Abstract
The functions of the cathepsin B-like proteases in liver flukes are unknown and analysis has been hindered by a lack of protein for study, since the protein is produced in small amounts by juvenile flukes. To circumvent this, we isolated and characterized a cDNA encoding the major secreted cathepsin B from Fasciola hepatica. The predicted preproprotein is 339 amino acids in length, with the mature protease predicted to be 254 amino acids long, and shows significant similarity to parasite and mammalian cathepsin B. Only one of the two conserved histidine residues required for cathepsin B exopeptidase activity is predicted to be present. Recombinant preproprotein was produced in yeast, and it was shown that the recombinant proprotein can undergo a degree of self-processing in vitro to the mature form, which is active against gelatin and synthetic peptide substrates. The recombinant protein is antigenic in vaccinated rats, and antibodies to the protein are detected early after infection of rats and sheep with F. hepatica. The kinetics of the response to cathepsin B and cathepsin L after infection of sheep and rats confirm the temporal expression of these proteins during the life cycle of the parasite.
The existence of secreted proteolytic enzymes in a wide variety of parasitic organisms implies that they are required for various mechanisms necessary for parasitism. The function of secreted proteases is primarily the digestion of host substrates, although some secreted proteases may be required for the activation of other parasite enzymes (e.g., see reference 6). Secreted proteases are prime targets for control of parasitic infections (39). Among the main candidate antigens for protection against Fasciola spp. (Fasciola hepatica is the most studied) are the proteases secreted from this parasite (33, 44). F. hepatica proteases of both secreted and somatic origin have been shown to have activity against a variety of natural substrates, such as gelatin (13), hemoglobin (21), collagen (3, 21, 41), immunoglobulin (4, 10, 21), globin, albumin (29), laminin, fibronectin (3), and CD4 on human and ovine T cells (36). The range of host substrates digested by these proteases implies a diverse range of action by the parasite, from feeding and facilitation of migration to immune suppression and/or evasion.
Secreted proteases released from adult Fasciola organisms are predominantly cathepsin L-like, based on substrate specificity and primary sequence similarity (see references 5 and 23 for summaries). Over 20 full sequences are available in the GenBank database for Fasciola cathepsin L-like enzymes expressed in adult parasites. These proteases form a monophyletic group among animals but can be further divided into four clades that correspond to observed and predicted enzymatic activities (23). However, there is also substantial proteolytic activity present in the newly excysted juvenile (NEJ) and immature stages of F. hepatica, and this has been demonstrated to be mainly cathepsin protease activity, as is the case for the adult fluke stage (8, 13, 21, 32, 49). Some of this activity is due to cathepsin L-like proteases, and two cDNA clones encoding cathepsin L from NEJ have been isolated (GenBank sequences AJ279091 and AJ279093). Cathepsin B-like activity has also been demonstrated for F. hepatica. Cathepsin B gene fragments have been amplified from adult F. hepatica RNA (18), and a protein with N-terminal sequence similarity to cathepsin B has been identified in somatic extracts of NEJ (45). Creaney et al. (12) localized cathepsin B to the gut of NEJ. Wilson et al. (49) showed by biochemical studies that the major protease activity in the excretory-secretory (ES) material of NEJ of F. hepatica was cathepsin B, and they isolated a cDNA clone encoding mature cathepsin B from F. hepatica. For this report we have cloned, sequenced, and characterized a full-length cDNA clone corresponding to this enzyme.
The functions of cathepsin B-like proteases in liver flukes are unknown. For F. hepatica, the observation that the cathepsin B (FhCatB) protease is released by the early, migratory stage of the fluke up to 5 weeks postinfection suggests that this protease is involved in the excystment and/or migration of the juvenile fluke into host tissues (49). Two pieces of evidence support the notion that FhCatB is important for fluke virulence. Expression of FhCatB is down-regulated in radiation-attenuated juvenile flukes, suggesting that attenuation may be associated with loss of FhCatB activity (12). It has also been demonstrated that incubation of juvenile flukes with a cathepsin B inhibitor in vitro can kill the parasites within 24 h (S. Beckham, T. Spithill, D. Piedrafita, J. McKerrow, and R. Pike, unpublished data). This lethal effect is most probably due to an inability to feed or to undergo development, as effects upon migration or immune evasion are not relevant in vitro. Therefore, FhCatB is a potential vaccine candidate and/or drug target against fasciolosis, since neutralization of its activity may reduce the ability of the juvenile fluke to establish infection and hence eliminate the disease before damage is done to the host liver by fluke migration. The present study demonstrates that FhCatB expressed as a recombinant protein in Saccharomyces cerevisiae or from a DNA vaccine can induce an immune response in rats and that FhCatB is immunogenic after infection of rats or sheep with F. hepatica. The implications of these findings for the vaccine potential of FhCatB are discussed.
MATERIALS AND METHODS
Metacercariae.
F. hepatica metacercariae were purchased from Baldwin Aquatics, Monmouth, Oregon, or from Compton Paddock Laboratories, Compton, United Kingdom.
Isolation of a full-length cDNA clone.
The clones constructed for this study are listed in Table 1. mRNA was isolated from 5-week-old parasites recovered from infected sheep, and cDNA extended at the 5′ end was prepared by rapid amplification of cDNA ends (RACE)-PCR (Clontech Laboratories, Inc.), as described by Reed et al. (37). This procedure positions an adapter primer at the 5′ end of each of the extended cDNAs. An aliquot of cDNA was subjected to PCR with the adapter-specific primer and a primer derived from the known sequence of the mature cathepsin B coding region (49). The resultant PCR product was cloned into pBluescript to form pCatB-5′. A full-length clone was then constructed by digesting pCatB-5′ and the clone encoding the 3′ portion of the coding region (pTPZA4) (49) with XhoII and BamHI. The resulting fragment was isolated from pTPZA4 and inserted into pCatB-5′ to create pBS-CatB, which encodes the entire coding region.
TABLE 1.
List of constructs used for this study
| Clone | Vector | Coding region (product) | Presence of glycosylation sites | Function |
|---|---|---|---|---|
| pBS-CatB | pBluescript | Prepromature | Yes | Holding vector |
| pVRCatB | pVR1012 | Prepromature | Yes | DNA vaccine |
| pFLAG/CatB-WT | pYEpFLAG | Promature | Yes | Template for mutagenesis |
| pFCatB | pYEpFLAG | Promature | No | Expression in yeast |
| pFCatB(pro) | pYEpFLAG | Prodomain only | No | Expression in yeast |
Tree construction and analysis.
