL-5 IFN-γ double-KO mice.
The activation status of neutrophils was assessed by determining neutrophil chemotaxis. Casein-induced neutrophils from the uninfected KO mice used for chemotaxis showed migration to the chemotactic stimulus platelet-activating factor that was significantly reduced compared to that seen with neutrophils from BALB/c mice (Table 2). Moreover, neutrophils from the thoracic cavity cell suspension of infected KO mice also exhibited reduced migration levels in comparison to the BALB/c mice. The migration of neutrophils from γ-KO and γ/5-KO mice demonstrated the lowest level of chemotactic activity in comparison to that of neutrophils from 5-KO mice (Table 2). In summary, a deficiency of IFN-γ leads to migration activity levels of neutrophils from infected mice lower than those seen with a deficiency of IL-5.
TABLE 2.
Chemotaxis analysis of neutrophils
| Experiment and strain | Chemotaxis
resultsa
|
|
|---|---|---|
| Proportion (%) of neutrophils | No. (%) of migrating neutrophils | |
| Casein induction | ||
| BALB/c | 54 | 1.4 × 104 (26) |
| γ-KO | 56 | 0.8 × 104 (14)b |
| 5-KO | 52 | 0.7 × 104 (13)b |
| γ/5-KO | 51 | 0.8 × 104 (14)b |
| L. sigmodontis infection | ||
| BALB/c | 36.5 | 2.9 × 104 (82) |
| γ-KO | 21.5 | 0.3 × 104 (13)b,c |
| 5-KO | 17 | 0.7 × 104 (39)b |
| γ/5-KO | 16.5 | 0.3 × 104 (18)b,c |
The number of cells used as input for each experiment and strain was 105. Cells were taken from the peritoneal cavity of uninfected mice subjected to casein induction or from the thoracic cavity of mice infected with L. sigmodontis.
Significantly different from results for BALB/c control mice (P < 0.05).
Significantly different from results for 5-KO mice (P < 0.05).
Impaired phagocytosis activity from neutrophils of IL-5, IFN-γ, and IL-5 IFN-γ double-KO mice.
An important marker for the activation status of neutrophils was to examine the phagocytosis activity of neutrophils, which were generated by casein injection into the peritoneal cavity. Zymosan was used as a marker for the phagocytosis activity. The neutrophils from KO mice showed a significantly reduced capacity to ingest zymosan particles (Table 3) at concentrations of 0.5 and 1.5 mg of zymosan. Neutrophils from γ-KO and 5 KO mice had a lower level of phagocytosis activity than those of wild-type mice at concentrations of 0.5 mg of zymosan only, while γ/5 KO mice had the lowest level of phagocytosis activity (Table 3). A dose of 1.0 mg of zymosan resulted in an increase in phagocytosis activity from single KO mice to levels comparable to those of the wild-type, while γ/5 KO mice still showed a significantly lower level of activity compared to those of all other groups. At a zymosan concentration of 1.5 mg, no KO mice strain showed phagocytosis activity significantly different from that seen with any other KO mice strain but the wild-type mice showed a level of activity significantly higher than that of any of the KO mice groups. These experiments demonstrated that the dose-dependent effect of the phagocytosis activity is dependent on the ingested zymosan concentration. The analyses of the uptake of zymosan particles at these three different zymosan concentrations were done after Giemsa staining by counting the number of ingested zymosan particles in 100 neutrophils under light microscopy (magnification, ×1,000). No significant differences between the four different mouse strains at the three different zymosan concentrations were found (data not shown).
TABLE 3.
