Abstract
In the title chiral aldimine, C19H17N, the azomethine group is not fully conjugated with the phenyl substituent: the dihedral angle between phenyl and C*—N=C mean planes is ϕ3 = 23.0 (2)°. Compared with the earlier DFT-B3LYP/6–31 G(d) computations from the literature, the C=N—C*—C(naphthyl) torsion angle, found at ϕ2 = −118.0 (2)° in the X-ray structure, does not match the angle calculated for the potential minimum energy at ϕ2 = 0°. However, this angle is close to the second potential energy minimum at ϕ2 = −120° which is ca. 8.5 kJ mol−1 above the global energy minimum. Thus, the reported X-ray structure corresponds to the second most likely (according to DFT) conformer, allowing the existence of other polymorphs to be anticipated.
Related literature
For a typical synthesis of the title compound, see: Lee & Ahn (2002 ▶). For general background to solvent-free synthesis, see: Tanaka & Toda (2000 ▶). For the structures of related imines, see: Espinosa Leija et al. (2009 ▶); Bernès et al. (2010 ▶). For the DFT study of the title compound (R enantiomer), see: Fukuda et al. (2007 ▶).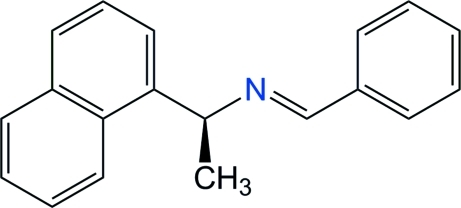
Experimental
Crystal data
C19H17N
M r = 259.34
Monoclinic,

a = 8.0761 (8) Å
b = 7.7874 (8) Å
c = 11.7760 (11) Å
β = 95.033 (7)°
V = 737.76 (13) Å3
Z = 2
Mo Kα radiation
μ = 0.07 mm−1
T = 298 K
0.4 × 0.2 × 0.2 mm
Data collection
Siemens P4 diffractometer
2470 measured reflections
1595 independent reflections
1276 reflections with I > 2σ(I)
R int = 0.017
3 standard reflections every 97 reflections intensity decay: 1%
Refinement
R[F 2 > 2σ(F 2)] = 0.034
wR(F 2) = 0.094
S = 1.02
1595 reflections
183 parameters
1 restraint
H-atom parameters constrained
Δρmax = 0.09 e Å−3
Δρmin = −0.08 e Å−3
Data collection: XSCANS (Siemens, 1996 ▶); cell refinement: XSCANS; data reduction: XSCANS; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: Mercury (Macrae et al., 2006 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811012980/ld2008sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811012980/ld2008Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
Support from VIEP-UAP (GUPJ-NAT10-G) is acknowledged.
supplementary crystallographic information
Comment
Schiff base compounds are widely studied and used, attracting much attention in both organic synthesis and metal ion complexation. Recently, we have focused our attention on the synthesis of chiral and achiral Schiff bases (Espinosa Leija et al., 2009; Bernès et al., 2010). In continuation of this work, we synthesized the title compound using the solvent-free approach (Tanaka & Toda, 2000). The reaction occurs under mild conditions and requires easier workup procedures and simpler equipment, compared to similar reactions carried out in solution, for example - in refluxing CH2Cl2 (Lee & Ahn, 2002).
In the title molecule (Fig. 1), all distances and bond angles have expected values. The imine group has a sterically favored E conformation, and it is rotated by 23.0 (2)° relative to the phenyl group (the dihedral angle between planes of N1/C2/C9 and C3···C8 groups). The dihedral angle between aromatic phenyl and naphthyl groups is 70.7 (1)°. This molecular conformation is significantly different from one observed in the solid-state for a related imine bearing a thiophene group instead of the phenyl (Espinosa Leija et al., 2009), in which corresponding angles are 5.1 (8) and 83.79 (13)°.
