Abstract
Purpose
The profound hypogonadism due to androgen deprivation therapy for prostate cancer results in complications such as sexual dysfunction, poor quality of life, vasomotor symptoms and altered cognition. Since estrogen is associated with cardiovascular risks, phytoestrogens are being increasingly evaluated as a potential treatment for these adverse effects. We evaluated the effects of high dose isoflavones, equivalent to that consumed by Asian populations, on the aforementioned consequences of androgen deprivation therapy.
Materials and Methods
A total of 33 men undergoing androgen deprivation therapy for prostate cancer were enrolled in this randomized, double-blind, placebo controlled, 12-week pilot trial. Participants were randomly assigned to receive 20 gm soy protein containing 160 mg total isoflavones (17) vs taste matched placebo, that is 20 gm whole milk protein (16). The study was performed at a tertiary care center in the United States.
Results
At baseline the groups were well matched in demographic parameters, sleep quality, cognition and overall quality of life. However, men in the isoflavone group had a higher baseline prevalence of hot flashes and poor intercourse satisfaction compared to those on placebo. At 12 weeks there were no significant differences between the 2 groups in any outcome measure.
Conclusions
This pilot study of high dose isoflavones in androgen deprived men showed no significant improvement in cognition, vasomotor symptoms or any other aspect of quality of life measures compared to placebo. Future studies should use variable doses of isoflavones for a longer period before ruling out beneficial isoflavone effects in this population.
Keywords: prostate, prostatic neoplasms, antiandrogens, isoflavones, quality of life
Prostate cancer is the most common malignancy in men.1 ADT has traditionally been given for locally advanced and metastatic PCa, for which it has shown a survival advantage (with radiation therapy) and improved QOL, respectively.2 Recent reports suggest that its use has significantly increased and approximately 600,000 men in the United States alone receive it.3,4 Despite its benefits the resulting profound hypogonadism is associated with adverse effects, such as osteoporosis, unfavorable body composition, sexual dysfunction, metabolic perturbations, cognitive changes, hot flashes and decreased QOL.5-7
Although castrate testosterone is implicated as the etiology of these symptoms, men on ADT also have low to undetectable estradiol. Hence, the relative contribution of sex steroids to these adverse effects remains unclear. Men with congenital aromatase deficiency who have undetectable estradiol have osteoporosis, sexual dysfunction and metabolic syndrome despite normal to increased testosterone.8 Indeed, decreased estradiol due to ADT is associated with decreased verbal fluency, visual memory and visual recognition, which are cognitive tasks believed to be influenced by estradiol.9 Furthermore, estrogen therapy in men on ADT shows significant improvement in vasomotor symptoms.10 Although these observations make estrogen an attractive option in these men, investigators are hesitant since a previous study showed an increased incidence of cardiovascular death in men on the synthetic estrogen diethylstilbestrol for advanced PCa.11
Hence, the quest continues for alternative agents that may provide beneficial effects similar to those of endogenous estrogen while lacking the adverse effects of synthetic estrogen. Phytoestrogens (plant estrogens) are nonsteroidal, naturally occurring compounds that can exert estrogenic effects.12 They are structurally similar to natural and synthetic estrogens, and bind to estrogen receptors, specifically estrogen receptor-β.13 The common classes of phytoestrogens are isoflavones, lignans and coumestans. Isoflavones are present in the highest amount in soybeans, flaxseed and legumes with genistein, daidzein and glycitein the most common types. Soy is a staple of Asian diets, in which the daily intake is at least 40 times higher than in Western populations.14 Estimates suggest that the average daily intake of isoflavones in the Chinese population is 100 to 150 mg per day compared to 1 mg per day in the United States.14
Animal studies of isoflavones revealed improved cognition after treatment.15 In postmenopausal women a few studies showed improved cognition, QOL and vasomotor symptoms.16 Such studies in men are limited. A previous series showed improved cognition in men on 100 mg isoflavones per day.17 To our knowledge no study to date has evaluated the influence of soy on sexual function, hot flashes, sleep scores, QOL or cognition in men on ADT for PCa. We performed this pilot study to evaluate these outcomes.
METHODS
Participants
Participants were recruited from The Johns Hopkins medical and radiation oncology clinics. English speaking men 21 years old or older undergoing medical or surgical ADT for at least 3 months were included in analysis. Study exclusion criteria were hepatic, renal, thyroid or neurological disease, active psychiatric disorder, current chemotherapy or glucocorticoids, appetite or weight promoting agents, substance abuse and triglycerides greater than 500 mg/dl. Men allergic to soy protein or cow milk were also excluded from study. Men already on soy supplements were washed out for at least 3 months before enrollment. After enrollment men were instructed to refrain from ingesting any kind of soy product during the 12-week study period (fig. 1).