Phagocytosis analysis of neutrophils after casein induction
| Strain | Phagocytosis results
|
|||
|---|---|---|---|---|
| Proportion (%) of neutrophils | % of neutrophils with
ingested particles at injected zymosan concn (mg) of:
|
|||
| 0.5 | 1.0 | 1.5 | ||
| BALB/c | 54 | 33 ± 2.2 | 47 ± 5.7 | 88 ± 4.3 |
| γ-KO | 56 | 21 ± 2.6a | 41 ± 5.6 | 51 ± 6.2a |
| 5-KO | 52 | 20 ± 3.3a | 49 ± 7.1 | 57 ± 9.5a |
| γ/5-KO | 51 | 0.8 ± 1.5a,b | 28 ± 4.6a,b | 53 ± 4.5a |
Significantly different from results for BALB/c control mice (P < 0.05).
Significantly different from results for γ-KO and 5-KO mice (P < 0.05).
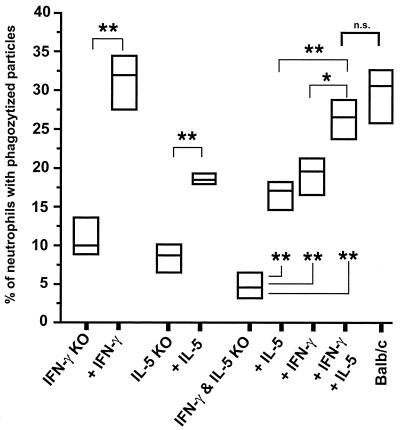
Next, an attempt was made to reconstitute the phagocytosis activity of neutrophils from KO mice by the injection of 0.25 μg of recombinant IFN-γ and/or IL-5. In vivo injection showed a significant increase in the phagocytosis activity level (Fig. 4). Importantly, while intraperitoneal injection of 0.25 μg of either IFN-γ or IL-5 into γ/5 KO mice led to a partial increase in the level of phagocytosis activity, the injection of both cytokines into γ/5 KO mice was required for a level of phagocytosis activity to occur that was comparable to that seen with the wild-type mice (Fig. 4).
FIG. 4.
In vivo reconstitution of phagocytosis activity by injection of 0.25 μg of IL-5 and/or 0.25 μg of IFN-γ in the peritoneal cavity of the KO mice. Zymosan was used as a marker for the phagocytosis activity of the neutrophils. A reconstitution of the 5-KO mice with 0.25 μg of IL-5 and a reconstitution of the γ-KO mice with 0.25 μg of IFN-γ led to a significant increase in the level of phagocytosis activity of the neutrophils. Moreover, a reconstitution of the γ/5-KO mice with 0.25 μg of IL-5 or 0.25 μg of IFN-γ led to a partial increase in the level of phagocytosis activity of the neutrophils. Only the injection of 0.25 μg of IL-5 and 0.25 μg of IFN-γ restored the complete phagocytosis activity of the neutrophils from γ/5-KO mice. The results from one of two consistent experiments is shown. Five mice were used for each group (*, P < 0.05; **, P < 0.03 [Mann-Whitney U test]). n.s., not significant.
DISCUSSION
That Th2 cytokines are important factors for an antifilarial immune response that leads to decreases in parasite worm burden is accepted (20, 25). Previous results showed that BALB/c 5-KO and γ-KO mice develop higher worm burdens than wild-type mice (35, 46). In this study we found novel data demonstrating that the Th1 cytokine IFN-γ and the Th2 cytokine IL-5 act synergistically during an infection with L. sigmodontis. In γ/5-KO mice, infection with L. sigmodontis resulted in a worm burden higher than that seen with the single KO mice (Table 1). Only 5-KO and γ/5-KO mice had higher levels of microfilaremia than wild-type and γ-KO mice, with no significant differences between 5-KO and γ/5-KO mice (Fig. 1). In summary, the synergistic effect of IL-5 and IFN-γ double deficiency is restricted to adult worms. With human lymphatic filariasis, moreover, it was shown that IL-5 and IFN-γ levels were reduced in patients with microfilaremia (36), suggesting that during the infection these two cytokines were actively down regulated.