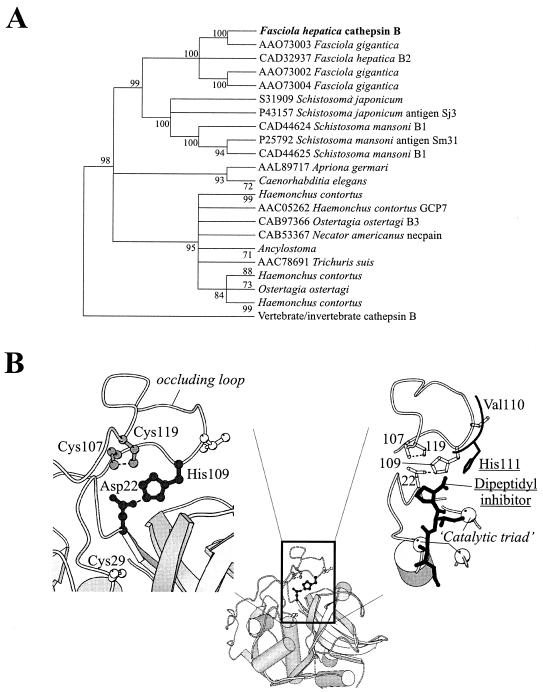
A PSI-BLAST (1) search of the National Center for Biotechnology Information nonredundant protein database using the translated sequence of F. hepatica cathepsin B identified 727 putative homologues (E < 10−6). Based on a histogram of the distribution of pairwise identities (which revealed two distinct sequence classes), 110 sequences sharing >35% identity were retained. These were aligned with CLUSTALW (19) guided by the secondary structure profile of human cathepsin B (protein database accession no. 1gmy [17]). The MOLPHY (J. Adachi and M. Hasegawa, Institute of Statistical Mathematics, Tokyo, Japan) and PHYLIP (J. Felsenstein, University of Washington, Seattle) packages were used to generate 1,000 bootstrap neighbor-joining trees, and a maximally informative reduced partition (48) was selected from those generated by use of the partition cluster method (22); this partition dictated the 71 sequences in the final reported majority bootstrap tree (see Fig. 2A) constructed by use of MEGA software (26). Branches with <80% support were collapsed and used as input into the aaml program of the PAML3.1 package (50) for maximum likelihood reconstruction of ancestral sequences (applying the empirical JTT (Jones, Taylor, Thornton) substitution matrix).
FIG. 2.
Evolutionary analysis and molecular modeling of FhCatB. (A) A bootstrap neighbor-joining tree (implemented by use of MEGA software [35]) is shown; the majority-rule bootstrap maximum parsimony tree was in agreement with it but was not as well resolved. Branches for which there was <80% support were collapsed. (B) A molecular model of F. hepatica cathepsin B, constructed as explained in the text, is presented, highlighting the constraints that are predicted to act within its occluding loop. (Left) The His109-Asp22 salt bridge (black ball-and-stick model) and the disulfide bond between Cys107 and Cys119 (gray ball-and-stick model) are shown; the active site Cys29 (white ball-and-stick model) also appears as a reference. (Right) For comparative purposes, the His111 residue of human cathepsin B (replaced by Val110 in the F. hepatica enzyme) and the dipeptidyl inhibitor with which it interacts are shown in black. In F. hepatica cathepsin B, the histidine-mediated stabilization of the substrate's carboxy terminus is predicted to be absent. Some elements of secondary structure have been stripped away and the positions of the catalytic residues are shown (white spheres). This figure was prepared with the use of MOLSCRIPT.
Construction of homology model.
A molecular model of F. hepatica cathepsin B, built by the MODELLER program (40) and based on human cathepsin B (accession no. 1gmy [17]), was used to predict the role of the remaining His109 of the consensus occluding-loop His pair. The quality of the model was determined by use of WHATCHECK (20); the stereochemistry was found to be acceptable and all buried polar side chains could be accounted for. CHARMm energy minimization was conducted by use of the QUANTA package (Accelrys Inc., San Diego, Calif.), with distance constraints promoting the following alternative interactions: a salt bridge between His109 and Asp22 or a salt bridge between His109 and the C terminus of a dipeptide inhibitor (superimposed from accession no. 1csb [46]). In general, residues were constrained to their initial positions, with the exception of all of those in the occluding loop and their close neighbors. The disulfide bond between Cys107 and Cys119 was also enforced.
Cloning and mutagenesis of FhCatB cDNA in YEpFLAG.
Recombinant FhCatB expression was performed in an analogous fashion to that previously described for F. hepatica cathepsin L5 (43). Briefly, the FhCatB pro-mature coding region was amplified by using pBS-CatB as a template for PCR with oligonucleotides 5′GGAAACCAAACCATAAACCGC and 5′CGCGGATCCTCAATGGTGATGGTGATGGTGAAGACGTCACTTCAACTGG, which also incorporated the coding sequence for a C-terminal hexahistidine tag. The product was digested with BamHI. YEpFLAG was digested with EcoRI and the overhang was rendered blunt by use of mung bean nuclease, and subsequently the plasmid was digested with BamHI. The amplified FhCatB coding region was inserted into this vector, creating pFLAG/CatB-WT.
Mutagenesis of pFLAG/CatB-WT was performed to remove potential N-linked glycosylation sites, Asn-X-Ser/Thr, from the predicted protein prior to expression in S. cerevisiae. There are three such sites in the coding region of cathepsin B, at codons 180, 223, and 234. Examination of a molecular model of FhCatB indicated that the least disruptive substitutions to eliminate these sites were changing codon 182 from specifying Thr to specifying Ala and changing the remaining two Asn codons (223 and 234) to Gln. In vitro mutagenesis was performed with the following oligonucleotides (nucleotide substitutions are underlined): Mut1 (T182A), 5′GGGAAAATCGAGCTGGTTGTCAGC; and Mut2 (N223Q and N234Q), 5′GTTGTAAGATGATTGTCCGTAAAACTTATCTTGCTCGTACGTTTTTTGATATCCAGTTTGG.
A PCR was performed with Mut1 and Mut2, with pFLAG/CatB-WT as the template. The resultant 188-bp product was used as a primer with 5′AGGGGTACCTTTGGATAAAAGAG (this primer is complementary to the pFLAG plasmid sequence encompassing the unique KpnI site) in a second-round PCR. The final product was digested with KpnI and BamHI and inserted into pFLAG that had been digested with the same enzymes. Throughout this report, the resultant clone will be referred to as pFCatB.
Expression of FhCatB in a DNA vaccine vector.
The coding region from pBS-CatB was excised by digestion with NotI and BamHI and cloned into pVR1012 (VICAL Inc.) digested with the same enzymes. The resultant clone was termed pVRCatB. Note that the entire pre-pro-mature coding region is included, and potential N-linked glycosylation sites remain encoded by this construct. Expression of FhCatB from the DNA vaccines was verified by transfecting plasmids into COS7 cells by mediation with Lipofectamine (Gibco BRL) as previously described (42). Briefly, 2 × 105 cells were transfected with 1 μg of plasmid DNA, and the culture was continued for 72 h in RPMI medium to allow secretion of FhCatB. Medium was analyzed for the presence of FhCatB by Western blotting.
Construction of pFCatB(pro).
The proregion of F. hepatica CatB was subcloned into YEpFLAG for expression in yeast as follows. Oligonucleotides 5′ GCGGATCCGAATTCAAACCAAACCATAAACCG and 5′ GCGGATCCGCGGCCGCTAATGATGATGATGATGATGATCGTTTTTCGATATGTC were used in a PCR to amplify the proregion of FhCatB from the template pFCatB, simultaneously adding a C-terminal hexahistidine tag coding region. The resulting PCR product was inserted into YEpFLAG as an EcoRI and NotI fragment. The resultant clone is referred to as pFCatB(pro).
Preparation of recombinant proteins from yeast.
Recombinant yeast BJ3505/pFCatB was prepared by transformation of S. cerevisiae strain BJ3505 (mata ura3-52 trp1-Δ101 lys2-208 gal2 can1 pep4::HIS3 prbΔ1.6R) (Kodak, USA) by facilitation with PEG-Bicine (24). Recombinant yeast cells were selected by culture in minimal medium containing uracil and lysine. After 5 days, positive colonies were picked and replated onto the same medium prior to growth in liquid medium. A 10-ml starter culture of BJ3505/pFCatB yeast in medium containing 0.67% Bacto yeast nitrogen base without amino acids, 2% dextrose, 20 μg of uracil per liter, and 30 μg of l-lysine-HCl per liter was grown at 28°C with shaking for 48 h. This culture was diluted 1:20 into medium containing 1% dextrose, 3% glycerol, 1% yeast extract, 8% peptone, and 20 mM CaCl2 and grown for 48 h for expression of recombinant protein.