Figure 1.
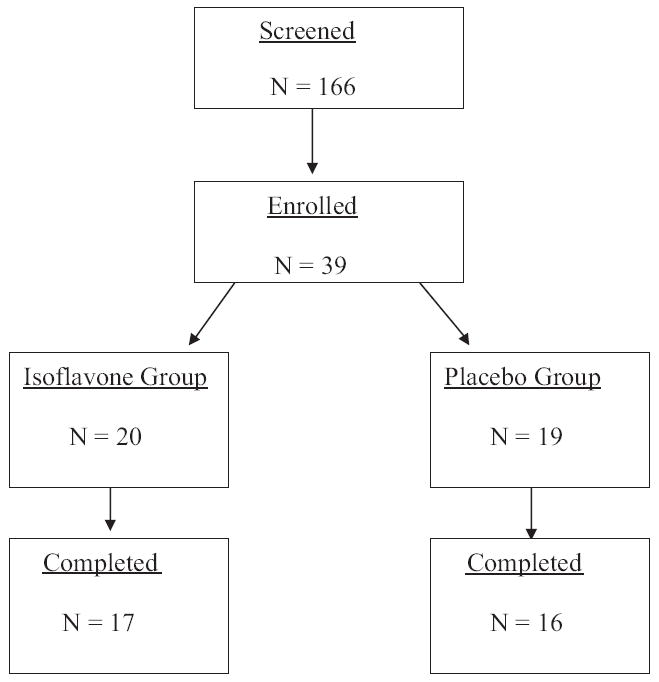
Study flow
Randomization
A list of randomized numbers was generated by a personal computer. Men were randomly assigned to the isoflavone vs placebo group by personnel at the clinical trial unit at our institution who were blinded to the current trial. Patients and study personnel remained blinded to group assignment during the study.
Intervention
The intervention contained 20 gm Revival® soy protein consisting of 160 mg total isoflavones as powder to be mixed with beverages. Each batch is tested for isoflavone content. The concentration of individual isoflavones was 64 mg genistein, 63 mg diadzein and 34 mg glycitein. Placebo contained 20 gm whole milk protein and similar nutrients (31 to 35 gm carbohydrate, 2.0 to 2.5 gm fat and 600 mg calcium) as the intervention except for isoflavones. Active and placebo powders appeared and tasted similar and were available in vanilla and chocolate flavors, dispensed based on patient preference. Supplements were ingested once daily for 12 weeks, and dispensed at the baseline and 6-week visits.
Measurement Schedule
Data were gathered at study baseline, and weeks 6 and 12. Blood was collected between 8 and 10 a.m. after an overnight fast. Weight and height were measured in a standardized way and BMI was calculated.
Outcome Measures
Cognition
All participants were administered an identical battery of the neuropsychological tests, including the National Adult Reading Test, Cube Comparison Test, Identical Pictures Test, verbal fluency test, Trail Making Test and Grooved Pegboard Test.
Sexual function and QOL
The Watts and International Index of Erectile Function questionnaires were used to evaluate sexual function.18,19 Overall QOL was assessed using the standardized SF-36™. Sleep quality was determined using the Epsworth Sleepiness Scale.20 Since there is no widely validated tool to evaluate distress due to vasomotor symptoms in men, they were administered the Blatt-Kupperman questionnaire, which is used in post-menopausal women.21
Laboratory data
PSA, TSH, complete blood count and routine chemistry values were determined as part of patient safety at the screening visit. Laboratory samples were measured at the Johns Hopkins Core Laboratory.
Statistical Analysis
Before testing hypotheses and modeling, the normality of continuous variables was inspected by plotting histograms and the Shapiro-Wilks test, and the need for transformation or nonparametric analysis was determined. No outliers were identified for any outcome measure or demographic variable. For comparison between treatment groups chi-square analysis was done for categorical demographic variables. Based on the distributional properties of the continuous demographic variables the 2-sample t or Wilcoxon rank sum test was used. For body composition, cognitive function, sexual function and QOL comparison across visits in each treatment group was done by 1-way ANOVA or the nonparametric Kruskal-Wallis test. Comparison between treatment groups at each visit was done by the t or Wilcoxon rank sum test. A mixed model was used to compare the treatment effect on body composition, cognitive function, sexual function and QOL after adjusting for baseline measurements. All analyses were done using SAS version 9.1.3.