In C57BL/6 mice, which are naturally resistant to L. sigmodontis, lack of IL-4 results in susceptibility (26). When a nonsusceptible model and the filarial parasite B. malayi were used, it was detected that lack of IFN-γ or IL-4 prolongs the survival of the parasite (5). During intestinal helminth infections with Nippostrongylus brasiliensis, in contrast, only a strong Th2 immune response is effective against worms, while treatment with IFN-γ leads to a significantly delayed intestinal expulsion of adult worms (43). Mice with other helminth infections (including, for example, S. mansoni infections) also acquire a strong Th2 immune response for survival. A deficiency of IL-4 results in increased concentrations of inflammatory mediators and the death of mice (8, 9, 15). A strong Th1 immune response is necessary to control infections with L. major (1, 14, 38), M. leprae (23, 29, 44), and Mycobacterium tuberculosis (10, 11, 16). All these studies are examples of investigations of polarized immune defense mechanisms. In contrast to the results of the reports cited above, in our study a lack of IL-5 as well as of IFN-γ led to a higher level of susceptibility (Table 1). Similar to the results of IL-5 deficiency (3), the absence of IFN-γ (35) also results in a decreased accumulation of neutrophils in the thoracic cavity during infection (Fig. 2). Neutrophils played an important role in defense against adult worms, because a depletion of granulocyte colony-stimulating factor, which results in reduced numbers of neutrophils in the thoracic cavity, leads to higher adult worm levels due to a lack of inflammatory nodule formation (3). The product of endobacteria (LPS) seems to be the major stimulus for the neutrophils to surround and form the innermost layer around live adult worms (33, 47). This encapsulation process leads to nodule formation, which inhibits the motility of the worms. Moreover, it was shown that neutrophils were able to attack worms in vitro (21) and were important cells for defense against human filarial infections (6).
IL-5 and IFN-γ had different influences on the migration of neutrophils in the thoracic cavity. A lack of IL-5 results in a drastic reduction of numbers of neutrophils in the thoracic cavity compared to the results seen with γ-KO and BALB/c mice. In contrast, γ-ΚΟ mice showed an level of accumulation of neutrophils in the thoracic cavity lower than that seen with wild-type mice but higher than that seen with 5-KO mice. Interestingly, γ/5-KO mice demonstrated numbers of neutrophils in the thoracic cavity equivalent to 5-KO mice (Fig. 2) and therefore show no synergistic effect concerning the numbers of neutrophils in the thoracic cavity. CD4 T-cell levels were reduced in all groups of KO mice, while levels of eosinophils, NK cells, and CD4 T cells were diminished only in 5-KO and γ/5-KO mice (Fig. 2).
IL-5 and also IL-4 are cytokines that are known to be typically Th2 associated. An important difference between the cytokines IL-5 and IL-4 in the defense against filarial infection is that IL-4-deficient mice did not have reduced IL-5 levels in the thoracic cavity (45). Moreover, neutrophils accumulate in IL-4-deficient mice to levels similar to the levels seen with wild-type mice, which might explain why adult worm killing is not impaired (45). IL-4 deficiencies in mice have no influence on nodule formation in the adult worms. These mice show only higher levels of prolonged microfilaremia. In contrast, 5-KO mice, which also showed higher levels of prolonged microfilaremia, showed impaired nodule formation and prolonged survival of adult worms (45). These data allow the conclusion that IL-5 and IL-4 are independently regulated in filarial infections (22, 45).
Although our data suggested that neutrophils are a major cell population that is influenced by a deficiency of IL-5 and IFN-γ, other mechanisms which may influence worm load that are dependent on these cytokines should also be mentioned. Cystatins of filarial nematodes induce NO production in IFN-γ-activated macrophages (18). Adult worms and microfilariae were shown to be substantially affected by NO released in vitro from IFN-γ-activated macrophages or NO donors (31, 40, 41). So there is a possibility that due to interruption of the NO pathway, a deficiency of IFN-γ can lead to higher levels of worm loads. However, a blockade of NO in vivo during L. sigmodontis infection has no effect on the worm load and microfilaremia (31, 35).