Yeast culture supernatant containing secreted FhCatB was dialyzed against four changes of buffer A (25 mM NaH2PO4, 10 mM imidazole, 250 mM NaCl, pH 7.6), and recombinant protein was purified by incubation with Ni-nitrilotriacetic acid (NTA) (Qiagen) in batch format. After washing the resin with 10 bed volumes of buffer A, FhCatB was eluted from Ni-NTA with buffer A containing 250 mM imidazole. The purified enzyme was stored at −20°C in 50% glycerol. Preparation of the recombinant proregion of FhCatB protein expressed from pFCatB(pro) was carried out essentially as described above for pFCatB. F. hepatica CatL5 was prepared from the recombinant yeast culture supernatant as described previously (43).
Preparation of F. hepatica ES material.
Adult liver flukes recovered from the livers of infected animals were washed and briefly incubated in phosphate-buffered saline at 37°C to remove blood from their guts. They were then incubated in RPMI 1640 medium (Sigma) at 50 flukes per 100 ml at 37°C for 2 h. The supernatant was collected as the adult ES material. Juvenile ES material was collected similarly, as described elsewhere (49).
Protein analysis and manipulations.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out in Tris-glycine gels. Enzyme activity was routinely assayed at 37°C with the substrates Z-Phe-Arg-NHMec, tosyl-Gly-Pro-ArgNHMec, and Z-Arg-Arg-NHMec (Sigma) by the method described by Barrett and Kirschke (2) in buffer B containing 0.1 M sodium acetate, 1 mM EDTA, 200 mM NaCl, 10 mM dithiothreitol, and 50 μg of dextran sulfate per ml (pH 4.5). The amount of protein in an enzyme preparation was estimated by the Bradford method using the Bio-Rad protein dye reagent concentrate, with bovine serum albumin as the standard. Gelatin substrate gel analysis was performed essentially as described previously (47).
Autoactivation of FhCatB (proprotein) was performed by incubating the purified enzyme in buffer B at 37°C for up to 3 h as indicated. Pepsin digestion was carried out by incubation of the purified protein (100 μg/ml) in a buffer containing 250 mM sodium acetate, pH 4.5 or 3, at 37°C for 30 min in the presence of 0.17 or 0.3 μg, respectively, of pepsin per ml. Analysis of the N-terminal amino acid sequences of the digested protein was carried out by Edman degradation in an Advanced Biosystem gas-phase Sequenator or by mass spectrometry analysis of the 32- and 28-kDa products, respectively.
Immunizations, immunoassays, and infections.
For vaccination of rats with FhCatB, four groups of eight Sprague-Dawley male rats (9 weeks old) were injected twice (3 weeks apart) intramuscularly in the thigh. In the vaccinated groups, each animal was given 20 μg of FhCatB in phosphate-buffered saline each time, in the presence of 100 μg of Quil A or Freund's adjuvant (complete adjuvant for the first injection and incomplete adjuvant for the second injection), while the control groups received adjuvant alone. A total volume of 500 μl per animal was injected each time. Blood was taken from the tail vein before and 3 weeks after each injection. Sera were collected and tested for reactivity to FhCatB.
A group of four Sprague-Dawley male rats (9 weeks old) were infected by oral gavage with metacercariae (50 per animal) suspended in 0.4% carboxymethyl cellulose. Blood samples were taken from the tail every 2 to 3 weeks up to 10 weeks postinfection. A group of eight Merino wethers were infected with 250 metacercariae by the same method. Blood samples were taken from the jugular vein. Sera were tested for immunoreactivity to FhCatB by enzyme-linked immunosorbent assay (ELISA) and Western blotting.
For ELISA, microtiter plates were coated with recombinant proteins at 2 to 4 μg/ml in carbonate buffer, pH 9.6. ELISA analysis on sera from individual animals was performed as previously described (42). Titers were defined as the highest dilution yielding an optical density at 450 nm of 0.2. For Western blotting, proteins were separated by SDS-PAGE (12%) and transferred onto nitrocellulose in 3-(cyclohexylamino)-1-propanesulfonic acid (CAPS) buffer containing 10 mM CAPS (pH 11) and 15% methanol at 100 V for 1 h at 4°C. The nitrocellulose was blocked with 5% skim milk-TBS (25 mM Tris [pH 7.4], 137 mM NaCl, 2.7 mM KCl) at 42°C for 1 h. Sera from vaccinated animals and infected animals were used at 1/500 and 1/200 dilutions, respectively, in 5% skim milk-TBS and were incubated at room temperature (RT) for 2 h with mixing, followed by three washes in TBS. Secondary antibodies (rabbit anti-rat [Sigma] or donkey anti-sheep [Silenus]) conjugated to horseradish peroxidase were diluted 1/1,000 and incubated at RT for 1 h with mixing, followed by five washes in TBS. The bound antibody was visualized by the enhanced chemiluminescence (ECL; Amersham) detection method according to the manufacturer's specifications.
RESULTS
Characterization of the full-length FhCatB cDNA clone and predicted protein.
The FhCatB clone created by the fusion of CatB-5′ and pTPZa2 is 1,116 nucleotides long. Within this clone is a reading frame encoding a protein of 339 amino acids, of which 15 are predicted to comprise the signal peptide and 70 to comprise the propeptide sequence (predicted to begin at K16). The mature protein is predicted to be 254 amino acids long, beginning at L86 of the complete sequence specified by the cDNA clone (see below). The predicted peptide has significant similarity with parasite and mammalian cathepsin B, as shown in Fig. 1. An alignment is illustrated with a second F. hepatica cathepsin L (CAD32973) and one of the three recently identified Fasciola gigantica cDNA clones encoding cathepsin B-like enzymes (AA073002). Intriguingly, another F. gigantica cathepsin B has only three amino acid differences from the F. hepatica protein reported here (AAO73003). The significance of this is not clear, although it should be noted that both of the cDNAs were isolated from NEJ mRNA. The other two F. gigantica cDNAs, which have 64 to 65% identity to FhCatB, were isolated from adult and metacercaria mRNA, respectively.
FIG. 1.
Alignment of cathepsin B-like enzymes. Shown are three Fasciola CatB sequences and sequences for S. mansoni, O. ostertagia, and three mammalian CatBs. Completely conserved residues are shaded in black and highly conserved residues are shown in gray. The two arrows position the predicted cleavage sites of the pre- and pro- sequences in F. hepatica CatB. The region delineated by the black line is the occluding loop, and asterisks identify the two essential histidine residues within this loop. The active site cysteine residue is shown by the filled triangle.
Primary sequence identity with cathepsin B from species other than liver flukes ranges from 39% with Ostertagia ostertagia to 50% with Schistosoma mansoni. Identity with the human protein is 46%. As expected, conservation occurs less in the preproregions of the predicted proteins than in the mature region. A bootstrap neighbor-joining tree data set, analyzed by use of the partition cluster method (22), revealed that the Fasciola cathepsin B sequence presented here is more closely related to cathepsin B enzymes from nematodes and trematodes than to mammalian homologues (98% confidence). This precludes the possibility of lateral gene transfer between the parasite and its host.
A reduced joining-joining majority-rule bootstrap tree is shown in Fig. 2A. The Fasciola sequences form a clade distinct from other organisms, indicating that they share a single common ancestor with the related Schistosoma spp. Interspersion of F. hepatica and F. gigantica proteins indicates that at least one gene duplication occurred prior to the speciation that gave rise to these two organisms around 19 million years ago (23).