Interestingly, there is a study on conformational flexibility of the title compound that has been published on the basis of DFT calculations at B3LYP/6–31 G(d) level (Fukuda et al., 2007). The potential energies for internal rotations around σ bonds C9*—C11 (φ1), C9*—N1 (φ2) and C2—C3 (φ3) were computed (see Fig. 1 for the angle notations, hereafter assumed for the S enantiomer). The dihedral angle φ1 related to the orientation of the naphthyl group has two energy minima, with the global minimum at φ1 = 40°, close to that found by X-ray diffraction (φ1 = N1—C9—C11—C12 = -25.3 (3)°). Similarly, the orientations for the phenyl ring are consistent between DFT and X-ray data: φ3 = 0° vs. φ3 = N1—C2—C3—C8 = 19.9 (4)°. In contrast, internal rotation φ2 computed by DFT presents a minimum at φ2 = 0°, far different from the angle observed in the crystal structure: φ2 = C2—N1—C9—C11 = -118.0 (2)°. However, on the φ2 potential curve published by Fukuda et al., there are two lesser minima, at φ2 = -120° and φ2 = 110°. The first one is consistent with the conformer observed in the solid-state (φ2 = -118°) and is placed only 2 kcal/mol above the φ2 = 0° minimum. It may thus be expected that the title molecule could be crystallized in different polymorphic phases, derived from conformers with different values for the angle φ2.
Experimental
The title compound was prepared by reacting (S)-(–)-(1-naphthyl)ethylamine and benzaldehyde (Lee & Ahn, 2002), but at room temperature and using no solvent. The crude was recrystallized from EtOH affording colorless crystals of the title compound. Yield 94%; mp 79–81 oC. Analysis: [α]D25 = +233 (c 1, CHCl3). FT—IR (KBr): 1641 cm-1 (C=N). 1H NMR (400 MHz, CDCl3/TMS) δ = 1.71 (d, 3H, CH—CH3,), 5.31 (q, 1H, Ar—CH), 7.34–8.24 (m, 12H, Ar), 8.36 (s, 1H, H-C=N). 13C NMR (100 MHz, CDCl3/TMS) δ = 24.51 (CCH3), 65.51 (CHCH3), 123.56 (Ar), 123.97 (Ar), 125.26 (Ar), 125.64(Ar), 125.74 (Ar), 127.28 (Ar), 128.21 (Ar), 128.46 (Ar), 128.88 (Ar), 130.52 (Ar), 130.60 (Ar), 133.94 (Ar), 136.43 (Ar),141.12 (Ar), 159.55 (HC=N). MS—EI: m/z= 259 (M+).
Refinement
All C-bonded H atoms were placed in idealized positions and refined as riding to their carrier C atoms, with bond lengths fixed to 0.93 (aromatic CH), 0.96 (methyl CH3), and 0.98 Å (methine CH). Isotropic displacement parameters were calculated as Uiso(H) = 1.5Ueq(C10) for the methyl group and Uiso(H) = 1.2Ueq(carrier atom) otherwise. The methyl group C10 was considered as a rigid group but was allowed to rotate about C9—C10 bond. The absolute configuration was assigned from the known configuration of the chiral amine used as the starting material and confirmed by measring the optical rotation and comparing with rotations reported in the litterature for both enantiomers. All measured Friedel pairs (223) were merged.
Figures
Fig. 1.
The title molecule with displacement ellipsoids for non-H atoms shown at the 30% probability level. The scheme indicates the torsion angles used in the DFT study of Fukuda et al. (2007).
