Abstract
In the title compound, [CdCl2(C13H10N6)], the 2-amino-4,6-bis(pyridin-2-yl)-1,3,5-triazine (HABPT) ligand adopts a tridentate tripyridyl coordination mode. The CdII atom is five-coordinated by three N atoms from the HABPT ligand and two chloride ions. In the crystal, molecules are linked via N—H⋯N, N—H⋯Cl and C—H⋯Cl hydrogen bonds into a supramolecular network.
Related literature
For asymmetric ligands containing a triazine ring, see: Drew et al. (2000 ▶); Boubals et al. (2002 ▶); Medlycott et al. (2007 ▶); Chi et al. (2006 ▶); Cao et al. (2008 ▶, 2009 ▶). For the synthesis of the HABPT ligand, see: Case & Koft (1959 ▶). For metal complexes of the HABPT ligand, see: Drew et al. (2000 ▶); Boubals et al. (2002 ▶); Cao et al. (2009 ▶). For the diverse coordination modes of rigid multidentate polypyridyl ligands containing a triazine ring as a bridge, see: Zhou, Li, Wu & Zhang (2006 ▶); Zhou, Li, Zheng, Zhang & Wu (2006 ▶).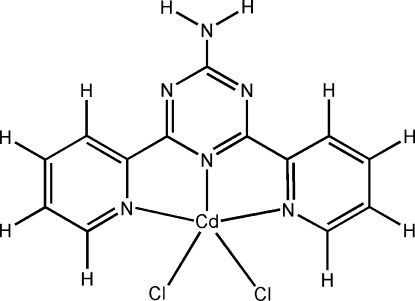
Experimental
Crystal data
[CdCl2(C13H10N6)]
M r = 433.58
Triclinic,

a = 8.8750 (6) Å
b = 9.2010 (7) Å
c = 10.2677 (7) Å
α = 82.5151 (9)°
β = 65.636 (1)°
γ = 82.798 (1)°
V = 754.89 (9) Å3
Z = 2
Mo Kα radiation
μ = 1.80 mm−1
T = 293 K
0.34 × 0.31 × 0.28 mm
Data collection
Bruker SMART APEX CCD detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶) T min = 0.581, T max = 0.636
5102 measured reflections
2588 independent reflections
2499 reflections with I > 2σ(I)
R int = 0.014
Refinement
R[F 2 > 2σ(F 2)] = 0.020
wR(F 2) = 0.056
S = 1.01
2588 reflections
199 parameters
H-atom parameters constrained
Δρmax = 0.28 e Å−3
Δρmin = −0.30 e Å−3
Data collection: SMART (Bruker, 2005 ▶); cell refinement: SAINT (Bruker, 2005 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811012517/zq2094sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811012517/zq2094Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N6—H6A⋯N3i | 0.91 | 2.31 | 3.183 (3) | 162 |
| N6—H6B⋯Cl2ii | 0.91 | 2.45 | 3.334 (2) | 165 |
| C12—H12A⋯Cl1iii | 0.97 | 2.76 | 3.671 (2) | 158 |
| C2—H2A⋯Cl1iv | 0.97 | 2.75 | 3.705 (3) | 166 |
| C11—H11A⋯Cl2v | 0.97 | 2.82 | 3.596 (2) | 138 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  .
.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21001031) and the special research fund for PhDs of Guangdong University of Education (10ARF05).
supplementary crystallographic information
Comment
The rigid multidentate polypyridyl ligands containing a triazine ring as a bridge have attracted greatly our attention due to their coordination diversity (Zhou, Li, Wu & Zhang, 2006); Zhou, Li, Zheng, Zhang & Wu, 2006). Although coordination chemistry of the symmetrical ligands like tri(2-pyridyl)-l,3,5-triazine (TPT) has been well explored, the observations on the asymmetric ligands containing triazine ring are still rare (Drew et al., 2000; Boubals et al., 2002; Medlycott et al., 2007; Chi et al., 2006; Cao et al., 2008, 2009).
The ligand 2-amino-4,6-bis(2-pyridyl)-l,3,5-triazine (HABPT) has five potential coordinate sites, it may offer a tridentate chelating mode or bis-bidentate binding mode with the capability of bridging two metal ions in chelating way (Drew et al. 2000; Boubals et al., 2002; Cao et al., 2009). As a contribution to the synthesis and structural studies of coordination abilities of the ligand (Case et al., 1959; Drew et al., 2000; Boubals et al., 2002 and Cao et al., 2009), we present here the crystal structure of the title compound, a new cadmium(II) complex with the HABPT ligand.