The FhCatB predicted amino acid sequence shares several important features conserved throughout cathepsin B-like proteins. These are highlighted in Fig. 1. The conserved cysteine residues and the amino acids comprising the occluding loop (required for the exopeptidase activity of cathepsin B) are present. However, of the two key His residues that are required for exopeptidase activity (110 and 111 in the human sequence [25]), only one is present in the Fasciola proteins (amino acid 109 in the mature protein, corresponding to amino acid 110 in mature human cathepsin B). In each of the remaining species represented in Fig. 1, the histidine doublet is present. In human cathepsin B, His110 has been shown to mediate binding via a salt bridge to Asp22, an interaction which plays a vital role in stabilizing the position of the occluding loop and also in the exopeptidase activity of the enzyme (25). The corresponding Asp residue is present in FhCatB. His111 is thought to play a role in stabilizing the carboxyl group of substrates and thus contributes to, but is not vital for, exopeptidase activity.
The evolutionary loss of the second His residue was examined. A majority-rule consensus tree, with poorly supported (<80%) branches collapsed, was used to reconstruct ancestral sequences. The analysis revealed that the occluding loop His111 (mammalian numbering) was most probably present at the split between Schistosoma spp. and Fasciola spp. (L = 0.95) but was subsequently lost prior to the gene duplication event giving rise to the present-day Fasciola cathepsin B sequences.
Homology model building in conjunction with energy minimization was used to explore the alternative possibilities for interactions with the remaining histidine residue: (i) a salt bridge exists between His109 and Asp22, as seen with mammalian cathepsin B structures; or (ii) a salt bridge exists between His109 and the C terminus of a dipeptide inhibitor (transferred from accession no. 1csb [46]), in which case His109 will have taken up the role of the lost His111 (mammalian numbering) residue.
The minimization trials predicted that His109 would be able to form an interaction with Asp22 without requiring the violation of main chain stereochemistry (Fig. 2B). In contrast, an interaction between His109 and the C terminus of the inhibitor required an unacceptable perturbation of the protein backbone between Cys107 and His110 due to the constraint imposed by the disulfide. Therefore, from the model it appears more likely that the remaining His109 residue is involved in stabilization of the occluding loop rather than promotion of exopeptidase activity.
Expression of FhCatB in yeast.
A variant form of the proFhCatB coding sequence, carrying mutations Thr97Ala (Thr182 in the full sequence), Asn138Gln (Asn223 in the full sequence), and Asn149Gln (Asn149 in the full sequence) (to remove the putative glycosylation sites [Asn-X-Ser/Thr]) and a hexahistidine tag at the C terminus, was cloned into the yeast expression vector YEpFLAG-1. The recombinant plasmid (pFCatB) was transformed into S. cerevisiae BJ3505 for protein expression. The resultant secreted protein will contain an N-terminal FLAG epitope, the pro-mature region of FhCatB, and a C-terminal hexahistidine tag. The presence of FhCatB protein in the yeast culture supernatant was monitored by use of an anti-FLAG monoclonal antibody, M2 (Sigma). Conditions for the optimal expression of protein were determined by dot blot assays using the M2 monoclonal antibody.
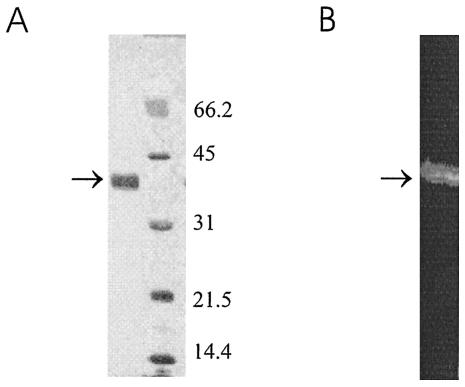
For purification of the recombinant protein, Ni-NTA resin was used to capture the hexahistidine-tagged protein in the culture supernatant in a batch format, with an average yield of about 2 mg/liter. The purified protein was analyzed by SDS-PAGE followed by silver staining, which revealed a single predominant species with a relative mobility of 38 kDa (Fig. 3A); this result is consistent with the calculated molecular mass of a secreted protein containing proFhCatB with an N-terminal FLAG epitope and a C-terminal hexahistidine tag (38.641 kDa). The identity of the 38-kDa protein was confirmed by N-terminal sequencing by Edman degradation (data not shown), showing that the N-terminal residues correspond with those of the FLAG epitope. Purified FhCatB was shown to be enzymatically active in a gelatin substrate gel (Fig. 3B), and optimal activity was found after incubation at pH 4.5 in the presence of dextran sulfate (not shown). Further studies have shown that, like native FhCatB isolated from juvenile ES material, the purified recombinant protein is active as judged by cleavage of fluorescent synthetic substrates in the order Z-Phe-Arg-NHMec > Tos-Gly-Pro-ArgNHMec > Z-Arg-Arg-NHMec (data not shown).
FIG. 3.
Characterization of recombinant proCat. Recombinant proFhCatB was purified from yeast culture supernatant by immobilized metal affinity chromatography using Ni-NTA. The purified protein (arrow) was analyzed by SDS-PAGE (12%) under reducing conditions, followed by silver staining (A) and a gelatin substrate gel (12% gel under nonreducing conditions) (B). Molecular sizes are shown to the right of the gel in panel A.
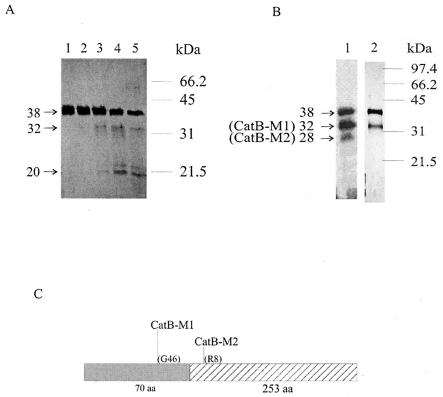
That the purified proFhCatB is active suggests that the recombinant protein undergoes self-processing upon incubation at pH 4.5. To test this assumption, samples were treated under the same conditions as for the gelatin gel digestion for up to 3 h and analyzed by SDS-PAGE. As shown in Fig. 4A, small amounts of low-molecular-mass species (30 to 32 kDa) were generated after incubation, suggesting that self-processing of proFhCatB did indeed take place during incubation. Prolonged incubation of up to 3 h (lane 5) failed to increase the production of these low-molecular-mass species, while the amount of the starting proFhCatB diminished, suggesting both that the processing is not very efficient and that the processed products may not be stable.
FIG. 4.
Processing of proFhCatB. (A) Purified recombinant proFhCatB-H6 (38 kDa) was incubated at pH 4.5 for 0, 30, 60, 120, and 180 min (lanes 1 to 5, respectively). Samples were analyzed by SDS-12% PAGE under denaturing conditions. A number of processed products, including 32- and 20-kDa products, are indicated. (B) proFhCatB was also treated with pepsin at pHs 4.5 and 3 (lanes 1 and 2, respectively) and analyzed as for panel A; two major products were obtained, of 32 kDa (FhCatB-M1) and 28 kDa (FhCatB-M2). (C) Results of N-terminal sequence analysis of these two species. Filled box, proregion; hatched box, mature FhCatB. Also marked are the first amino acids of the processed products and their positions (relative to the N termini of the pro- and mature proteins, respectively).