Crystal data
| C19H17N | F(000) = 276 |
| Mr = 259.34 | Dx = 1.167 Mg m−3 |
| Monoclinic, P21 | Melting point: 352 K |
| Hall symbol: P 2yb | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.0761 (8) Å | Cell parameters from 80 reflections |
| b = 7.7874 (8) Å | θ = 4.9–12.3° |
| c = 11.7760 (11) Å | µ = 0.07 mm−1 |
| β = 95.033 (7)° | T = 298 K |
| V = 737.76 (13) Å3 | Irregular, colourless |
| Z = 2 | 0.4 × 0.2 × 0.2 mm |
Data collection
| Siemens P4 diffractometer | Rint = 0.017 |
| Radiation source: fine-focus sealed tube | θmax = 26.2°, θmin = 2.5° |
| graphite | h = −10→3 |
| 2θ/ω scans | k = −1→9 |
| 2470 measured reflections | l = −14→14 |
| 1595 independent reflections | 3 standard reflections every 97 reflections |
| 1276 reflections with I > 2σ(I) | intensity decay: 1% |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.034 | H-atom parameters constrained |
| wR(F2) = 0.094 | w = 1/[σ2(Fo2) + (0.0477P)2 + 0.0347P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max < 0.001 |
| 1595 reflections | Δρmax = 0.09 e Å−3 |
| 183 parameters | Δρmin = −0.08 e Å−3 |
| 1 restraint | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 constraints | Extinction coefficient: 0.063 (8) |
| Primary atom site location: structure-invariant direct methods | Absolute structure: 223 Friedel pairs merged |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.3971 (2) | 0.6059 (3) | 0.71942 (14) | 0.0646 (5) | |
| C2 | 0.4226 (3) | 0.6094 (3) | 0.61588 (18) | 0.0643 (6) | |
| H2A | 0.3363 | 0.6432 | 0.5631 | 0.077* | |
| C3 | 0.5833 (3) | 0.5624 (3) | 0.57416 (18) | 0.0662 (6) | |
| C4 | 0.6202 (4) | 0.6121 (4) | 0.4665 (2) | 0.0906 (8) | |
| H4A | 0.5440 | 0.6761 | 0.4203 | 0.109* | |
| C5 | 0.7722 (5) | 0.5659 (5) | 0.4274 (3) | 0.1090 (11) | |
| H5A | 0.7989 | 0.6028 | 0.3561 | 0.131* | |
| C6 | 0.8809 (4) | 0.4676 (5) | 0.4930 (3) | 0.1058 (12) | |
| H6A | 0.9813 | 0.4356 | 0.4660 | 0.127* | |
| C7 | 0.8442 (3) | 0.4155 (5) | 0.5982 (3) | 0.0977 (10) | |
| H7A | 0.9188 | 0.3468 | 0.6423 | 0.117* | |
| C8 | 0.6973 (3) | 0.4640 (4) | 0.6394 (2) | 0.0748 (7) | |
| H8A | 0.6745 | 0.4301 | 0.7121 | 0.090* | |
| C9 | 0.2278 (2) | 0.6405 (3) | 0.74826 (16) | 0.0583 (5) | |
| H9A | 0.1547 | 0.6558 | 0.6781 | 0.070* | |
| C10 | 0.1713 (3) | 0.4844 (3) | 0.8128 (2) | 0.0747 (7) | |
| H10A | 0.1743 | 0.3844 | 0.7654 | 0.112* | |
| H10B | 0.0599 | 0.5025 | 0.8328 | 0.112* | |
| H10C | 0.2442 | 0.4681 | 0.8809 | 0.112* | |