We postulate that one reason for the reduced accumulation of neutrophils is that the lack of eosinophils results in decreased chemotactic (7) and antiapoptotic (IL-4) factors (17, 34). Another important factor for the stronger inhibition of neutrophil accumulation in the KO mice may be the reduced production of neutrophil-activating cytokine TNF-α by macrophages. In our study, macrophages were not only reduced in numbers in 5-KO and γ/5-KO mice but also produced significantly less TNF-α in vitro (Fig. 3). It is also important that in human filaria infections, macrophages produce neutrophil chemotactic cytokines in response to microfilaria-producing Onchocerca volvulus worms (6). Moreover, macrophages incubated with soluble extracts of the human filarial parasite B. malayi produce TNF-α (39, 40). One conclusion from this study was that reduction of TNF-α is responsible for a lower level of neutrophil accumulation. However, other interpretations of these results concerning the role of TNF-α are also possible, because TNF-α is a key cytokine which is involved in many parts of the immune response. Furthermore, TNF-α is not the only chemotactic active factor for neutrophils. Other important components are KC (homolog to human IL-8) (32) and granulocyte colony-stimulating factor (3), which are produced at much lower levels in anti-IL-5-treated mice (3).
The active component of the soluble extract of the human filarial parasite B. malayi, which is responsible for chemotactic activity of neutrophils, is heat stable and can be inhibited by polymyxin B. These results strongly suggest that Wolbachia LPS might be responsible for the inflammatory response (39). In conducting our study, we assumed that there is a positive-feedback mechanism between TNF-α and IFN-γ or IL-5 concerning the inflammatory response of the macrophages. A positive interaction between IFN-γ and TNF-α was described for other systems of infection with Listeria (42).
Furthermore, neutrophils taken from uninfected KO mice showed a reduced level of migration (Table 2). Differences in migration motility levels between 5-KO, γ-KO, and γ/5-KO neutrophils were observed by using neutrophils from infected mice (Table 2). Neutrophils taken from γ-KO and γ/5-KO mice demonstrated a significantly lower level of migration activity than neutrophils from 5-KO mice. Previous studies already showed a lower level of migration of neutrophils taken from γ-KO mice (35). This report also demonstrated that IL-5 deficiency leads to significant reduction concerning the migration activity of neutrophils (Table 2). Taken together, the data show that both IFN-γ and IL-5 seem to be essential for migration of neutrophils and that a deficiency of IFN-γ results in inhibition stronger than that seen with neutrophils which were deficient of IL-5 (Table 2). Analyses of the phagocytic activity from neutrophils shows that casein-induced neutrophils taken from uninfected γ/5-KO mice have a significantly lower level of phagocytic activity than neutrophils from single KO mice (Table 3). Reconstitution analyses demonstrated that cytokines IL-5 and IFN-γ contribute equivalently for a normal phagocytic activity of the neutrophils (Fig. 4).
In summary, these data suggest that IL-5 and IFN-γ have a synergistic effect during infection with filarial worms. A deficiency of both cytokines results in drastically reduced levels of migration and phagocytic activity of neutrophils, which constitute one important population of the cells that play a role in the defense against filarial infections. The data show synergism in vivo for the first time and add to the general picture that Th1 and Th2 immune responses are not always antagonistic. Rather, immunosuppressive responses involving IL-10 and TGF-β, in part orchestrated by T-regulatory cells (13, 37), seem to antagonize both Th1 and Th2 in filariasis (19).
Acknowledgments
We thank T. Schüler, B. Richter, and Y. Richter for organization of the animal facility.