A second approach to generate mature FhCatB from recombinant proFhCatB utilized pepsin digestion. This approach has been successfully applied to activate recombinant human proCatB and S. mansoni proCatB expressed in yeast (28, 34, 38). When incubation was carried out at pH 4.5 with 1.7 μg of pepsin per ml at 37°C for 30 min, two digested products, of 32 kDa (CatB-M1) and 28 kDa (CatB-M2), were observed (Fig. 4B, lane 1). The N-terminal sequences of the 32- and 28-kDa products were determined and revealed that neither of these two products were processed correctly to the full-length mature FhCatB, as shown in Fig. 4C. The N-terminal sequence of the 32-kDa CatB-M1 was resolved by Edman degradation; it contains the mature sequence plus an additional 25 amino acids from the prosequence beginning at the sequence GALS (the calculated molecular mass is 32.38 kDa). The N-terminal sequence of the 28-kDa CatB-M2 was resolved by mass spectrometry following trypsin digestion, and it contains the mature sequence without the first seven amino acids (i.e., beginning with RSQW; the calculated molecular mass is 28.08 kDa). Subsequent efforts to generate complete digestion of proFhCatB into CatB-M1 and CatB-M2 with pepsin by prolonged incubations or higher concentrations of pepsin were not successful (data not shown). This suggests that the proFhCatB preparation consists of a polypeptide mixture of different conformations, such that only a proportion of the protein could be cleaved by pepsin under the conditions studied.
Immunoreactivity of proFhCatB.
One of the major advantages of the availability of recombinant FhCatB is the ability to perform immunization and diagnostic analyses. Secreted cathepsin B from NEJ or immature liver flukes is available in only small quantities, making such analyses difficult. As a prelude to vaccine trials in animals, immunoreactivity experiments after recombinant FhCatB immunization were performed. We have previously shown that vaccination with juvenile ES material (which contains a high proportion of FhCatB) induces a low-level antibody response in sheep when used with Freund's adjuvant (L. Wilson and T. Spithill, unpublished observations), suggesting that FhCatB might not induce a potent humoral response.
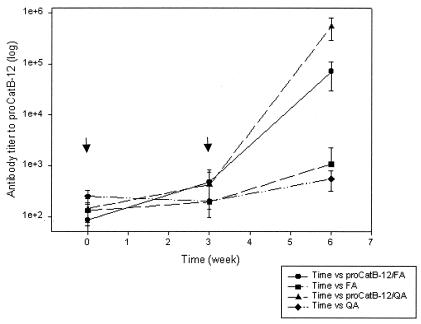
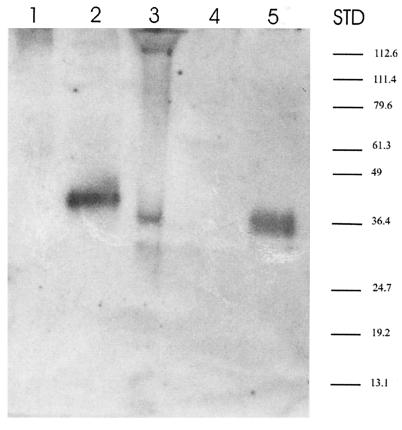
Groups of eight rats were immunized with 20 μg of purified proFhCatB protein formulated in either Freund's or Quil A adjuvant. Two injections were given 3 weeks apart, and sera were taken before and 3 weeks after each injection. The level of the serum antibody response to FhCatB was tested by ELISA, and the results are shown in Fig. 5. At week 9 (3 weeks after the second injection), the reciprocal antibody titers of the immunized animals were on the order of 106 and 105 in the Quil A and Freund's adjuvant groups, respectively. Further immunization experiments carried out with other species, including rabbits and mice, also induced high antibody titers (data not shown). Using Western blotting, we tested the reactivities of rat antisera with FhCatB from various sources (Fig. 6). We found that rat antiserum reacts with the recombinant FhCatB used for immunization (lane 5) as well as with FhCatB in the supernatant of transfected COS7 cells (lane 2; ∼41 kDa), whereas no signal was detected for the vector-only control (lane 1), indicating that this signal is specific to FhCatB expression. The COS7-expressed proFhCatB migrates slightly slower than the recombinant proFhCatB (lane 5), which could be a result of glycosylation of the unmodified gene product, as this clone encodes potential glycosylation sites (Table 1). Lanes 3 and 4 contain ES material from juvenile and adult F. hepatica, respectively. A positive signal is detected from juvenile ES material but not from adult ES material, confirming our previous observation that secreted FhCatB is a juvenile-specific secreted protease (49). In the case of juvenile ES material, a 36-kDa species and a 31-kDa species were detectable. It is probable that the 36-kDa band represents FhCatB with the proregion and the 31-kDa band represents mature FhCatB.
FIG. 5.
Humoral response to proFhCatB following vaccination of rats. Animals (n = 8) were injected intramuscularly with 20 μg of proFhCatB protein in the presence of Freund's adjuvant (FA) or Quil A (QA) twice as indicated by the arrows. Blood was taken at 3 weeks postinjection and tested individually for anti-proFhCatB antibody by ELISA using plates coated with 2 μg of proFhCatB per ml.
FIG. 6.
Western blot analysis of the immunoreactivity of native and recombinant proFhCatB. Sera from rats immunized with proFhCatB were tested for activity on a Western blot using FhCatB expressed in different systems. Lanes 1 and 2, supernatants of COS7 cells transfected with pVR1012 vector alone and pVRCatB, respectively; lanes 3 and 4, ES material from F. hepatica juveniles and adults, respectively; lane 5, recombinant proFhCatB. Samples were separated by SDS-PAGE (12%) under nonreducing conditions and then transferred to nitrocellulose. STD, standard molecular size marker.
Kinetics of the humoral response to FhCatB in animals during liver fluke infection.
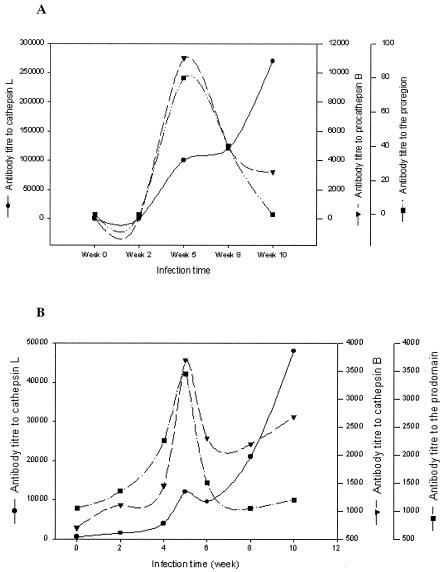
To determine if antibody to FhCatB is induced in animals infected with liver flukes and, if so, the time during which the antibody to FhCatB is present, we studied the time course of humoral responses to FhCatB in infected animals. Responses to FhCatL5 were also analyzed for comparison. A group of four Sprague-Dawley rats were infected with 50 metacercariae, and sera were collected at 2- to 3-week intervals up to 10 weeks postinfection. Also studied was a group of eight Merino sheep infected with 250 metacercariae, with sera collected at 2-week intervals up to 10 weeks postinfection. The immune responses were analyzed by ELISA, with pooled sera assayed for antibodies to FhCatL5, FhCatB, and FhCatB(pro) (Fig. 7).
FIG. 7.
Humoral response to FhCatB, FhCatL, and proregion of FhCatB following fluke infection in rats and sheep. Pools of rat (n = 4) (A) and sheep (n = 8) (B) sera collected from 0 to 10 weeks postinfection were tested for reactivity to cathepsin L, proFhCatB, and the proregion of CatB by ELISA, using plates coated with antigens at 4 μg/ml. The titers were plotted against time in weeks postinfection. Note that antibody titers to different antigens are illustrated using different scales.
In infected rats, antibody to FhCatB was first detected at 5 weeks postinfection and decreased thereafter (Fig. 7A). The highest titer detected was about 104 at week 5. Interestingly, a similar pattern of antibody responses specific for the FhCatB proregion was also detected, although the titer was lower (i.e., 100-fold less than that against complete pro-mature FhCatB). FhCatL antibodies, which are a hallmark of liver fluke infection in ruminants (11), were also measured. These appeared at 5 weeks postinfection, and the levels continued to increase throughout the 10-week period, reaching a titer of >105 by the 10th week.