Within the title compound, C13H10CdCl2N6, the CdII center is five-coordinated respectively by three N atoms [Cd—N1 2.387 (2), Cd—N2 2.2679 (18), Cd—N5 2.433 (2) Å] from the HABPT ligand and two Cl atoms [Cd—Cl1 2.4176 (7) and Cd—Cl2 2.4431 (7) Å]. The ligand adopts a tridentate tripyridyl mode to coordinate to the CdII ion. Two chloride ligands are posited up and down the plane of the ligand that further accept hydrogen bonds from other ligands, the deviations values of Cd, Cl1 and Cl2 from the least-squares plane (rms deviation 0.122 Å for all non-H atoms of the planar tridentate ligand) are -0.603 (1), 0.540 (2) and -2.997 (1) Å, respectively. Viewed from the whole crystal structure, molecules are linked by intermolecular N—H···N, N—H···Cl and C—H···Cl hydrogen bonds to form a supramolecular structure. A weak intermolecular π–π interaction between the triazine ring and one pyridyl ring is also observed with a centroid-centroid distance of 3.976 (1) Å.
Experimental
The ligand HABPT was prepared according to previously reported procedures (F.H. Case et al., 1959). To a suspension of HABPT (0.025 g, 0.1 mmol) in 7 ml of ethanol was added the solution of CdCl2 (0.018 g, 0.1 mmol) in 7 ml distilled water. The resulting mixture was vibrated under ultrasonic condition for 20 min and then filtered. The obtained colorless filtrate was allowed to stay at ambient temperature for one week giving 0.026 g (60% yield, based on the ligand) of colourless crystals suitable for a structural determination.
Anal. Calcd. for C13H10Cl2N6Cd (%): C 36.01, H 2.31, N 19.38; found (%): C 36.12, H 2.40, N 19.31.
Refinement
All H atoms were fixed geometrically and treated as riding with C—H = 0.96–0.99 Å and N—H = 0.90-0.91 Å with Uiso(H) = 1.2 Ueq(C or N).
Figures
Fig. 1.
View of the title compound with the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level. H atoms are represented as small spheres of arbitrary radii.
Crystal data
| [CdCl2(C13H10N6)] | Z = 2 |
| Mr = 433.58 | F(000) = 424 |
| Triclinic, P1 | Dx = 1.908 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.8750 (6) Å | Cell parameters from 2588 reflections |
| b = 9.2010 (7) Å | θ = 2.2–25.0° |
| c = 10.2677 (7) Å | µ = 1.80 mm−1 |
| α = 82.5151 (9)° | T = 293 K |
| β = 65.636 (1)° | Block, colourless |
| γ = 82.798 (1)° | 0.34 × 0.31 × 0.28 mm |
| V = 754.89 (9) Å3 |
Data collection
| Bruker SMART APEX CCD detector diffractometer | 2588 independent reflections |
| Radiation source: fine-focus sealed tube | 2499 reflections with I > 2σ(I) |
| graphite | Rint = 0.014 |
| φ and ω scans | θmax = 25.0°, θmin = 2.2° |