RESULTS
A total of 17 men on isoflavones and 16 on placebo who were undergoing ADT for PCa for at least 3 months completed this randomized, double-blind, placebo controlled pilot study. Three of the initially enrolled 39 men were excluded from analysis based on screening laboratory values and 3 withdrew from study due to personal reasons (2) and dislike of the compound taste (1).
Baseline Data
Mean age was similar between the placebo and treatment groups (p = 0.94). Overall 80% of the men were white (table 1). Mean ADT duration in each group was approximately 2 years (p = 0.70). Most men were on medical ADT and greater than 80% also received radiation therapy. Only 4 men were on combined androgen blockade. The 2 groups were well matched in age, weight, BMI, TSH and comorbidity. Men in the placebo group had higher mean PSA but this was not significantly different vs the isoflavone group (p = 0.30). This high mean value was driven by 4 men in the placebo group with PSA between 100 and 600 ng/ml. During the study there were no significant changes in PSA, weight or BMI in either group (data not shown).
Table 1.
Baseline comparison
| Variable | Placebo | Treatment | p Value |
|---|---|---|---|
| Mean ± SE age | 69.0 ± 2.2 | 69.2 ± 2.5 | 0.94 |
| No. white/black | 11/5 | 15/2 | |
| Mean ± SE wt (kg) | 97.39 ± 4.73 | 90.2 ± 4.01 | 0.25 |
| Mean ± SE BMI (kg/m2) | 30.05 ± 1.44 | 28.71 ± 1.24 | 0.48 |
| Mean ± SE PSA (ng/ml) | 45.05 ± 39.54 | 3.9 ± 2.58 | 0.30 |
| Mean ± SE TSH (mIU/ml) | 1.98 ± 0.22 | 1.81 ± 0.24 | 0.60 |
| ADT duration (yrs) | 1.96 ± 0.64 | 2.37 ± 0.37 | 0.70 |
| No. gonadotropin-releasing hormone analogues/orchiectomy | 16/0 | 16/1 | |
| No. androgen receptor antagonist | 1 | 3 | |
| No. radiation therapy | 11 | 13 | |
| No. metastasis history | 7 | 7 | |
| Mean ± SE No. system review abnormalities | 3.19 ± 0.42 | 3.41 ± 0.52 | 0.74 |
Cognition
Table 2 lists cognitive data. There was a significant test session effect for performance on the Hidden Figures test in men assigned to the placebo or the active treatment group. These results indicate a general learning effect across test sessions for this measure. No other cognitive task revealed a learning effect in the 2 treatment groups with time.
Table 2.
Cognitive function
| Variable | Mean ± SE Baseline Score | Mean ± SE 6-Wk Score | Mean ± SE 12-Wk Score | p Value |
|---|---|---|---|---|
| General intelligence | ||||
| National Adult Reading Test: | ||||
| Placebo | 45.2 ± 2.12 | 42.43 ± 2.66 | 43.69 ± 2.83 | 0.733 |
| Active | 39.6 ± 3.02 | 41.29 ± 3.25 | 41.57 ± 3.21 | 0.89 |
| p Value | 0.14 | 0.788 | 0.627 | |
| Hopkins verbal learning memory | ||||
| Total recall: | ||||
| Placebo | 24.67 ± 1.67 | 24.87 ± 1.57 | 25.85 ± 1.56 | 0.864 |
| Active | 21.6 ± 1.26 | 22.53 ± 0.96 | 22.47 ± 1.39 | 0.832 |
| p Value | 0.154 | 0.214 | 0.116 | |
| Recognition: | ||||
| Placebo | 22.13 ± 0.46 | 20.4 ± 1 | 22.77 ± 0.53 | 0.067 |
| Active | 20.8 ± 0.87 | 21.47 ± 1 | 22.67 ± 0.67 | 0.307 |