| C11 | 0.2202 (3) | 0.7992 (3) | 0.82185 (16) | 0.0538 (5) | |
| C12 | 0.3561 (3) | 0.8538 (3) | 0.88883 (18) | 0.0648 (6) | |
| H12A | 0.4567 | 0.7968 | 0.8856 | 0.078* | |
| C13 | 0.3473 (3) | 0.9946 (3) | 0.9628 (2) | 0.0760 (7) | |
| H13A | 0.4412 | 1.0271 | 1.0092 | 0.091* | |
| C14 | 0.2046 (3) | 1.0830 (3) | 0.96724 (19) | 0.0729 (7) | |
| H14A | 0.2011 | 1.1765 | 1.0161 | 0.087* | |
| C15 | 0.0608 (3) | 1.0349 (3) | 0.89846 (17) | 0.0595 (5) | |
| C16 | −0.0910 (3) | 1.1256 (3) | 0.90111 (19) | 0.0729 (7) | |
| H16A | −0.0950 | 1.2215 | 0.9478 | 0.088* | |
| C17 | −0.2301 (3) | 1.0758 (4) | 0.8373 (2) | 0.0776 (7) | |
| H17A | −0.3285 | 1.1369 | 0.8407 | 0.093* | |
| C18 | −0.2257 (3) | 0.9330 (4) | 0.7667 (2) | 0.0731 (7) | |
| H18A | −0.3217 | 0.8987 | 0.7233 | 0.088* | |
| C19 | −0.0826 (2) | 0.8434 (3) | 0.76058 (18) | 0.0618 (6) | |
| H19A | −0.0822 | 0.7490 | 0.7122 | 0.074* | |
| C20 | 0.0660 (2) | 0.8899 (3) | 0.82582 (16) | 0.0529 (5) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0602 (10) | 0.0696 (13) | 0.0646 (10) | 0.0073 (10) | 0.0089 (8) | −0.0043 (10) |
| C2 | 0.0694 (13) | 0.0571 (13) | 0.0670 (12) | 0.0072 (12) | 0.0095 (11) | −0.0043 (11) |
| C3 | 0.0726 (14) | 0.0565 (12) | 0.0718 (12) | −0.0021 (11) | 0.0185 (11) | −0.0127 (11) |
| C4 | 0.121 (2) | 0.0679 (16) | 0.0887 (16) | 0.0076 (18) | 0.0422 (16) | −0.0017 (14) |
| C5 | 0.139 (3) | 0.084 (2) | 0.116 (2) | −0.017 (2) | 0.074 (2) | −0.023 (2) |
| C6 | 0.0783 (18) | 0.100 (2) | 0.145 (3) | −0.0151 (19) | 0.0411 (19) | −0.059 (2) |
| C7 | 0.0638 (15) | 0.112 (3) | 0.117 (2) | 0.0058 (16) | 0.0057 (15) | −0.052 (2) |
| C8 | 0.0638 (14) | 0.0823 (17) | 0.0782 (13) | 0.0047 (14) | 0.0061 (11) | −0.0211 (13) |
| C9 | 0.0544 (11) | 0.0602 (12) | 0.0606 (11) | 0.0038 (11) | 0.0073 (9) | −0.0060 (11) |
| C10 | 0.0847 (16) | 0.0558 (14) | 0.0842 (15) | −0.0062 (13) | 0.0114 (12) | −0.0021 (13) |
| C11 | 0.0571 (11) | 0.0528 (12) | 0.0530 (10) | −0.0038 (10) | 0.0127 (9) | 0.0041 (9) |
| C12 | 0.0600 (13) | 0.0637 (13) | 0.0708 (12) | −0.0029 (12) | 0.0060 (11) | 0.0000 (12) |
| C13 | 0.0773 (17) | 0.0729 (17) | 0.0765 (14) | −0.0175 (15) | −0.0008 (12) | −0.0106 (14) |
| C14 | 0.0912 (17) | 0.0565 (14) | 0.0723 (13) | −0.0121 (13) | 0.0155 (12) | −0.0119 (11) |
| C15 | 0.0730 (14) | 0.0493 (11) | 0.0587 (11) | −0.0047 (11) | 0.0209 (10) | 0.0048 (10) |
| C16 | 0.0884 (18) | 0.0584 (13) | 0.0768 (14) | 0.0041 (14) | 0.0341 (13) | −0.0024 (13) |