In infected sheep (Fig. 7B), the patterns of antibody responses to the three antigens were qualitatively similar to the rat responses, but with some important differences. In contrast to the titers in rats, similar titers against FhCatB and FhCatB(pro) were observed which peaked at about 5 weeks postinfection (titer of 3,500 against both antigens), whereas in rats the response to the proregion was poor. This indicates that the proregion is more immunogenic in sheep than in rats, relative to the mature region. The antibody titers to FhCatB dropped after the 5th week postinfection and began to rise again 8 to 10 weeks after infection. On the other hand, FhCatL antibody appeared at 2 to 4 weeks postinfection, increasing steadily throughout the 10-week period to reach a titer of about 5 × 104 by week 10 postinfection.
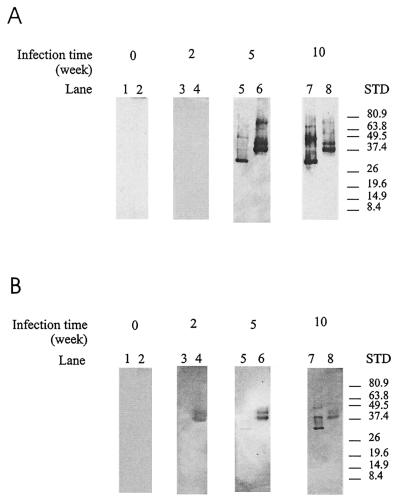
Western blot analysis was used to confirm the ELISA results. Recombinant FhCatL5 and FhCatB were transferred to nitrocellulose after SDS-PAGE and were probed with pooled rat and sheep sera collected at weeks 0, 2, 5, and 10 postinfection. In rats (Fig. 8A), both FhCatB (lanes 2, 4, 6, and 8) and FhCatL5 (lanes 1, 3, 5, and 7) were recognized by antibodies in sera collected at 5 and 10 weeks postinfection whereas no specific recognition of either antigen was observed with week 2 sera. These results are in agreement with the ELISA data (Fig. 6A) and demonstrate that the signal detected by the ELISA method was indeed antigen specific. In contrast to the results with rats, FhCatB was detectable by the sheep sera at 2 weeks postinfection (Fig. 8B, lane 4), suggesting that the sheep host was more responsive to the fluke FhCatB. FhCatB was also recognized by sheep antibody collected at 5 to 10 weeks postinfection (Fig. 8B). Reactivity with FhCatL5 was observed at weeks 5 (low-level response) and 10 (Fig. 8B, lanes 5 and 7).
FIG. 8.
Western blot analysis of antibody responses to FhCatB and FhCatL following fluke infection in rats and sheep. One microgram of CatL (lanes 1, 3, 5, and 7) and 5 μg of proFhCatB (lanes 2, 4, 6, and 8) were separated by SDS-PAGE (12%) under nonreducing conditions and transferred to nitrocellulose. Sera were collected from rats (n = 4) (A) and sheep (n = 8) (B) after liver fluke infection, pooled, and tested for antibodies to FhCatL and proFhCatB. Sera tested were taken at weeks 0, 2, 5, and 10 postinfection (lanes 1 and 2, 3 and 4, 5 and 6, and 7 and 8, respectively). STD, standard molecular size marker.
DISCUSSION
We have expressed and characterized a recombinant prepro-cathepsin B that corresponds to the major cathepsin B protein secreted by juvenile flukes (49). Wilson et al. identified two N-terminal sequences (LPES and VPAS) for native cathepsin B in ES material from juvenile parasites, with LPES being the more abundant sequence, suggesting that juvenile flukes secrete at least two cathepsin B isoforms. In other studies, the N terminus of a somatic NEJ cathepsin B protein as well as a secreted NEJ cathepsin B was identified as DLPES (45; R. Law, unpublished data), which from the sequence presented here would equate to a mature protease of 255 amino acids. The biochemistry of the maturation of cathepsin B in F. hepatica remains to be elucidated. The process of maturation of the cathepsin B family of enzymes is complex, with pH-dependent autoactivation (30) proposed to be the primary mechanism of activation of mammalian (e.g., human) CatB enzymes. It is also possible that maturation of the secreted FhCatB is facilitated by other proteases. In particular, asparaginyl endopeptidases (legumains) have been identified in the ES material of S. mansoni (6, 14). S. mansoni legumain demonstrates absolute specificity for asparagine at P1 and can trans-process recombinant S. mansoni cathepsin B (31). In Fasciola cathepsin B, the sequence around the maturation site is ISKN↓DLPES, which may be a suitable target for a putative Fasciola legumain-like enzyme that is yet to be reported.
Evidence to date suggests that F. hepatica expresses a number of cathepsin B proteins, as is the case for cathepsin L-like enzymes (16, 23, 43). In a PCR-based study using material from adult parasites, Heussler and Dobbelaere (18) isolated a series of transcripts encoding cathepsin-like proteases from F. hepatica, including two cathepsin B-like enzymes showing 33 and 60% identity with the FhCatB protein sequence described here. These partial cDNA clones were derived from RNA of adult flukes, and Northern blot analysis shows very low levels of expression of these genes. Whether functional cathepsin B-like proteins are expressed in adult flukes from the genes corresponding to these partial transcripts is not known. Recently, a second CatB cDNA sequence for juvenile F. hepatica was deposited in GenBank (CAD32937), as were three cDNAs from F. gigantica (AAO73002 to -4), isolated from adult, NEJ, and metacercariae, respectively. The possibility of redundancy of cathepsin B in Fasciola mirrors that found in many other nematodes and trematodes, including Caenorhabditis elegans (27) and Schistosoma (7), and the functions of these various cathepsin B enzymes remain to be determined.
Cathepsin B enzymes possess a unique feature in their active sites referred to as the occluding loop. This structure partially occludes the active site of the enzyme, restricting access of extended substrates and inhibitors such as the cystatins. Cathepsin B enzymes have been shown to display dipeptidyl peptidase or exopeptidase activity, which is dependent on the maintenance of bonding patterns mediated by two His residues found at positions 110 and 111 (mammalian numbering, corresponding to positions 109 and 110 in the mature Fasciola protein) in most cathepsin B sequences. Sequence comparisons of FhCatB with other cathepsin B molecules suggest that FhCatB is probably missing the His111 residue. Figure 2 indicates that the remaining His residue forms a salt bridge to Asp22, which is vital for the maintenance of the occluding structure, rather than stabilizing the carboxyl group of the substrate. The structure of the occluding loop is likely to be intact, however, implying that access to extended substrates and inhibitors would be impaired, as found for other cathepsin B enzymes investigated to date. The presence of the vital salt bridge suggests that FhCatB may still display exopeptidase activity, although the level of this activity might be somewhat impaired in the absence of the second His residue. When viewed in the context of the gene tree (Fig. 2A), the consequent predicted decrease in exopeptidase activity most likely developed at some time following the divergence of liver flukes from schistosomes, but prior to the speciation that gave rise to F. hepatica and F. gigantica lineages.
The kinetics of the humoral response to the FhCatB protein after infection of both rats and sheep confirm that this secreted protease is expressed by juvenile parasites during the establishment of infection and show that the protease is antigenic within 2 to 5 weeks of infection in sheep and within 5 weeks in rats. FhCatB may therefore be useful for the diagnosis of fasciolosis during the acute stage of infection. The kinetics of the humoral response to cathepsin L5 are quite different from those to cathepsin B and reflect the continuing expression of FhCatL throughout the mammalian cycle of the parasite, as seen in other studies using cathepsin L to diagnose fasciolosis in sheep (9, 11, 15). The significance of the second surge of FhCatB antibody in infected sheep around 8 to 10 weeks of infection is not known, but the surge may result from the expression of an adult form of cathepsin B, such as those putatively encoded by transcripts identified by Heussler and Dobbelaere (18), which are sufficiently similar to share epitopes with the FhCatB sequence reported here. Antibodies to the proregion of FhCatB were observed by ELISA at week 5 for infected sheep, but very low titers to the proregion were observed for rats. In contrast, no reactivity with the proregion of FhCatB was observed by Western blotting with the rat or sheep infection sera (data not shown), suggesting that the antibodies observed by ELISA react with a conformational epitope(s) in the FhCatB proregion which is destroyed by SDS-PAGE.
