Abstract
In the title compound, C12H13N3O6, the dihydropyran ring adopts a near screw-boat conformation. The dihedral angle between the mean planes of the benzene and dihydropyran rings is 6.35 (5)°. An intramolecular N—H⋯O hydrogen bond generates an S(6) motif, which stabilizes the molecular conformation. In the crystal, weak intermolecular C—H⋯O, N—H⋯O and C—H⋯π hydrogen bonds contribute to the stabilization of the packing.
Related literature
For related structures, see: Gayathri et al. (2006 ▶); Bhaskaran et al. (2006 ▶). For the biological importance of 4H-chromene derivatives, see: Cai (2007 ▶, 2008 ▶); Cai et al. (2006 ▶); Gabor et al. (1988 ▶); Brooks (1998 ▶); Valenti et al. (1993 ▶); Hyana & Saimoto (1987 ▶); Tang et al. (2007 ▶); Wang et al. (2000 ▶). For ring puckering analysis, see: Cremer & Pople (1975 ▶). For C—H⋯π interactions, see: Desiraju & Steiner (1999 ▶).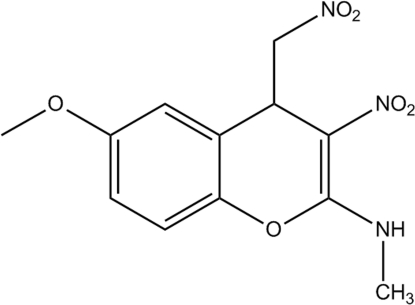
Experimental
Crystal data
C12H13N3O6
M r = 295.25
Monoclinic,

a = 6.8354 (2) Å
b = 9.4363 (2) Å
c = 19.9332 (4) Å
β = 90.777 (2)°
V = 1285.59 (5) Å3
Z = 4
Mo Kα radiation
μ = 0.12 mm−1
T = 293 K
0.4 × 0.4 × 0.2 mm
Data collection
Oxford Diffraction Xcalibur Eos diffractometer
Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2009 ▶) T min = 0.966, T max = 1.000
13856 measured reflections
2256 independent reflections
1804 reflections with I > 2σ(I)
R int = 0.040
Refinement
R[F 2 > 2σ(F 2)] = 0.041
wR(F 2) = 0.116
S = 1.01
2256 reflections
192 parameters
H-atom parameters constrained
Δρmax = 0.28 e Å−3
Δρmin = −0.36 e Å−3
Data collection: CrysAlis CCD (Oxford Diffraction, 2009 ▶); cell refinement: CrysAlis RED (Oxford Diffraction, 2009 ▶); data reduction: CrysAlis RED; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶) and PLATON (Spek, 2009 ▶); software used to prepare material for publication: PLATON.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811015595/hq2002sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811015595/hq2002Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811015595/hq2002Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg is the centroid of the C1–C6 benzene ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O2 | 0.86 | 2.01 | 2.6169 (17) | 127 |
| N1—H1⋯O3i | 0.86 | 2.26 | 2.9808 (18) | 142 |
| C11—H11A⋯O2ii | 0.97 | 2.49 | 3.4366 (19) | 165 |
| C10—H10A⋯Cgiii | 0.96 | 2.61 | 3.548 (2) | 164 |
| C10—H10C⋯Cgiv | 0.96 | 2.86 | 3.706 (2) | 148 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Acknowledgments
RK, JM, and PM thank the Centre for Bioinformatics (funded by the Department of Biotechnology and the Department of Information Technology, New Delhi, India) and Pondicherry University for providing computational facilities. AP thanks Pondicherry University for a fellowship and PM thanks the University Grants Commission (UGC) for a fellowship. HSP thanks the UGC for the SAP and the Department of Science and Technology (DST) for the FIST.
supplementary crystallographic information
Comment
4H-Chromene derivatives exhibit anti-viral, anti-fungal, anti-inflammatory, anti-diabetic, cardionthonic, anti-anaphylactic and anti-cancer activity (Cai, 2008; Cai, 2007; Cai et al., 2006; Gabor et al., 1988; Brooks, 1998; Valenti et al., 1993; Hyana & Saimoto, 1987; Tang et al., 2007). Functionally substituted 4H-Chromene derivatives are a new class of compound that binds to Bcl-2 protein and induces apoptosis or programmed cell death in cancer cells (Wang et al., 2000). In order to examine the activity relationship between their molecular structure and biology, a single-crystal of the title compound was prepared for X-ray diffraction studies.