| Absorption correction: multi-scan (SADABS; Bruker, 2005) | h = −10→10 |
| Tmin = 0.581, Tmax = 0.636 | k = −10→10 |
| 5102 measured reflections | l = −12→12 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.020 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.056 | H-atom parameters constrained |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.0354P)2 + 0.2818P] where P = (Fo2 + 2Fc2)/3 |
| 2588 reflections | (Δ/σ)max = 0.001 |
| 199 parameters | Δρmax = 0.28 e Å−3 |
| 0 restraints | Δρmin = −0.30 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cd | 0.21372 (2) | 0.386656 (17) | 0.788019 (17) | 0.03420 (8) | |
| Cl1 | 0.22830 (10) | 0.26193 (9) | 1.00530 (7) | 0.05660 (19) | |
| Cl2 | 0.41551 (8) | 0.26525 (8) | 0.58040 (7) | 0.04683 (16) | |
| N1 | 0.2999 (2) | 0.6218 (2) | 0.7893 (2) | 0.0354 (4) | |
| N2 | 0.0652 (2) | 0.5692 (2) | 0.7109 (2) | 0.0316 (4) | |
| N3 | 0.0354 (2) | 0.8157 (2) | 0.6236 (2) | 0.0307 (4) | |
| N4 | −0.1444 (2) | 0.6385 (2) | 0.6264 (2) | 0.0332 (4) | |
| N5 | −0.0378 (2) | 0.2992 (2) | 0.7962 (2) | 0.0352 (4) | |
| N6 | −0.1636 (3) | 0.8735 (2) | 0.5305 (2) | 0.0404 (5) | |
| H6A | −0.1322 | 0.9668 | 0.5070 | 0.048* | |
| H6B | −0.2468 | 0.8494 | 0.5095 | 0.048* | |
| C1 | 0.4108 (3) | 0.6446 (3) | 0.8398 (3) | 0.0444 (6) | |
| H1A | 0.4555 | 0.5567 | 0.8820 | 0.053* | |
| C2 | 0.4571 (3) | 0.7822 (3) | 0.8394 (3) | 0.0472 (6) | |
| H2A | 0.5370 | 0.7896 | 0.8800 | 0.057* | |
| C3 | 0.3859 (3) | 0.9019 (3) | 0.7847 (3) | 0.0467 (6) | |
| H3A | 0.4194 | 0.9989 | 0.7835 | 0.056* | |
| C4 | 0.2714 (3) | 0.8806 (3) | 0.7307 (3) | 0.0377 (5) | |
| H4A | 0.2264 | 0.9664 | 0.6864 | 0.045* | |
| C5 | 0.2318 (3) | 0.7398 (3) | 0.7343 (2) | 0.0303 (5) | |
| C6 | 0.1044 (3) | 0.7081 (2) | 0.6848 (2) | 0.0290 (5) | |
| C7 | −0.0883 (3) | 0.7746 (3) | 0.5937 (2) | 0.0321 (5) | |
| C8 | −0.0629 (3) | 0.5416 (2) | 0.6838 (2) | 0.0303 (5) | |
| C9 | −0.1177 (3) | 0.3903 (2) | 0.7273 (2) | 0.0311 (5) | |
| C10 | −0.2455 (3) | 0.3474 (3) | 0.7005 (3) | 0.0381 (5) | |
| H10A | −0.3033 | 0.4136 | 0.6498 | 0.046* | |
| C11 | −0.2923 (3) | 0.2053 (3) | 0.7456 (3) | 0.0481 (6) | |
| H11A | −0.3814 | 0.1696 | 0.7309 | 0.058* | |
| C12 | −0.2127 (3) | 0.1126 (3) | 0.8178 (3) | 0.0478 (6) | |
| H12A | −0.2415 | 0.0132 | 0.8551 | 0.057* | |
| C13 | −0.0864 (3) | 0.1634 (3) | 0.8405 (3) | 0.0427 (6) | |
| H13A | −0.0270 | 0.0998 | 0.8914 | 0.051* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cd | 0.03892 (12) | 0.02984 (12) | 0.03786 (12) | 0.00290 (8) | −0.02185 (9) | −0.00009 (8) |
| Cl1 | 0.0672 (4) | 0.0623 (5) | 0.0399 (3) | 0.0064 (4) | −0.0279 (3) | 0.0077 (3) |