| C17 | 0.0715 (16) | 0.0747 (17) | 0.0901 (15) | 0.0131 (14) | 0.0269 (13) | 0.0072 (14) |
| C18 | 0.0605 (13) | 0.0809 (17) | 0.0794 (14) | 0.0022 (13) | 0.0145 (11) | 0.0021 (13) |
| C19 | 0.0601 (13) | 0.0612 (13) | 0.0655 (11) | −0.0024 (11) | 0.0139 (10) | −0.0031 (11) |
| C20 | 0.0591 (11) | 0.0495 (11) | 0.0520 (9) | −0.0052 (9) | 0.0166 (8) | 0.0045 (9) |
Geometric parameters (Å, °)
| N1—C2 | 1.255 (2) | C10—H10C | 0.9600 |
| N1—C9 | 1.462 (3) | C11—C12 | 1.362 (3) |
| C2—C3 | 1.473 (3) | C11—C20 | 1.436 (3) |
| C2—H2A | 0.9300 | C12—C13 | 1.405 (3) |
| C3—C8 | 1.379 (3) | C12—H12A | 0.9300 |
| C3—C4 | 1.383 (3) | C13—C14 | 1.348 (3) |
| C4—C5 | 1.395 (4) | C13—H13A | 0.9300 |
| C4—H4A | 0.9300 | C14—C15 | 1.407 (3) |
| C5—C6 | 1.355 (5) | C14—H14A | 0.9300 |
| C5—H5A | 0.9300 | C15—C16 | 1.418 (3) |
| C6—C7 | 1.361 (5) | C15—C20 | 1.420 (3) |
| C6—H6A | 0.9300 | C16—C17 | 1.352 (3) |
| C7—C8 | 1.373 (3) | C16—H16A | 0.9300 |
| C7—H7A | 0.9300 | C17—C18 | 1.391 (4) |
| C8—H8A | 0.9300 | C17—H17A | 0.9300 |
| C9—C11 | 1.514 (3) | C18—C19 | 1.358 (3) |
| C9—C10 | 1.525 (3) | C18—H18A | 0.9300 |
| C9—H9A | 0.9800 | C19—C20 | 1.414 (3) |
| C10—H10A | 0.9600 | C19—H19A | 0.9300 |
| C10—H10B | 0.9600 | ||
| C2—N1—C9 | 117.30 (18) | H10A—C10—H10C | 109.5 |
| N1—C2—C3 | 122.9 (2) | H10B—C10—H10C | 109.5 |
| N1—C2—H2A | 118.5 | C12—C11—C20 | 118.99 (19) |
| C3—C2—H2A | 118.5 | C12—C11—C9 | 121.04 (19) |
| C8—C3—C4 | 118.6 (2) | C20—C11—C9 | 119.90 (18) |
| C8—C3—C2 | 121.2 (2) | C11—C12—C13 | 121.4 (2) |
| C4—C3—C2 | 120.2 (2) | C11—C12—H12A | 119.3 |
| C3—C4—C5 | 119.8 (3) | C13—C12—H12A | 119.3 |
| C3—C4—H4A | 120.1 | C14—C13—C12 | 120.8 (2) |
| C5—C4—H4A | 120.1 | C14—C13—H13A | 119.6 |
| C6—C5—C4 | 120.2 (3) | C12—C13—H13A | 119.6 |
| C6—C5—H5A | 119.9 | C13—C14—C15 | 120.5 (2) |
| C4—C5—H5A | 119.9 | C13—C14—H14A | 119.8 |
| C5—C6—C7 | 120.4 (3) | C15—C14—H14A | 119.8 |
| C5—C6—H6A | 119.8 | C14—C15—C16 | 121.8 (2) |
| C7—C6—H6A | 119.8 | C14—C15—C20 | 119.5 (2) |
| C6—C7—C8 | 120.2 (3) | C16—C15—C20 | 118.7 (2) |
| C6—C7—H7A | 119.9 | C17—C16—C15 | 121.5 (2) |
| C8—C7—H7A | 119.9 | C17—C16—H16A | 119.2 |
| C7—C8—C3 | 120.8 (3) | C15—C16—H16A | 119.2 |
| C7—C8—H8A | 119.6 | C16—C17—C18 | 119.9 (2) |
| C3—C8—H8A | 119.6 | C16—C17—H17A | 120.1 |
| N1—C9—C11 | 111.65 (18) | C18—C17—H17A | 120.1 |
| N1—C9—C10 | 107.2 (2) | C19—C18—C17 | 120.6 (2) |
| C11—C9—C10 | 109.68 (15) | C19—C18—H18A | 119.7 |