| p Value | 0.185 | 0.458 | 0.907 | |
| % Retained: | ||||
| Placebo | 100.88 ± 5.98 | 94.31 ± 2.38 | 94.68 ± 3.63 | 0.482 |
| Active | 89.82 ± 5.84 | 94.08 ± 4.3 | 95.21 ± 5.5 | 0.748 |
| p Value | 0.196 | 0.964 | 0.939 | |
| Verbal fluency | ||||
| FAS: | ||||
| Placebo | 58.79 ± 3.65 | 64.47 ± 4.51 | 63.31 ± 4.43 | 0.603 |
| Active | 55.36 ± 5.08 | 56.93 ± 4.78 | 57.93 ± 4.42 | 0.929 |
| p Value | 0.588 | 0.261 | 0.401 | |
| Grooved pegboard hand fine motor | ||||
| Dominant time (secs): | ||||
| Placebo | 101.5 ± 8.14 | 89.93 ± 4.32 | 90.75 ± 6.05 | 0.362 |
| Active | 91.8 ± 8.7 | 78.73 ± 3.34 | 83.8 ± 5.72 | 0.346 |
| p Value | 0.424 | 0.049 | 0.415 | |
| No. dominant drops: | ||||
| Placebo | 0.64 ± 0.27 | 0.14 ± 0.1 | 0.09 ± 0.09 | 0.074 |
| Active | 0.4 ± 0.19 | 0.33 ± 0.21 | 0.2 ± 0.11 | 0.715 |
| p Value | 0.463 | 0.43 | 0.466 | |
| Nondominant time (secs): | ||||
| Placebo | 115.43 ± 9.75 | 98.86 ± 7.76 | 102.73 ± 7.43 | 0.347 |
| Active | 112.8 ± 11.25 | 89.2 ± 3.05 | 88.67 ± 6.91 | 0.055 |
| p Value | 0.862 | 0.245 | 0.184 | |
| No. nondominant drops: | ||||
| Placebo | 0.21 ± 0.11 | 0.07 ± 0.07 | 0.45 ± 0.28 | 0.269 |
| Active | 0.6 ± 0.4 | 0.4 ± 0.13 | 0.27 ± 0.12 | 0.646 |
| p Value | 0.376 | 0.04 | 0.504 | |
| Visual-spatial | ||||
| Mental 3-dimensional rotation processing: | ||||
| Placebo | 8.4 ± 0.82 | 8.07 ± 1.17 | 13.69 ± 2.06 | 0.012 |
| Active | 7.33 ± 0.9 | 8.6 ± 1.16 | 8.27 ± 0.74 | 0.623 |
| p Value | 0.388 | 0.751 | 0.015 | |
| Rey complex figure immediate memory recall: | ||||
| Placebo | 20.87 ± 1.5 | 24.33 ± 1.4 | 25.92 ± 1.52 | 0.058 |
| Active | 16.03 ± 2.21 | 22.33 ± 1.62 | 25.13 ± 1.91 | 0.006 |
| p Value | 0.082 | 0.357 | 0.754 | |
| Rey complex figure delayed memory recall: | ||||
| Placebo | 20.37 ± 1.44 | 23.07 ± 1.43 | 24.42 ± 1.79 | 0.184 |
| Active | 15 ± 2.27 | 20.43 ± 1.61 | 24.27 ± 2.04 | 0.008 |
| p Value | 0.056 | 0.232 | 0.955 | |
Treatment by test session analysis revealed a significant group difference on the 3-Dimensional Mental Rotation test after 12 weeks of treatment with men on placebo outperforming men on isoflavones. Also, at week 6 men on soy completed the Grooved Pegboard dominant hand task more rapidly than those on placebo but this difference failed to persist after 12 weeks of treatment. At 6 weeks men on isoflavones dropped more pegs when completing the Grooved Pegboard task with the nondominant hand than men on placebo. This group difference also failed to persist after 12 weeks of treatment. No other group differences or group by test session interaction were observed on the cognitive tests.
Sexual Function and QOL
The groups were well matched at baseline. There was no significant improvement in libido or erectile function in men on isoflavones compared to those on placebo (table 3). For QOL there was no significant improvement in physical or emotional parameters in the isoflavone vs placebo group (table 4).
Table 3.