| Cl2 | 0.0430 (3) | 0.0568 (4) | 0.0433 (3) | 0.0013 (3) | −0.0188 (3) | −0.0137 (3) |
| N1 | 0.0387 (10) | 0.0315 (11) | 0.0427 (11) | 0.0007 (8) | −0.0245 (9) | −0.0014 (8) |
| N2 | 0.0321 (10) | 0.0272 (10) | 0.0403 (10) | −0.0013 (8) | −0.0199 (8) | −0.0015 (8) |
| N3 | 0.0352 (10) | 0.0268 (10) | 0.0355 (10) | −0.0027 (8) | −0.0204 (8) | 0.0005 (8) |
| N4 | 0.0390 (10) | 0.0284 (10) | 0.0389 (10) | −0.0042 (8) | −0.0236 (9) | 0.0023 (8) |
| N5 | 0.0368 (10) | 0.0294 (11) | 0.0401 (11) | −0.0015 (8) | −0.0180 (9) | 0.0021 (8) |
| N6 | 0.0485 (12) | 0.0314 (11) | 0.0551 (13) | −0.0068 (9) | −0.0372 (11) | 0.0088 (9) |
| C1 | 0.0432 (14) | 0.0467 (16) | 0.0534 (16) | 0.0046 (12) | −0.0312 (13) | −0.0062 (12) |
| C2 | 0.0435 (14) | 0.0562 (18) | 0.0551 (16) | −0.0070 (12) | −0.0313 (13) | −0.0081 (13) |
| C3 | 0.0494 (15) | 0.0447 (16) | 0.0565 (16) | −0.0134 (12) | −0.0295 (13) | −0.0030 (12) |
| C4 | 0.0429 (13) | 0.0329 (13) | 0.0436 (13) | −0.0066 (10) | −0.0237 (11) | 0.0003 (10) |
| C5 | 0.0294 (11) | 0.0331 (13) | 0.0303 (11) | −0.0029 (9) | −0.0141 (9) | −0.0025 (9) |
| C6 | 0.0315 (11) | 0.0275 (12) | 0.0300 (11) | −0.0012 (9) | −0.0147 (9) | −0.0026 (9) |
| C7 | 0.0358 (12) | 0.0307 (12) | 0.0325 (11) | −0.0027 (9) | −0.0172 (10) | −0.0001 (9) |
| C8 | 0.0328 (11) | 0.0278 (12) | 0.0317 (11) | −0.0017 (9) | −0.0149 (9) | −0.0017 (9) |
| C9 | 0.0323 (11) | 0.0279 (12) | 0.0333 (11) | −0.0016 (9) | −0.0136 (9) | −0.0024 (9) |
| C10 | 0.0375 (12) | 0.0340 (13) | 0.0467 (14) | −0.0033 (10) | −0.0211 (11) | −0.0027 (10) |
| C11 | 0.0459 (15) | 0.0414 (15) | 0.0608 (17) | −0.0142 (12) | −0.0233 (13) | −0.0007 (13) |
| C12 | 0.0489 (15) | 0.0310 (14) | 0.0585 (17) | −0.0117 (11) | −0.0168 (13) | 0.0048 (12) |
| C13 | 0.0440 (14) | 0.0315 (13) | 0.0474 (14) | −0.0008 (11) | −0.0166 (12) | 0.0065 (11) |
Geometric parameters (Å, °)
| Cd—N2 | 2.2679 (18) | C1—C2 | 1.378 (4) |
| Cd—N1 | 2.387 (2) | C1—H1A | 0.9845 |
| Cd—Cl1 | 2.4176 (7) | C2—C3 | 1.373 (4) |
| Cd—N5 | 2.433 (2) | C2—H2A | 0.9723 |
| Cd—Cl2 | 2.4431 (7) | C3—C4 | 1.386 (3) |
| N1—C1 | 1.336 (3) | C3—H3A | 0.9727 |
| N1—C5 | 1.349 (3) | C4—C5 | 1.376 (3) |
| N2—C6 | 1.331 (3) | C4—H4A | 0.9838 |
| N2—C8 | 1.337 (3) | C5—C6 | 1.489 (3) |
| N3—C6 | 1.324 (3) | C8—C9 | 1.485 (3) |
| N3—C7 | 1.362 (3) | C9—C10 | 1.384 (3) |
| N4—C8 | 1.311 (3) | C10—C11 | 1.385 (4) |
| N4—C7 | 1.356 (3) | C10—H10A | 0.9818 |
| N5—C13 | 1.333 (3) | C11—C12 | 1.375 (4) |
| N5—C9 | 1.348 (3) | C11—H11A | 0.9664 |
| N6—C7 | 1.321 (3) | C12—C13 | 1.381 (4) |
| N6—H6A | 0.9078 | C12—H12A | 0.9670 |