The availability of recombinant F. hepatica cathepsin B protein has enabled us to determine that the proprotein is immunogenic in both a laboratory animal (the rat) and a definitive host (sheep) after immunization with an appropriate adjuvant. We are currently performing challenge experiments to determine if the immunogenic responses induced are protective. We have also demonstrated that the immune systems of rats and sheep can mount a response to FhCatB within 5 weeks of infection. Prophylactic vaccination with FhCatB may be an approach to successfully combat subsequent fluke infections in animals.
Acknowledgments
The first two authors contributed equally to this work.
This work was supported by the CRC for Vaccine Technology (Australia), an ARC SPIRT scholarship to Nick Kennedy, the Australian Centre for International Agricultural Research (Canberra), and Monash University.
We also thank Michael Reed for providing RACE-amplified cDNA and Simone Beckham for her constructive comments.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett, A. J., and H. Kirschke. 1981. Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol. 80:535-561. [DOI] [PubMed] [Google Scholar]
- 3.Berasain, P., F. Goni, S. McGonigle, A. Dowd, J. P. Dalton, B. Frangione, and C. Carmona. 1997. Proteinases secreted by Fasciola hepatica degrade extracellular matrix and basement membrane components. J. Parasitol. 83:1-5. [PubMed] [Google Scholar]
- 4.Berasain, P., C. Carmona, B. Frangione, J. P. Dalton, and F. Goni. 2000. Fasciola hepatica: parasite-secreted proteinases degrade all human IgG subclasses: determination of the specific cleavage sites and identification of the immunoglobulin fragments produced. Exp. Parasitol. 94:99-110. [DOI] [PubMed] [Google Scholar]
- 5.Brady, C. P., A. J. Dowd, J. Tort, L. Roche, B. Condon, S. M. O'Neill, P. J. Brindley, and J. P. Dalton. 1999. The cathepsin L-like proteinases of liver fluke and blood fluke parasites of the trematode genera Fasciola and Schistosoma. Biochem. Soc. Trans. 27:740-745. [DOI] [PubMed] [Google Scholar]
- 6.Caffrey, C. R., M. A. Mathieu, A. M. Gaffney, J. P. Salter, M. Sajid, K. D. Lucas, C. Franklin, M. Bogyo, and J. H. McKerrow. 2000. Identification of a cDNA encoding an active asparaginyl endopeptidase of Schistosoma mansoni and its expression in Pichia pastoris. FEBS Lett. 466:244-248. [DOI] [PubMed] [Google Scholar]
- 7.Caffrey, C. R., J. P. Salter, K. D. Lucas, D. Khiem, I. Hsieh, K. C. Lim, A. Ruppel, J. H. McKerrow, and M. Sajid. 2002. SmCB2, a novel tegumental cathepsin B from adult Schistosoma mansoni. Mol. Biochem. Parasitol. 121:49-61. [DOI] [PubMed] [Google Scholar]
- 8.Carmona, C., A. J. Dowd, A. M. Smith, and J. P. Dalton. 1993. Cathepsin L proteinase secreted by Fasciola hepatica in vitro prevents antibody-mediated eosinophil attachment to newly excysted juveniles. Mol. Biochem. Parasitol. 62:9-17. [DOI] [PubMed] [Google Scholar]
- 9.Carnevale, S., M. I. Rodriguez, E. A. Guarnera, C. Carmona, T. Tanos, and S. O. Angel. 2001. Immunodiagnosis of fasciolosis using recombinant procathepsin L cystein proteinase. Diagn. Microbiol. Infect. Dis. 41:43-49. [DOI] [PubMed] [Google Scholar]
- 10.Chapman, C. B., and G. F. Mitchell. 1982. Proteolytic cleavage of immunoglobulin by enzymes released by Fasciola hepatica. Vet. Parasitol. 11:165-178. [DOI] [PubMed] [Google Scholar]
- 11.Cornelissen, J. B., C. P. Gaasenbeek, F. H. Borgsteede, W. G. Holland, M. M. Harmsen, and W. J. Boersma. 2001. Early immunodiagnosis of fasciolosis in ruminants using recombinant Fasciola hepatica cathepsin L-like protease. Int. J. Parasitol. 31:728-737. [DOI] [PubMed] [Google Scholar]
- 12.Creaney, J., L. Wilson, M. Dosen, R. M. Sandeman, T. W. Spithill, and J. C. Parsons. 1996. Fasciola hepatica: irradiation-induced alterations in carbohydrate and cathepsin-B protease expression in newly excysted juvenile liver fluke. Exp. Parasitol. 83:202-215. [DOI] [PubMed] [Google Scholar]
- 13.Dalton, J. P., and M. Heffernan. 1989. Thiol proteases released in vitro by Fasciola hepatica. Mol. Biochem. Parasitol. 35:161-166. [DOI] [PubMed] [Google Scholar]
- 14.Dalton, J. P., L. Hola-Jamriska, and P. J. Brindley. 1995. Asparaginyl endopeptidase activity in adult Schistosoma mansoni. Parasitology 111:575-580. [DOI] [PubMed] [Google Scholar]
- 15.Dixit, A. K., S. C. Yadav, and R. L. Sharma. 2002. 28 kDa Fasciola gigantica cysteine proteinase in the diagnosis of prepatent ovine fasciolosis. Vet. Parasitol. 109:233-247. [DOI] [PubMed] [Google Scholar]
- 16.Dowd, A. J., A. M. Smith, S. McGonigle, and J. P. Dalton. 1994. Purification and characterisation of a second cathepsin L proteinase secreted by the parasitic trematode Fasciola hepatica. Eur. J. Biochem. 223:91-98. [DOI] [PubMed] [Google Scholar]
- 17.Greenspan, P. D., K. L. Clark, R. A. Tommasi, S. D. Cowen, L. W. McQuire, D. L. Farley, J. H. van Duzer, R. L. Goldberg, H. Zhou, Z. Du, J. J. Fitt, D. E. Coppa, Z. Fang, W. Macchia, L. Zhu, M. P. Capparelli, R. Goldstein, A. M. Wigg, J. R. Doughty, R. S. Bohacek, and A. K. Knap. 2001. Identification of dipeptidyl nitriles as potent and selective inhibitors of cathepsin B through structure-based drug design. J. Med. Chem. 44:4524-4534. [DOI] [PubMed] [Google Scholar]
- 18.Heussler, V. T., and D. A. Dobbelaere. 1994. Cloning of a protease gene family of Fasciola hepatica by the polymerase chain reaction. Mol. Biochem. Parasitol. 64:11-23. [DOI] [PubMed] [Google Scholar]
- 19.Higgins, D. G., J. D. Thompson, and T. J. Gibson. 1996. Using CLUSTAL for multiple sequence alignments. Methods Enzymol. 266:383-402. [DOI] [PubMed] [Google Scholar]
- 20.Hooft, R. W., G. Vriend, C. Sander, and E. E. Abola. 1996. Errors in protein structures. Nature 381:272. [DOI] [PubMed] [Google Scholar]
- 21.Howell, R. M. 1966. Collagenase activity of immature Fasciola hepatica. Nature 209:713-714. [DOI] [PubMed] [Google Scholar]
- 22.Irving, J. A., D. J. Askew, and G. A. Silverman. Computational analysis of evolution and conservation in a protein superfamily. Methods, in press. [DOI] [PubMed]
- 23.Irving, J. A., T. W. Spithill, R. N. Pike, J. C. Whisstock, and P. M. Smooker. 2003. The evolution of enzyme specificity in Fasciola spp. J. Mol. Evol. 57:1-15. [DOI] [PubMed] [Google Scholar]