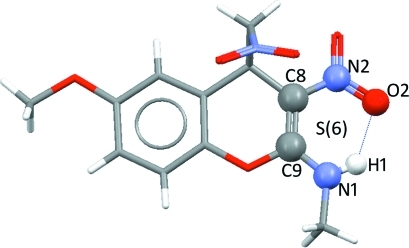
In the title compound (Fig. 1), the methoxy substituent at the C4 atom forms the torsion angle of -180 (14) ° [(-) anti-periplanar conformation] with the atom set O6/C4/C3/C2. From the puckering analysis (Cremer & Pople, 1975), the dihydropyran ring (O1/C1/C6/C7/C8/C9) is very similar to the screw-boat conformation (S form) with puckering parameters of Q = 0.1798 (15) Å, θ = 100.8 (5)° and Φ = 20.1 (5)°. Three intramolecular interactions N1—H1···O2 (symmetry code: x, y, z), N1—H1···N2 and C11—H11A···O3 are observed to contribute to the stability of the title compound, in which an N1—H1···O2 interaction generates a characteristic intramolecular S (6) motif with an N···O distance of 2.617 (17) Å (Fig. 2). The stabilization of crystal packing (Fig. 3) is influenced by intermolecular hydrogen bonding such as N1—H1···O3 (symmetry code: -x + 1/2, y - 1/2, -z + 1/2) and C11—H11A···O2 (symmetry code: -x + 1/2, y + 1/2, -z + 1/2). The C—H···pi interactions (Fig. 4) observed between C10—H10A···Cg (symmetry code:-x, 1 - y,-z, Cg is the centroid of the benzene ring C1—C6, C···Cg distance: 3.548 (2) Å, H-Perp: -2.56 Å) and C10—H10C···Cg (symmetry code: 1 - x,1 - y,-z, C···Cg distance: 3.706 (2) Å, H-Perp: 2.65 Å) also contribute to the crystal packing. The bond distances of the C—H···π interactions agree with those described by Desiraju & Steiner (1999). An intermolecular N1—H1···O3 interaction generates a C (6) motif with an N···O distance of 2.981 (18) Å (Fig. 5).
Experimental
(E)-4-Methoxy-2-(2-nitrovinyl)phenol (200 mg, 1.024 mmol) was taken in a 25 ml round bottom flask in methanol (5 ml). To this solution, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (15 mg, 0.102 mmol) was added and stirred thoroughly for 10 minutes at room temperature. To this stirred solution, ((E) N-methyl-1-(methylthio)-2-nitroethenamine) (NMSM) was added and stirred for 8 h for completion (TLC, hexane:ethyl acetate, 3:2, Rf of I = 1/2). The reaction mixture was then kept in a refrigerator for 3 h to afford racemic mixture of the product (I)as a white precipitate, which was filtered. Good crystals were obtained by recrystallization with a solution of dichloromethane:hexane (9:3 v/v).
Refinement
All hydrogen atoms were placed in calculated positions, with N—H = 0.86 and C—H = 0.97 and included in the final cycles of refinement using a riding model with Uiso(H) = 1.2 Ueq(C).
TITL
CELL 0.71073 6.8354 9.4363 19.9332 90.000 90.777 90.000
ZERR 4.00 0.0002 0.0002 0.0004 0.000 0.002 0.000
LATT 1
SYMM 1/2 - X, 1/2 + Y, 1/2 - Z
SFAC C H N O
UNIT 48 52 12 24
MERG 2
OMIT -2 50
ACTA
CONF
FMAP 2
PLAN 20
BOND $H
EQIV $1 1/2-X, -1/2+Y, 1/2-Z
EQIV $2 1/2-X, 1/2+Y, 1/2-Z
HTAB N1 O3_$1
HTAB C11 O2_$2
L.S. 4
WGHT 0.087000
FVAR 5.43518
Figures
Fig. 1.
The molecular structure of (I), showing the atom-numbering scheme and displacement ellipsoids drawn at the 50% probability level.
Fig. 2.
A view of the intramolecular S (6) motif formed by N—H···O interaction in Compound (I). The motif forming atoms are shown in ball and stick model and the hydrogen bond is shown as a blue dashed line.