| N6—H6B | 0.9080 | C13—H13A | 0.9815 |
| N2—Cd—N1 | 68.97 (6) | C4—C3—H3A | 122.2 |
| N2—Cd—Cl1 | 141.58 (5) | C5—C4—C3 | 118.9 (2) |
| N1—Cd—Cl1 | 100.97 (5) | C5—C4—H4A | 122.6 |
| N2—Cd—N5 | 69.07 (7) | C3—C4—H4A | 118.4 |
| N1—Cd—N5 | 134.84 (7) | N1—C5—C4 | 122.3 (2) |
| Cl1—Cd—N5 | 101.44 (5) | N1—C5—C6 | 115.38 (19) |
| N2—Cd—Cl2 | 108.57 (5) | C4—C5—C6 | 122.3 (2) |
| N1—Cd—Cl2 | 109.67 (5) | N3—C6—N2 | 124.7 (2) |
| Cl1—Cd—Cl2 | 109.69 (3) | N3—C6—C5 | 120.0 (2) |
| N5—Cd—Cl2 | 98.80 (5) | N2—C6—C5 | 115.24 (19) |
| C1—N1—C5 | 117.9 (2) | N6—C7—N4 | 116.1 (2) |
| C1—N1—Cd | 124.73 (17) | N6—C7—N3 | 118.9 (2) |
| C5—N1—Cd | 117.40 (14) | N4—C7—N3 | 124.9 (2) |
| C6—N2—C8 | 116.34 (19) | N4—C8—N2 | 124.9 (2) |
| C6—N2—Cd | 122.13 (14) | N4—C8—C9 | 118.8 (2) |
| C8—N2—Cd | 121.48 (15) | N2—C8—C9 | 116.28 (19) |
| C6—N3—C7 | 114.17 (19) | N5—C9—C10 | 122.5 (2) |
| C8—N4—C7 | 114.62 (19) | N5—C9—C8 | 116.0 (2) |
| C13—N5—C9 | 117.9 (2) | C10—C9—C8 | 121.5 (2) |
| C13—N5—Cd | 125.93 (16) | C9—C10—C11 | 118.7 (2) |
| C9—N5—Cd | 115.21 (15) | C9—C10—H10A | 122.5 |
| C7—N6—H6A | 120.1 | C11—C10—H10A | 118.8 |
| C7—N6—H6B | 120.9 | C12—C11—C10 | 119.0 (2) |
| H6A—N6—H6B | 119.0 | C12—C11—H11A | 118.6 |
| N1—C1—C2 | 123.1 (2) | C10—C11—H11A | 122.4 |
| N1—C1—H1A | 115.9 | C11—C12—C13 | 118.9 (2) |
| C2—C1—H1A | 121.0 | C11—C12—H12A | 123.4 |
| C3—C2—C1 | 118.6 (2) | C13—C12—H12A | 117.7 |
| C3—C2—H2A | 123.3 | N5—C13—C12 | 123.0 (2) |
| C1—C2—H2A | 118.0 | N5—C13—H13A | 116.2 |
| C2—C3—C4 | 119.2 (2) | C12—C13—H13A | 120.8 |
| C2—C3—H3A | 118.6 | ||
| N2—Cd—N1—C1 | 174.8 (2) | C7—N3—C6—N2 | 2.9 (3) |
| Cl1—Cd—N1—C1 | 33.5 (2) | C7—N3—C6—C5 | −175.0 (2) |
| N5—Cd—N1—C1 | 152.02 (19) | C8—N2—C6—N3 | −6.0 (3) |
| Cl2—Cd—N1—C1 | −82.2 (2) | Cd—N2—C6—N3 | 171.45 (17) |
| N2—Cd—N1—C5 | −5.55 (16) | C8—N2—C6—C5 | 172.00 (19) |
| Cl1—Cd—N1—C5 | −146.86 (16) | Cd—N2—C6—C5 | −10.6 (3) |
| N5—Cd—N1—C5 | −28.3 (2) | N1—C5—C6—N3 | −177.2 (2) |
| Cl2—Cd—N1—C5 | 97.44 (16) | C4—C5—C6—N3 | 5.9 (3) |
| N1—Cd—N2—C6 | 8.74 (16) | N1—C5—C6—N2 | 4.8 (3) |
| Cl1—Cd—N2—C6 | 89.73 (18) | C4—C5—C6—N2 | −172.2 (2) |
| N5—Cd—N2—C6 | 171.65 (19) | C8—N4—C7—N6 | 177.5 (2) |
| Cl2—Cd—N2—C6 | −95.81 (17) | C8—N4—C7—N3 | −3.8 (3) |
| N1—Cd—N2—C8 | −173.96 (19) | C6—N3—C7—N6 | −179.0 (2) |
| Cl1—Cd—N2—C8 | −92.97 (18) | C6—N3—C7—N4 | 2.3 (3) |
| N5—Cd—N2—C8 | −11.05 (17) | C7—N4—C8—N2 | 0.3 (3) |
| Cl2—Cd—N2—C8 | 81.49 (17) | C7—N4—C8—C9 | 178.3 (2) |
| N2—Cd—N5—C13 | −179.5 (2) | C6—N2—C8—N4 | 4.2 (3) |
| N1—Cd—N5—C13 | −156.69 (19) | Cd—N2—C8—N4 | −173.20 (17) |
| Cl1—Cd—N5—C13 | −38.3 (2) | C6—N2—C8—C9 | −173.76 (19) |