Sexual function
| Variable | Mean ± SE Baseline Score | Mean ± SE 6-Wk Score | Mean ± SE 12-Wk Score | p Value |
|---|---|---|---|---|
| Watts Questionnaire | ||||
| Totals: | ||||
| Placebo | 62.92 ± 3.18 | 63.21 ± 2.3 | 59.7 ± 3.39 | 0.66 |
| Active | 62 ± 3.71 | 63.83 ± 2.79 | 67 ± 3.68 | 0.58 |
| p Value | 0.852 | 0.864 | 0.167 | |
| Libido: | ||||
| Placebo | 22.21 ± 1.19 | 22.13 ± 1.15 | 21.42 ± 1.28 | 0.88 |
| Active | 21.4 ± 1.4 | 21.36 ± 1.2 | 22.93 ± 1.3 | 0.62 |
| p Value | 0.663 | 0.644 | 0.42 | |
| Erectile function: | ||||
| Placebo | 26.83 ± 1.35 | 26.29 ± 1.14 | 25.36 ± 1.65 | 0.76 |
| Active | 26 ± 1.24 | 25.92 ± 1.44 | 27.46 ± 1.5 | 0.68 |
| p Value | 0.655 | 0.844 | 0.356 | |
| Sexual arousal: | ||||
| Placebo | 3.92 ± 0.33 | 4.36 ± 0.23 | 3.45 ± 0.51 | 0.21 |
| Active | 3.79 ± 0.37 | 4.13 ± 0.27 | 4.31 ± 0.31 | 0.51 |
| p Value | 0.784 | 0.536 | 0.153 | |
| Sexual satisfaction: | ||||
| Placebo | 10.92 ± 0.77 | 11 ± 0.71 | 11.75 ± 0.68 | 0.68 |
| Active | 10.69 ± 0.71 | 11.92 ± 0.81 | 12.5 ± 0.69 | 0.22 |
| p Value | 0.832 | 0.399 | 0.45 | |
| International Index of Erectile Function | ||||
| Totals: | ||||
| Placebo | 21.33 ± 5.17 | 17.4 ± 4.6 | 17.08 ± 5 | 0.79 |
| Active | 11.31 ± 2.1 | 13.2 ± 2.24 | 15 ± 3.29 | 0.62 |
| p Value | 0.101 | 0.418 | 0.728 | |
| Erectile function: | ||||
| Placebo | 7.33 ± 2.44 | 5.87 ± 2.35 | 5.85 ± 2.44 | 0.88 |
| Active | 3.27 ± 1.07 | 3.67 ± 1.06 | 4.53 ± 1.55 | 0.76 |
| p Value | 0.139 | 0.4 | 0.644 | |
| Intercourse satisfaction: | ||||
| Placebo | 3.8 ± 1.35 | 2.33 ± 1.11 | 2.15 ± 1.25 | 0.59 |
| Active | 0.67 ± 0.46 | 1.2 ± 0.59 | 1.53 ± 0.74 | 0.60 |
| p Value | 0.036 | 0.375 | 0.664 | |
| Orgasmic function: | ||||
| Placebo | 1.73 ± 0.62 | 1.27 ± 0.54 | 1.46 ± 0.87 | 0.88 |
| Active | 0.47 ± 0.26 | 1.07 ± 0.5 | 1.13 ± 0.47 | 0.47 |
| p Value | 0.07 | 0.788 | 0.732 | |
| Sexual desire: | ||||
| Placebo | 3.73 ± 0.6 | 3.47 ± 0.52 | 3.54 ± 0.57 | 0.94 |
| Active | 2.8 ± 0.26 | 3.07 ± 0.33 | 2.93 ± 0.34 | 0.83 |
| p Value | 0.163 | 0.524 | 0.359 | |
| Sexual satisfaction: | ||||
| Placebo | 4.73 ± 0.73 | 4.47 ± 0.72 | 4.08 ± 0.61 | 0.80 |
| Active | 3.85 ± 0.66 | 4.2 ± 0.63 | 4.64 ± 0.86 | 0.74 |
| p Value | 0.38 | 0.782 | 0.601 | |
Table 4.