Fig. 3.
The crystal packing of Compound (I) viewed down the XO-axis, showing intermolecular hydrogen bonding interactions as dashed lines.
Fig. 4.
A view showing the weak C—H···pi intermolecular interactions in Compound (I). Cg is a centroid of the C1—C6 ring in the 4H-Chromene moiety.
Fig. 5.
A view of the intermolecular C (6) motif formed by the N—H···O interaction in Compound (I). The motif forming atoms are shown in ball and stick model and the hydrogen bond is shown as a blue dashed line.
Crystal data
| C12H13N3O6 | F(000) = 616 |
| Mr = 295.25 | Dx = 1.525 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 7594 reflections |
| a = 6.8354 (2) Å | θ = 3.0–29.3° |
| b = 9.4363 (2) Å | µ = 0.12 mm−1 |
| c = 19.9332 (4) Å | T = 293 K |
| β = 90.777 (2)° | Block, colorless |
| V = 1285.59 (5) Å3 | 0.4 × 0.4 × 0.2 mm |
| Z = 4 |
Data collection
| Oxford Diffraction Xcalibur Eos diffractometer | 2256 independent reflections |
| Radiation source: fine-focus sealed tube | 1804 reflections with I > 2σ(I) |
| graphite | Rint = 0.040 |
| Detector resolution: 15.9821 pixels mm-1 | θmax = 25.0°, θmin = 3.0° |
| ω scans | h = −8→8 |
| Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2009) | k = −11→11 |
| Tmin = 0.966, Tmax = 1.000 | l = −23→23 |
| 13856 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.041 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.116 | H-atom parameters constrained |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.087P)2] where P = (Fo2 + 2Fc2)/3 |
| 2256 reflections | (Δ/σ)max = 0.015 |
| 192 parameters | Δρmax = 0.28 e Å−3 |
| 0 restraints | Δρmin = −0.36 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F^2^ against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F^2^, conventional R-factors R are based on F, with F set to zero for negative F^2^. The threshold expression of F^2^ > σ(F^2^) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F^2^ are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.2620 (2) | 0.61723 (15) | −0.00254 (7) | 0.0299 (4) | |
| C2 | 0.2708 (2) | 0.58244 (17) | −0.06949 (8) | 0.0337 (4) | |
| H2 | 0.2594 | 0.4884 | −0.0830 | 0.040* | |
| C3 | 0.2967 (2) | 0.68797 (18) | −0.11657 (8) | 0.0356 (4) | |
| H3 | 0.3019 | 0.6656 | −0.1620 | 0.043* | |
| C4 | 0.3149 (2) | 0.82767 (17) | −0.09555 (8) | 0.0335 (4) | |
| C5 | 0.3093 (2) | 0.85951 (17) | −0.02791 (7) | 0.0329 (4) | |
| H5 | 0.3239 | 0.9532 | −0.0142 | 0.039* | |
| C6 | 0.2826 (2) | 0.75487 (16) | 0.01994 (7) | 0.0297 (4) | |
| C7 | 0.2766 (2) | 0.78954 (16) | 0.09386 (7) | 0.0323 (4) | |
| H7 | 0.3939 | 0.8453 | 0.1045 | 0.039* | |