- 24.Klebe, R. J., J. V. Harris, Z. D. Sharp, and M. G. Douglas. 1983. A general method for polyethylene-glycol-induced genetic transformation of bacteria and yeast. Gene 25:333-341. [DOI] [PubMed] [Google Scholar]
- 25.Krupa, J. C., S. Hasnain, D. K. Nagler, R. Menard, and J. S. Mort. 2002. S2′ substrate specificity and the role of His110 and His111 in the exopeptidase activity of human cathepsin B. Biochem. J. 361:613-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 27.Larminie, C. G., and I. L. Johnstone. 1996. Isolation and characterization of four developmentally regulated cathepsin B-like cysteine protease genes from the nematode Caenorhabditis elegans. DNA Cell Biol. 15:75-82. [DOI] [PubMed] [Google Scholar]
- 28.Lipps, G., R. Fullkrug, and E. Beck. 1996. Cathepsin B of Schistosoma mansoni. Purification and activation of the recombinant proenzyme secreted by Saccharomyces cerevisiae. J. Biol. Chem. 271:1717-1725. [DOI] [PubMed] [Google Scholar]
- 29.Locatelli, A., and C. Paoletti. 1969. Fasciola hepatica L. homogenate proteolytic activity: action against different substrates. Arch. Vet. Ital. 20:97-100. [PubMed] [Google Scholar]
- 30.Mach, L., J. S. Mort, and J. Glossl. 1994. Maturation of human procathepsin B. Proenzyme activation and proteolytic processing of the precursor to the mature proteinase, in vitro, are primarily unimolecular processes. J. Biol. Chem. 269:13030-13035. [PubMed] [Google Scholar]
- 31.Mathieu, M. A., M. Bogyo, C. R. Caffrey, Y. Choe, J. Lee, H. Chapman, M. Sajid, C. S. Craik, and J. H. McKerrow. 2002. Substrate specificity of schistosome versus human legumain determined by P1-P3 peptide libraries. Mol. Biochem. Parasitol. 121:99-105. [DOI] [PubMed] [Google Scholar]
- 32.McGinty, A., M. Moore, D. W. Halton, and B. Walker. 1993. Characterization of the cysteine proteinases of the common liver fluke Fasciola hepatica using novel, active-site directed affinity labels. Parasitology 106:487-493. [DOI] [PubMed] [Google Scholar]
- 33.Mulcahy, G., and J. P. Dalton. 2001. Cathepsin L proteinases as vaccines against infection with Fasciola hepatica (liver fluke) in ruminants. Res. Vet. Sci. 70:83-86. [DOI] [PubMed] [Google Scholar]
- 34.Nagler, D. K., A. C. Storer, F. C. Portaro, E. Carmona, L. Juliano, and R. Menard. 1997. Major increase in endopeptidase activity of human cathepsin B upon removal of occluding loop contacts. Biochemistry 36:12608-12615. [DOI] [PubMed] [Google Scholar]
- 35.Nei, M., S. Kumar, and K. Takahashi. 1998. The optimization principle in phylogenetic analysis tends to give incorrect topologies when the number of nucleotides or amino acids used is small. Proc. Natl. Acad. Sci. USA 95:12390-12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prowse, R. K., P. Chaplin, H. C. Robinson, and T. W. Spithill. 2002. Fasciola hepatica cathepsin L suppresses sheep lymphocyte proliferation in vitro and modulates surface CD4 expression on human and ovine T cells. Parasite Immunol. 24:57-66. [DOI] [PubMed] [Google Scholar]
- 37.Reed, M. B., M. Panaccio, R. A. Strugnell, and T. W. Spithill. 1998. Developmental expression of a Fasciola hepatica sequence homologous to ABC transporters. Int. J. Parasitol. 28:1375-1381. [DOI] [PubMed] [Google Scholar]
- 38.Rowan, A. D., P. Mason, L. Mach, and J. S. Mort. 1992. Rat procathepsin B. Proteolytic processing to the mature form in vitro. J. Biol. Chem. 267:15993-15999. [PubMed] [Google Scholar]
- 39.Sajid, M., and J. H. McKerrow. 2002. Cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 120:1-21. [DOI] [PubMed] [Google Scholar]
- 40.Sali, A., and T. L. Blundell. 1993. Comparative protein modelling by satisfaction of spacial restraints. J. Mol. Biol. 234:779-815. [DOI] [PubMed] [Google Scholar]
- 41.Simpkin, K. G., C. R. Chapman, and G. C. Coles. 1980. Fasciola hepatica: a proteolytic digestive enzyme. Exp. Parasitol. 49:281-287. [DOI] [PubMed] [Google Scholar]
- 42.Smooker, P. M., K. R. Steeper, D. R. Drew, R. A. Strugnell, and T. W. Spithill. 1999. Humoral responses in mice following vaccination with DNA encoding glutathione S-transferase of Fasciola hepatica: effects of mode of vaccination and the cellular compartment of antigen expression. Parasite Immunol. 21:357-364. [DOI] [PubMed] [Google Scholar]
- 43.Smooker, P. M., J. C. Whisstock, J. A. Irving, S. Siyaguna, T. W. Spithill, and R. N. Pike. 2000. A single amino acid substitution affects substrate specificity in cysteine proteinases from Fasciola hepatica. Protein Sci. 9:2567-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spithill, T. W., and J. P. Dalton. 1998. Progress in development of liver fluke vaccines. Parasitol. Today 14:224-228. [DOI] [PubMed] [Google Scholar]
- 45.Tkalcevic, J., K. Ashman, and E. Meeusen. 1995. Fasciola hepatica: rapid identification of newly excysted juvenile proteins. Biochem. Biophys. Res. Commun. 213:169-174. [DOI] [PubMed] [Google Scholar]
- 46.Turk, D., M. Podobnik, T. Popovic, N. Katunuma, W. Bode, R. Huber, and V. Turk. 1995. Crystal structure of cathepsin B inhibited with CA030 at 2.0-A resolution: a basis for the design of specific epoxysuccinyl inhibitors. Biochemistry 34:4791-4797. [DOI] [PubMed] [Google Scholar]
- 47.Wijffels, G. L., L. Salvatore, M. Dosen, J. Waddington, L. Wilson, C. Thompson, N. Campbell, J. Sexton, J. Wicker, F. Bowen, and T. W. Spithill. 1994. Vaccination of sheep with purified cysteine proteinases of Fasciola hepatica decreases worm fecundity. Exp. Parasitol. 78:132-148. [DOI] [PubMed] [Google Scholar]
- 48.Wilkinson, M. 1996. Majority-rule reduced consensus trees and their use in bootstrapping. Mol. Biol. Evol. 13:437-444. [DOI] [PubMed] [Google Scholar]
- 49.Wilson, L. R., R. T. Good, M. Panaccio, G. L. Wijffels, R. M. Sandeman, and T. W. Spithill. 1998. Fasciola hepatica: characterization and cloning of the major cathepsin B protease secreted by newly excysted juvenile liver fluke. Exp. Parasitol. 88:85-94. [DOI] [PubMed] [Google Scholar]
- 50.Yang, Z. 1997. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biol. Sci. 13:555-556. [DOI] [PubMed] [Google Scholar]