| Cl2—Cd—N5—C13 | 73.9 (2) | Cd—N2—C8—C9 | 8.8 (3) |
| N2—Cd—N5—C9 | 12.20 (16) | C13—N5—C9—C10 | −0.6 (4) |
| N1—Cd—N5—C9 | 35.0 (2) | Cd—N5—C9—C10 | 168.70 (18) |
| Cl1—Cd—N5—C9 | 153.32 (16) | C13—N5—C9—C8 | 178.2 (2) |
| Cl2—Cd—N5—C9 | −94.40 (16) | Cd—N5—C9—C8 | −12.4 (3) |
| C5—N1—C1—C2 | 0.6 (4) | N4—C8—C9—N5 | −174.8 (2) |
| Cd—N1—C1—C2 | −179.7 (2) | N2—C8—C9—N5 | 3.3 (3) |
| N1—C1—C2—C3 | 0.1 (4) | N4—C8—C9—C10 | 4.0 (3) |
| C1—C2—C3—C4 | −0.6 (4) | N2—C8—C9—C10 | −177.8 (2) |
| C2—C3—C4—C5 | 0.4 (4) | N5—C9—C10—C11 | −0.3 (4) |
| C1—N1—C5—C4 | −0.9 (3) | C8—C9—C10—C11 | −179.1 (2) |
| Cd—N1—C5—C4 | 179.46 (18) | C9—C10—C11—C12 | 1.2 (4) |
| C1—N1—C5—C6 | −177.8 (2) | C10—C11—C12—C13 | −1.2 (4) |
| Cd—N1—C5—C6 | 2.5 (3) | C9—N5—C13—C12 | 0.6 (4) |
| C3—C4—C5—N1 | 0.3 (4) | Cd—N5—C13—C12 | −167.4 (2) |
| C3—C4—C5—C6 | 177.1 (2) | C11—C12—C13—N5 | 0.3 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N6—H6A···N3i | 0.91 | 2.31 | 3.183 (3) | 162 |
| N6—H6B···Cl2ii | 0.91 | 2.45 | 3.334 (2) | 165 |
| C12—H12A···Cl1iii | 0.97 | 2.76 | 3.671 (2) | 158 |
| C2—H2A···Cl1iv | 0.97 | 2.75 | 3.705 (3) | 166 |
| C11—H11A···Cl2v | 0.97 | 2.82 | 3.596 (2) | 138 |
Symmetry codes: (i) −x, −y+2, −z+1; (ii) −x, −y+1, −z+1; (iii) −x, −y, −z+2; (iv) −x+1, −y+1, −z+2; (v) x−1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: ZQ2094).
References
- Boubals, N., Drew, M. G. B., Hill, C., Hudson, M. J., Iveson, P. B., Madic, C., Russell, M. L. & Youngs, T. G. A. (2002). J. Chem. Soc. Dalton Trans. pp. 55–62.
- Bruker (2005). SMART, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Cao, M.-L., Hao, H.-G. & Ye, B.-H. (2009). Cryst. Growth Des. 9, 546–554.
- Cao, M.-L., Hao, H.-G., Zhang, W.-X. & Ye, B.-H. (2008). Inorg. Chem. 47, 8126–8133. [DOI] [PubMed]
- Case, F. H. & Koft, E. (1959). J. Am. Chem. Soc. 81, 905–906.
- Chi, Y.-N., Huang, K.-L., Cui, F.-Y., Xu, Y.-Q. & Hu, C.-W. (2006). Inorg. Chem. 45, 10605–10612. [DOI] [PubMed]
- Drew, M. G. B., Hudson, M. J., Iveson, P. B., Madic, C. & Russell, M. L. (2000). J. Chem. Soc. Dalton Trans. pp. 2711–2720.
- Medlycott, E. A., Udachin, K. A. & Hanan, G. S. (2007). Dalton Trans. pp. 430–438. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Zhou, X.-P., Li, D., Wu, T. & Zhang, X. (2006). Dalton Trans. pp. 2435–2443. [DOI] [PubMed]
- Zhou, X.-P., Li, D., Zheng, S.-L., Zhang, X. & Wu, T. (2006). Inorg. Chem. 45, 7119–7125. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811012517/zq2094sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811012517/zq2094Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