A. Hoerauf acknowledges support by the Deutsche Forschungsgemeinschaft (Ho 2009/1-3).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alexander, J., A. R. Satoskar, and D. G. Russell. 1999. Leishmania species: models of intracellular parasitism. J. Cell Sci. 112:2993-3002. [DOI] [PubMed] [Google Scholar]
- 2.Al-Qaoud, K. M., B. Fleischer, and A. Hoerauf. 1998. The Xid defect imparts susceptibility to experimental murine filariosis—association with a lack of antibody and IL-10 production by B cells in response to phosphorylcholine. Int. Immunol. 10:17-25. [DOI] [PubMed] [Google Scholar]
- 3.Al-Qaoud, K. M., E. Pearlman, J. Klukowski, T. Hartung, B. Fleischer, and A. Hoerauf. 2000. A new mechanism for IL-5-dependent helminth control: neutrophil accumulation and neutrophil-mediated worm encapsulation in murine filariasis are abolished in the absence of IL-5. Int. Immunol. 12:899-908. [DOI] [PubMed] [Google Scholar]
- 4.Al-Qaoud, K. M., A. Taubert, H. Zahner, B. Fleischer, and A. Hoerauf. 1997. Infection of BALB/c mice with the filarial nematode Litomosoides sigmodontis: role of CD4+ T cells in controlling larval development. Infect. Immun. 65:2457-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babu, S., L. M. Ganley, T. R. Klei, L. D. Shultz, and T. V. Rajan. 2000. Role of gamma interferon and interleukin-4 in host defense against the human filarial parasite Brugia malayi. Infect. Immun. 68:3034-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brattig, N., D. W. Büttner, and A. Hoerauf. 2001. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microbes Infect. 3:439-446. [DOI] [PubMed] [Google Scholar]
- 7.Braun, R. K., M. Franchini, F. Erard, S. Rihs, I. J. De Vries, K. Blaser, T. T. Hansel, and C. Walker. 1993. Human peripheral blood eosinophils produce and release interleukin-8 on stimulation with calcium ionophore. Eur. J. Immunol. 23:956-960. [DOI] [PubMed] [Google Scholar]
- 8.Brunet, L. R., M. Beall, D. W. Dunne, and E. J. Pearce. 1997. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J. Immunol. 159:777-785. [PubMed] [Google Scholar]
- 9.Brunet, L. R., M. Beall, D. W. Dunne, and E. J. Pearce. 1999. Nitric oxide and the Th2 response combine to prevent severe hepatic damage during Schistosoma mansoni infection. J. Immunol. 163:4976-4984. [PubMed] [Google Scholar]
- 10.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper, A. M., J. Magram, J. Ferrante, and I. M. Orme. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 186:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 13.Doetze, A., J. Satoguina, G. Burchard, T. Rau, C. Löliger, B. Fleischer, and A. Hoerauf. 2000. Antigen-specific cellular hyporesponsiveness in generalized onchocerciasis is mediated by Th3/Tr1-type cytokines IL-10 and TGF-beta but not by a Th1 to Th2 shift. Int. Immunol. 12:623-630. [DOI] [PubMed] [Google Scholar]
- 14.Etges, R., and I. Muller. 1998. Progressive disease or protective immunity to Leishmania major infection: the result of a network of stimulatory and inhibitory interactions. J. Mol. Med. 76:372-390. [DOI] [PubMed] [Google Scholar]
- 15.Fallon, P. G., E. J. Richardson, G. J. McKenzie, and A. N. McKenzie. 2000. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 164:2585-2591. [DOI] [PubMed] [Google Scholar]