| C8 | 0.2891 (2) | 0.65553 (16) | 0.13472 (7) | 0.0310 (4) | |
| C9 | 0.2506 (2) | 0.52117 (16) | 0.10765 (7) | 0.0289 (4) | |
| C10 | 0.2007 (2) | 0.26444 (16) | 0.10926 (9) | 0.0379 (4) | |
| H10A | 0.0798 | 0.2655 | 0.0842 | 0.057* | |
| H10B | 0.1950 | 0.1928 | 0.1434 | 0.057* | |
| H10C | 0.3067 | 0.2442 | 0.0796 | 0.057* | |
| C11 | 0.0974 (3) | 0.88254 (16) | 0.11164 (8) | 0.0379 (4) | |
| H11A | 0.1119 | 0.9171 | 0.1573 | 0.046* | |
| H11B | 0.0916 | 0.9638 | 0.0819 | 0.046* | |
| C12 | 0.3430 (3) | 0.9162 (2) | −0.20753 (8) | 0.0543 (5) | |
| H12A | 0.4498 | 0.8543 | −0.2180 | 0.081* | |
| H12B | 0.3585 | 1.0045 | −0.2308 | 0.081* | |
| H12C | 0.2219 | 0.8728 | −0.2213 | 0.081* | |
| N1 | 0.2318 (2) | 0.40166 (13) | 0.14032 (6) | 0.0336 (3) | |
| H1 | 0.2383 | 0.4048 | 0.1834 | 0.040* | |
| N2 | 0.3329 (2) | 0.67051 (14) | 0.20171 (6) | 0.0356 (3) | |
| N3 | −0.0864 (2) | 0.80035 (15) | 0.10518 (7) | 0.0411 (4) | |
| O1 | 0.22704 (16) | 0.50418 (11) | 0.04132 (5) | 0.0358 (3) | |
| O2 | 0.3398 (2) | 0.56472 (13) | 0.24035 (5) | 0.0496 (4) | |
| O3 | 0.3669 (2) | 0.79190 (12) | 0.22406 (6) | 0.0484 (4) | |
| O4 | −0.1438 (2) | 0.73633 (16) | 0.15375 (8) | 0.0703 (5) | |
| O5 | −0.1686 (2) | 0.79552 (16) | 0.05057 (7) | 0.0641 (4) | |
| O6 | 0.34105 (19) | 0.94142 (13) | −0.13716 (6) | 0.0486 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0314 (8) | 0.0310 (8) | 0.0272 (8) | −0.0014 (6) | 0.0001 (6) | 0.0012 (7) |
| C2 | 0.0359 (9) | 0.0344 (9) | 0.0307 (9) | −0.0006 (7) | 0.0012 (7) | −0.0079 (7) |
| C3 | 0.0352 (9) | 0.0487 (10) | 0.0230 (8) | −0.0021 (7) | 0.0032 (7) | −0.0045 (7) |
| C4 | 0.0309 (8) | 0.0418 (9) | 0.0280 (8) | −0.0032 (7) | 0.0031 (6) | 0.0049 (7) |
| C5 | 0.0366 (9) | 0.0321 (8) | 0.0299 (8) | −0.0033 (7) | 0.0004 (7) | 0.0001 (7) |
| C6 | 0.0321 (8) | 0.0318 (8) | 0.0253 (8) | −0.0015 (6) | 0.0000 (6) | −0.0011 (6) |
| C7 | 0.0439 (9) | 0.0280 (8) | 0.0250 (8) | −0.0055 (7) | −0.0029 (7) | −0.0021 (6) |
| C8 | 0.0381 (9) | 0.0309 (8) | 0.0238 (8) | −0.0002 (7) | −0.0020 (6) | −0.0013 (6) |
| C9 | 0.0297 (8) | 0.0318 (8) | 0.0253 (8) | 0.0030 (6) | −0.0003 (6) | −0.0002 (6) |
| C10 | 0.0427 (10) | 0.0285 (9) | 0.0424 (10) | −0.0002 (7) | −0.0050 (8) | −0.0008 (7) |
| C11 | 0.0610 (11) | 0.0249 (8) | 0.0278 (9) | 0.0013 (7) | −0.0020 (7) | −0.0042 (7) |
| C12 | 0.0661 (13) | 0.0703 (14) | 0.0267 (9) | −0.0128 (10) | 0.0045 (8) | 0.0082 (9) |
| N1 | 0.0443 (8) | 0.0296 (7) | 0.0270 (7) | 0.0007 (6) | −0.0005 (6) | −0.0001 (6) |
| N2 | 0.0455 (8) | 0.0358 (8) | 0.0254 (7) | −0.0029 (6) | −0.0042 (6) | −0.0010 (6) |