SF-36
| Variable | Mean ± SE Baseline Score | Mean ± SE 6-Wk Score | Mean ± SE 12-Wk Score | p Value |
|---|---|---|---|---|
| Physical health summary: | ||||
| Placebo | 76.25 ± 5.59 | 74.73 ± 4.38 | 72.36 ± 5.45 | 0.869 |
| Active | 64.25 ± 6.17 | 65.75 ± 6.56 | 66.92 ± 5.63 | 0.954 |
| p Value | 0.161 | 0.272 | 0.497 | |
| Mental health summary: | ||||
| Placebo | 81.23 ± 4.62 | 77.37 ± 4.64 | 80.66 ± 4.87 | 0.817 |
| Active | 69.67 ± 7.16 | 71.36 ± 5.62 | 77.18 ± 4.02 | 0.642 |
| p Value | 0.186 | 0.416 | 0.584 | |
| Physical functioning: | ||||
| Placebo | 83.33 ± 5.53 | 82.14 ± 4.41 | 84.23 ± 3.34 | 0.952 |
| Active | 71.67 ± 6.74 | 71.33 ± 6.96 | 78.33 ± 8.07 | 0.747 |
| p Value | 0.192 | 0.208 | 0.528 | |
| Physical health role limitation: | ||||
| Placebo | 80 ± 9.82 | 71.67 ± 9.41 | 67.31 ± 12.46 | 0.69 |
| Active | 63.33 ± 11.67 | 61.67 ± 11.41 | 56.67 ± 10.76 | 0.91 |
| p Value | 0.284 | 0.504 | 0.522 | |
| Body pain: | ||||
| Placebo | 78 ± 5.37 | 74.33 ± 5.93 | 76.35 ± 5.31 | 0.893 |
| Active | 66 ± 6.2 | 73 ± 7.33 | 74 ± 6.48 | 0.657 |
| p Value | 0.155 | 0.889 | 0.786 | |
| General health: | ||||
| Placebo | 63.67 ± 5.24 | 65.67 ± 4.57 | 61.54 ± 5.2 | 0.848 |
| Active | 56 ± 3.94 | 57 ± 6.15 | 58.67 ± 4.77 | 0.931 |
| p Value | 0.252 | 0.268 | 0.687 | |
| Energy/fatigue: | ||||
| Placebo | 64 ± 5.65 | 63.67 ± 4.1 | 67.69 ± 4.62 | 0.819 |
| Active | 50.67 ± 8.24 | 49.67 ± 7.28 | 54 ± 5.88 | 0.906 |
| p Value | 0.193 | 0.105 | 0.085 | |
| Social functioning: | ||||
| Placebo | 94.17 ± 4.2 | 83.33 ± 6.06 | 89.42 ± 3.7 | 0.283 |
| Active | 78.33 ± 9 | 78.33 ± 8.75 | 88.33 ± 5.38 | 0.589 |
| p Value | 0.122 | 0.642 | 0.873 | |
| Emotional problem role limitation: | ||||
| Placebo | 82.22 ± 9.12 | 75.56 ± 10.01 | 76.92 ± 10.93 | 0.879 |
| Active | 68.89 ± 11.02 | 75.56 ± 8.27 | 80 ± 7.83 | 0.691 |
| p Value | 0.359 | 1 | 0.817 | |
| Emotional well-being: | ||||
| Placebo | 84.53 ± 2.7 | 86.93 ± 2.54 | 88.62 ± 2.43 | 0.542 |
| Active | 80.8 ± 3.87 | 81.87 ± 3.53 | 79.43 ± 3.7 | 0.899 |
| p Value | 0.435 | 0.254 | 0.052 |
Sleep Scale and Hot Flashes
The 2 groups had similar sleep scores at baseline. There was no significant improvement in sleep quality for men on isoflavones vs placebo (fig. 2, A). Men were not well matched for hot flashes with the isoflavone group reporting higher scores (increased distress) than men on placebo at baseline and at study end (fig. 2, B). However, within group analysis showed no significant changes in the vasomotor distress score in either group.
Figure 2.
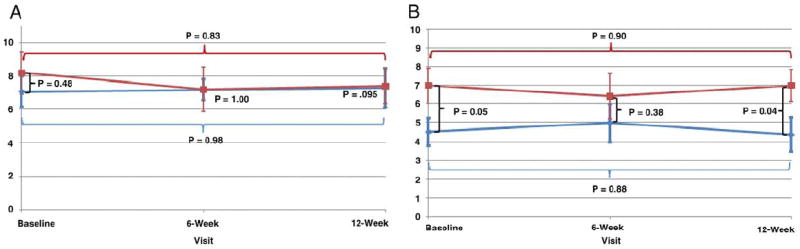
Scores in placebo (blue curve) and isoflavone (red curve) groups. A, sleep quality on Epworth Sleepiness Scale. B, vasomotor symptom scores on Blatt-Kupperman scale.
Safety and Compliance
There were no safety issues during the study and no significant changes in PSA, weight or BMI in either group (data not shown). Men tolerated the compound well with only 1 withdrawing from study because he disliked its taste. Overall compliance was high at approximately 80%. Compliance was based on the number of sachets returned by each patient at treatment weeks 6 and 12.
DISCUSSION
In this double-blind, randomized, placebo controlled pilot study administering high dose isoflavones to men with PCa undergoing ADT did not show any benefit in cognition, QOL, vasomotor symptoms, sleep quality or sexual function. To our knowledge this is the first study in the English literature using high dose isoflavones in this patient population.