- 16.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girard, D., R. Paquin, and A. D. Beaulieu. 1997. Responsiveness of human neutrophils to interleukin-4: induction of cytoskeletal rearrangements, de novo protein synthesis and delay of apoptosis. Biochem. J. 325:147-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartmann, S., A. Schönemeyer, B. Sonnenburg, B. Vray, and R. Lucius. 2002. Cystatins of filarial nematodes up-regulate the nitric oxide production of interferon-γ-activated murine macrophages. Parasite Immunol. 24:253-262. [DOI] [PubMed] [Google Scholar]
- 19.Hoerauf, A., and N. Brattig. 2002. Resistance and susceptibility in human onchocerciasis—beyond Th1 vs. Th2. Trends Parasitol. 18:25-31. [DOI] [PubMed] [Google Scholar]
- 20.Hoerauf, A., and B. Fleischer. 1997. Immune responses to filarial infection in laboratory mice. Med. Microbiol. Immunol. 185:207-215. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, E. H., M. Irvine, P. H. Kass, J. Browne, M. Abdullai, A. M. Prince, and S. Lustigman. 1994. Onchocerca volvulus: in vitro cytotoxic effects of human neutrophils and serum on third-stage larvae. Trop. Med. Parasitol. 45:331-335. [PubMed] [Google Scholar]
- 22.Johnson, E. H., S. Schynder-Candrian, T. V. Rajan, F. K. Nelson, S. Lustigman, and D. Abraham. 1998. Immune responses to third stage larvae of Onchocerca volvulus in interferon-gamma and interleukin-4 knockout mice. Parasite Immunol. 20:319-324. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, K., M. Kai, M. Gidoh, N. Nakata, M. Endoh, R. P. Singh, T. Kasama, and H. Saito. 1998. The possible role of interleukin (IL)-12 and interferon-gamma-inducing factor/IL-18 in protection against experimental Mycobacterium leprae infection in mice. Clin. Immunol. Immunopathol. 88:226-231. [DOI] [PubMed] [Google Scholar]
- 24.Kopf, M., F. Brombacher, P. D. Hodgkin, A. J. Ramsay, E. A. Milbourne, W. J. Dai, K. S. Ovington, C. A. Behm, G. Kohler, I. G. Young, and K. I. Matthaei. 1996. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 4:15-24. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence, R. A. 1996. Lymphatic filariasis: what mice can tell us. Parasitol. Today 12:267-271. [DOI] [PubMed] [Google Scholar]
- 26.Le Goff, L., T. J. Lamb, A. L. Graham, Y. Harcus, and J. E. Allen. 2002. IL-4 is required to prevent filarial nematode development in resistant but not susceptible strains of mice. Int. J. Parasitol. 32:1277-1284. [DOI] [PubMed] [Google Scholar]
- 27.MacIvor, D. M., S. D. Shapiro, C. T. Pham, A. Belaaouaj, S. N. Abraham, and T. J. Ley. 1999. Normal neutrophil function in cathepsin G-deficient mice. Blood 94:4282-4293. [PubMed] [Google Scholar]
- 28.Marechal, P., L. Goff, G. Petit, M. Diagne, D. W. Taylor, and O. Bain. 1996. The fate of the filaria Litomosoides sigmodontis in susceptible and naturally resistant mice. Parasite 3:25-31. [DOI] [PubMed] [Google Scholar]
- 29.Modlin, R. L. 1994. Th1-Th2 paradigm: insights from leprosy. J. Investig. Dermatol. 102:828-832. [DOI] [PubMed] [Google Scholar]
- 30.Petit, G., M. Diagne, P. Marechal, D. Owen, D. Taylor, and O. Bain. 1992. Maturation of the filaria Litomosoides sigmodontis in BALB/c mice: comparative susceptibility of nine other inbred strains. Ann. Parasitol. Hum. Comp. 67:144-150. [DOI] [PubMed] [Google Scholar]
- 31.Pfaff, A. W., H. Schulz-Key, P. T. Soboslay, S. M. Geiger, and W. H. Hoffmann. 2000. The role of nitric oxide in the innate resistance to microfilariae of Litomosoides sigmodontis in mice. Parasite Immunol. 22:397-405. [DOI] [PubMed] [Google Scholar]
- 32.Rot, A. 1992. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol. Today 13:291-294. [DOI] [PubMed] [Google Scholar]
- 33.Rubio de Krömer, M. T., M. Krömer, K. Luersen, and N. W. Brattig. 1998. Detection of a chemotactic factor for neutrophils in extracts of female Onchocerca volvulus. Acta Trop. 71:45-56. [DOI] [PubMed] [Google Scholar]