| N1—C9—H9A | 109.4 | C17—C18—H18A | 119.7 |
| C11—C9—H9A | 109.4 | C18—C19—C20 | 121.7 (2) |
| C10—C9—H9A | 109.4 | C18—C19—H19A | 119.2 |
| C9—C10—H10A | 109.5 | C20—C19—H19A | 119.2 |
| C9—C10—H10B | 109.5 | C19—C20—C15 | 117.57 (19) |
| H10A—C10—H10B | 109.5 | C19—C20—C11 | 123.62 (18) |
| C9—C10—H10C | 109.5 | C15—C20—C11 | 118.81 (18) |
| C9—N1—C2—C3 | −174.6 (2) | C11—C12—C13—C14 | −1.9 (4) |
| N1—C2—C3—C8 | 19.9 (4) | C12—C13—C14—C15 | 0.6 (4) |
| N1—C2—C3—C4 | −162.2 (3) | C13—C14—C15—C16 | −179.9 (2) |
| C8—C3—C4—C5 | −1.6 (4) | C13—C14—C15—C20 | 1.4 (3) |
| C2—C3—C4—C5 | −179.5 (2) | C14—C15—C16—C17 | −178.0 (2) |
| C3—C4—C5—C6 | 2.3 (5) | C20—C15—C16—C17 | 0.7 (3) |
| C4—C5—C6—C7 | −1.1 (5) | C15—C16—C17—C18 | −0.3 (4) |
| C5—C6—C7—C8 | −0.8 (5) | C16—C17—C18—C19 | −0.4 (4) |
| C6—C7—C8—C3 | 1.6 (4) | C17—C18—C19—C20 | 0.6 (3) |
| C4—C3—C8—C7 | −0.3 (4) | C18—C19—C20—C15 | −0.1 (3) |
| C2—C3—C8—C7 | 177.6 (3) | C18—C19—C20—C11 | −179.8 (2) |
| C2—N1—C9—C11 | −118.0 (2) | C14—C15—C20—C19 | 178.3 (2) |
| C2—N1—C9—C10 | 121.9 (2) | C16—C15—C20—C19 | −0.5 (3) |
| N1—C9—C11—C12 | −25.3 (3) | C14—C15—C20—C11 | −2.0 (3) |
| C10—C9—C11—C12 | 93.4 (2) | C16—C15—C20—C11 | 179.16 (18) |
| N1—C9—C11—C20 | 157.71 (17) | C12—C11—C20—C19 | −179.5 (2) |
| C10—C9—C11—C20 | −83.6 (2) | C9—C11—C20—C19 | −2.5 (3) |
| C20—C11—C12—C13 | 1.1 (3) | C12—C11—C20—C15 | 0.8 (3) |
| C9—C11—C12—C13 | −175.92 (19) | C9—C11—C20—C15 | 177.88 (17) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LD2008).
References
- Bernès, S., Hernández, G., Portillo, R., Cruz, S. & Gutiérrez, R. (2010). Acta Cryst. E66, o1322–o1323. [DOI] [PMC free article] [PubMed]
- Espinosa Leija, A., Hernández, G., Portillo, R., Gutiérrez, R. & Bernès, S. (2009). Acta Cryst. E65, o1651. [DOI] [PMC free article] [PubMed]
- Fukuda, K., Suzuki, H., Tokita, M., Watanabe, J. & Kawauchi, S. (2007). J. Mol. Struct. (Theochem), 821, 95–100.
- Lee, T. & Ahn, Y. (2002). Bull. Korean Chem. Soc. 23, 1490–1492.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst. 39, 453–457.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siemens (1996). XSCANS Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Tanaka, K. & Toda, F. (2000). Chem. Rev. 100, 1025–1074. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811012980/ld2008sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811012980/ld2008Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