ADT use has significantly increased in recent years with approximately 600,000 men in the United States alone receiving it. It results in profound hypogonadism, which is associated with vasomotor symptoms, sexual dysfunction, decreased QOL and cognitive function changes.10,22 Although estrogen therapy results in improvement in some of these parameters, its use is associated with increased cardiovascular mortality.11 Since isoflavones have a slightly different mechanism of action via estrogen receptor-β and their use results in improvement in some of these parameters in postmenopausal women,16 the hope was that isoflavones would be beneficial in men on ADT. Soy is consumed in large amounts in Asian countries compared to that by the Western population.14 Fewer Asian postmenopausal women complain of hot flashes than Western women.23 Hence, we evaluated a higher dose of isoflavones at a concentration similar to that used by the Asian population in men on ADT.
Estrogen promotes synaptogenesis in the hippocampus and improves the overall neuronal glucose supply.24 In men on ADT decreased cognitive performance is associated with decreased serum estradiol.9 However, most studies of the isoflavone effect on cognition have been done in women, showing mixed results.16,25 In men 1 trial using 100 vs 0.5 mg isoflavones per day showed significant improvements in short-term and long-term memory in the high dose group.17 Although the English literature has a few conceptual reviews on isoflavones in men with PCa undergoing ADT,26 to our knowledge the current study is the first to investigate this association. Our study does not support the hypothesis that phytoestrogens appreciably influence cognitive performance in this population even when given at a high dose.
Although it is established that ADT results in sexual dysfunction,5 we further evaluated any additional beneficial or harmful isoflavone effects on libido and erectile function. Animal studies show that phytoestrogens bind to estrogen receptors in the corpus cavernosum and attenuate its relaxation in response to acetylcholine and nitroglycerin.27 Some groups suggested that isoflavones should be considered a novel risk factor for erectile dysfunction.28 We found that high dose isoflavones were neither beneficial nor harmful in terms of libido or erectile function. However, this does not rule out any negative sexual effects of isoflavones on healthy eugonadal men.
A major consequence of ADT is hot flashes, which significantly decrease QOL in this population and occur as a result of withdrawal of estrogens.10,29 Previous studies of estrogen in men on ADT showed significant improvement in hot flashes, further consolidating the role of estrogens.30 However, estrogen is associated with gynecomastia and thromboembolic disease.10 Studies of isoflavones in postmenopausal women revealed decreased hot flash severity compared to that in the placebo group.16 Hence, it was important to evaluate whether isoflavones would mitigate some of these symptoms in this unique population. Since to our knowledge there is no validated instrument to evaluate hot flashes in men, we used the Blatt-Kupperman scale, which is used in postmenopausal women. Men on isoflavones did not show any significant improvement in hot flashes compared to those on placebo. Since hot flashes can influence sleep quality,26 we also studied the effects of isoflavones on sleep quality using the Epworth Sleepiness Scale. We noted no notable treatment effect on sleep quality. We previously reported that overall QOL in men on ADT is significantly lower than that in eugonadal men with PCa and in age matched controls.5 In the current study we found no significant improvement in the QOL for men on isoflavones vs placebo.
CONCLUSIONS
In this randomized, double-blind, placebo controlled study we used a novel design of treating men on ADT with high dose isoflavones and evaluated a number of outcome measures that could be important to overall health and well-being in this population. We found no benefit of this treatment over placebo. However, this pilot study had a small sample size and short treatment duration. Future studies should use variable isoflavone doses for longer periods before ruling out any beneficial effects of isoflavones in this population.