- 34.Sabin, E. A., M. A. Kopf, and E. J. Pearce. 1996. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J. Exp. Med. 184:1871-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saeftel, M., L. Volkmann, S. Korten, N. Brattig, K. M. Al-Qaoud, B. Fleischer, and A. Hoerauf. 2001. Lack of IFN-γ confers impaired neutrophil granulocyte function and imparts prolonged survival of adult filarial worms in murine filariasis. Microbes Infect. 3:203-213. [DOI] [PubMed] [Google Scholar]
- 36.Sartono, E., Y. C. Kruize, A. Kurniawan, R. M. Maizels, and M. Yazdanbakhsh. 1997. Depression of antigen-specific interleukin-5 and interferon-gamma responses in human lymphatic filariasis as a function of clinical status and age. J. Infect. Dis. 175:1276-1280. [DOI] [PubMed] [Google Scholar]
- 37.Satoguina, J., M. Mempel, J. Larbi, M. Badusche, C. Loliger, O. Adjei, G. Gachelin, B. Fleischer, and A. Hoerauf. 2002. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis). Microbes Infect. 4:1291-1300. [DOI] [PubMed] [Google Scholar]
- 38.Scott, P. 1991. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J. Immunol. 147:3149-3155. [PubMed] [Google Scholar]
- 39.Taylor, M. J., H. F. Cross, and K. Bilo. 2000. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J. Exp. Med. 191:1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor, M. J., H. F. Cross, A. A. Mohammed, A. J. Trees, and A. E. Bianco. 1996. Susceptibility of Brugia malayi and Onchocerca lienalis microfilariae to nitric oxide and hydrogen peroxide in cell-free culture and from IFN-γ-activated macrophages. Parasitology 112:315-322. [DOI] [PubMed] [Google Scholar]
- 41.Thomas, R., M. McCrossan, and M. Selkrik. 1997. Cytostatic and cytotoxic effects of activated macrophages and nitric oxide donors on Brugia malayi. Infect. Immun. 65:2732-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unanue, E. R. 1997. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr. Opin. Immunol. 9:35-43. [DOI] [PubMed] [Google Scholar]
- 43.Urban, J. F. J., K. B. Madden, A. W. Cheever, P. P. Trotta, I. M. Katona, and F. D. Finkelman. 1993. IFN inhibits inflammatory responses and protective immunity in mice infected with the nematode parasite, Nippostrongylus brasiliensis. J. Immunol. 151:7086-7094. [PubMed] [Google Scholar]
- 44.Verhagen, C. E., T. C. van der Pouw Kraan, A. A. Buffing, M. A. Chand, W. R. Faber, L. A. Aarden, and P. K. Das. 1998. Type 1- and type 2-like lesional skin-derived Mycobacterium leprae-responsive T cell clones are characterized by coexpression of IFN-gamma/TNF-alpha and IL-4/IL-5/IL-13, respectively. J. Immunol. 160:2380-2387. [PubMed] [Google Scholar]
- 45.Volkmann, L., O. Bain, M. Saeftel, S. Specht, K. Fischer, F. Brombacher, K. I. Matthaei, and A. Hoerauf. 2002. Murine filariasis: interleukin 4 and interleukin 5 lead to containment of different developmental stages. Med. Microbiol. Immunol. 191:23-31. [DOI] [PubMed] [Google Scholar]
- 46.Volkmann, L., M. Saeftel, B. Fleischer, and A. Hoerauf. 2001. IL-4 is essential for the control of microfilariae in murine infection with the filaria Litomosoides sigmodontis. Infect. Immun. 69:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wildenburg, G., A. Plenge-Bönig, A. Renz, P. Fischer, and D. W. Büttner. 1997. Distribution of mast cells and their correlation with inflammatory cells around Onchocerca gutturosa, O. tarsicola, O. ochengi, and O. flexuosa. Parasitol. Res. 83:109-120. [DOI] [PubMed] [Google Scholar]