Abbreviations and Acronyms
- ADT
androgen deprivation therapy
- BMI
body mass index
- PCa
prostate cancer
- PSA
prostate specific antigen
- QOL
quality of life
- TSH
thyroid-stimulating hormone
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 3.Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab. 2008;93:2042. doi: 10.1210/jc.2007-2595. [DOI] [PubMed] [Google Scholar]
- 4.Smith MR. Androgen deprivation therapy for prostate cancer: new concepts and concerns. Curr Opin Endocrinol Diabetes Obes. 2007;14:247. doi: 10.1097/MED.0b013e32814db88c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basaria S, Lieb J, 2nd, Tang AM, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf) 2002;56:779. doi: 10.1046/j.1365-2265.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 6.Basaria S, Muller DC, Carducci MA, et al. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 7.Cherrier MM, Rose AL, Higano C. The effects of combined androgen blockade on cognitive function during the first cycle of intermittent androgen suppression in patients with prostate cancer. J Urol. 2003;170:1808. doi: 10.1097/01.ju.0000091640.59812.83. [DOI] [PubMed] [Google Scholar]
- 8.Carani C, Qin K, Simoni M, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337:91. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 9.Salminen EK, Portin RI, Koskinen AI, et al. Estradiol and cognition during androgen deprivation in men with prostate carcinoma. Cancer. 2005;103:1381. doi: 10.1002/cncr.20962. [DOI] [PubMed] [Google Scholar]
- 10.Harle LK, Maggio M, Shahani S, et al. Endocrine complications of androgen-deprivation therapy in men with prostate cancer. Clin Adv Hematol Oncol. 2006;4:687. [PubMed] [Google Scholar]
- 11.Blackard CE. The Veterans’ Administration Cooperative Urological Research Group studies of carcinoma of the prostate: a review. Cancer Chemother Rep. 1975;59:225. [PubMed] [Google Scholar]
- 12.Murkies AL, Wilcox G, Davis SR. Clinical review 92: phytoestrogens. J Clin Endocrinol Metab. 1998;83:297. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- 13.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 14.de Kleijn MJ, van der Schouw YT, Wilson PW, et al. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: the Framingham study. J Nutr. 2001;131:1826. doi: 10.1093/jn/131.6.1826. [DOI] [PubMed] [Google Scholar]
- 15.Lephart ED, Adlercreutz H, Lund TD. Dietary soy phytoestrogen effects on brain structure and aromatase in Long-Evans rats. Neuroreport. 2001;12:3451. doi: 10.1097/00001756-200111160-00015. [DOI] [PubMed] [Google Scholar]
- 16.Basaria S, Wisniewski A, Dupree K, et al. Effects of high-dose isoflavones on cognition, quality of life, androgens and lipoproteins in postmenopausal women. J Endocrinol Invest. 2009;32:150. doi: 10.1007/BF03345705. [DOI] [PubMed] [Google Scholar]
- 17.File SE, Jarrett N, Fluck E, et al. Eating soya improves human memory. Psychopharmacology (Berl) 2001;157:430. doi: 10.1007/s002130100845. [DOI] [PubMed] [Google Scholar]
- 18.Rosen RC, Riley A, Wagner G, et al. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 19.Watts RJ. Dimensions of sexual health. Am J Nurs. 1979;79:1568. [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Alder E. The Blatt-Kupperman menopausal index: a critique. Maturitas. 1998;29:19. doi: 10.1016/s0378-5122(98)00024-3. [DOI] [PubMed] [Google Scholar]
- 22.Basaria S. Androgen deprivation therapy, insulin resistance, and cardiovascular mortality: an inconvenient truth. J Androl. 2008;29:534. doi: 10.2164/jandrol.108.005454. [DOI] [PubMed] [Google Scholar]
- 23.Tang GW. The climacteric of Chinese factory workers. Maturitas. 1994;19:177. doi: 10.1016/0378-5122(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 24.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrinol Rev. 1999;20:279. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 25.Kreijkamp-Kaspers S, Kok L, Grobbee DE, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. 2004;292:65. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- 26.Moyad MA. Complementary/alternative therapies for reducing hot flashes in prostate cancer patients: reevaluating the existing indirect data from studies of breast cancer and postmenopausal women. Urology. 2002;59:20. doi: 10.1016/s0090-4295(02)01641-2. [DOI] [PubMed] [Google Scholar]
- 27.Srilatha B, Adaikan PG. Estrogen and phytoestrogen predispose to erectile dysfunction: do ER-alpha and ER-beta in the cavernosum play a role? Urology. 2004;63:382. doi: 10.1016/j.urology.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Pan L, Xia X, Feng Y, et al. Exposure to the phytoestrogen daidzein attenuates apomorphine-induced penile erection concomitant with plasma testosterone level reduction in dose- and time-related manner in adult rats. Urology. 2007;70:613. doi: 10.1016/j.urology.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Guise TA, Oefelein MG, Eastham JA, et al. Estrogenic side effects of androgen deprivation therapy. Rev Urol. 2007;9:163. [PMC free article] [PubMed] [Google Scholar]
- 30.Gerber GS, Zagaja GP, Ray PS, et al. Transdermal estrogen in the treatment of hot flashes in men with prostate cancer. Urology. 2000;55:97. doi: 10.1016/s0090-4295(99)00370-2. [DOI] [PubMed] [Google Scholar]