| N3 | 0.0534 (9) | 0.0347 (8) | 0.0353 (9) | 0.0109 (6) | 0.0022 (7) | −0.0042 (7) |
| O1 | 0.0538 (7) | 0.0289 (6) | 0.0244 (6) | −0.0057 (5) | −0.0031 (5) | −0.0018 (4) |
| O2 | 0.0772 (9) | 0.0417 (7) | 0.0297 (7) | −0.0045 (6) | −0.0105 (6) | 0.0074 (5) |
| O3 | 0.0775 (9) | 0.0378 (7) | 0.0297 (7) | −0.0097 (6) | −0.0054 (6) | −0.0087 (5) |
| O4 | 0.0762 (10) | 0.0754 (10) | 0.0597 (9) | −0.0071 (8) | 0.0115 (8) | 0.0222 (8) |
| O5 | 0.0679 (10) | 0.0781 (10) | 0.0460 (8) | 0.0039 (7) | −0.0138 (7) | −0.0154 (7) |
| O6 | 0.0696 (9) | 0.0479 (7) | 0.0285 (6) | −0.0079 (6) | 0.0065 (6) | 0.0080 (5) |
Geometric parameters (Å, °)
| C1—C2 | 1.376 (2) | C9—O1 | 1.3394 (17) |
| C1—C6 | 1.381 (2) | C10—N1 | 1.4496 (19) |
| C1—O1 | 1.4019 (17) | C10—H10A | 0.9600 |
| C2—C3 | 1.381 (2) | C10—H10B | 0.9600 |
| C2—H2 | 0.9300 | C10—H10C | 0.9600 |
| C3—C4 | 1.388 (2) | C11—N3 | 1.480 (2) |
| C3—H3 | 0.9300 | C11—H11A | 0.9700 |
| C4—O6 | 1.3696 (19) | C11—H11B | 0.9700 |
| C4—C5 | 1.382 (2) | C12—O6 | 1.423 (2) |
| C5—C6 | 1.387 (2) | C12—H12A | 0.9600 |
| C5—H5 | 0.9300 | C12—H12B | 0.9600 |
| C6—C7 | 1.511 (2) | C12—H12C | 0.9600 |
| C7—C8 | 1.506 (2) | N1—H1 | 0.8600 |
| C7—C11 | 1.551 (2) | N2—O3 | 1.2497 (17) |
| C7—H7 | 0.9800 | N2—O2 | 1.2613 (16) |
| C8—N2 | 1.3719 (19) | N3—O4 | 1.2111 (18) |
| C8—C9 | 1.401 (2) | N3—O5 | 1.2191 (19) |
| C9—N1 | 1.3095 (19) | ||
| C2—C1—C6 | 122.24 (14) | N1—C10—H10A | 109.5 |
| C2—C1—O1 | 115.67 (13) | N1—C10—H10B | 109.5 |
| C6—C1—O1 | 122.07 (13) | H10A—C10—H10B | 109.5 |
| C1—C2—C3 | 119.63 (14) | N1—C10—H10C | 109.5 |
| C1—C2—H2 | 120.2 | H10A—C10—H10C | 109.5 |
| C3—C2—H2 | 120.2 | H10B—C10—H10C | 109.5 |
| C2—C3—C4 | 119.43 (14) | N3—C11—C7 | 110.81 (12) |
| C2—C3—H3 | 120.3 | N3—C11—H11A | 109.5 |
| C4—C3—H3 | 120.3 | C7—C11—H11A | 109.5 |
| O6—C4—C5 | 115.20 (14) | N3—C11—H11B | 109.5 |
| O6—C4—C3 | 124.98 (14) | C7—C11—H11B | 109.5 |
| C5—C4—C3 | 119.81 (14) | H11A—C11—H11B | 108.1 |
| C6—C5—C4 | 121.45 (15) | O6—C12—H12A | 109.5 |
| C6—C5—H5 | 119.3 | O6—C12—H12B | 109.5 |
| C4—C5—H5 | 119.3 | H12A—C12—H12B | 109.5 |
| C1—C6—C5 | 117.41 (14) | O6—C12—H12C | 109.5 |
| C1—C6—C7 | 121.09 (13) | H12A—C12—H12C | 109.5 |
| C5—C6—C7 | 121.49 (13) | H12B—C12—H12C | 109.5 |
| C8—C7—C6 | 110.10 (12) | C9—N1—C10 | 124.87 (13) |
| C8—C7—C11 | 112.99 (13) | C9—N1—H1 | 117.6 |
| C6—C7—C11 | 112.17 (12) | C10—N1—H1 | 117.6 |
| C8—C7—H7 | 107.1 | O3—N2—O2 | 120.17 (13) |
| C6—C7—H7 | 107.1 | O3—N2—C8 | 118.59 (13) |
| C11—C7—H7 | 107.1 | O2—N2—C8 | 121.24 (13) |
| N2—C8—C9 | 120.36 (13) | O4—N3—O5 | 123.00 (17) |
| N2—C8—C7 | 116.74 (13) | O4—N3—C11 | 118.40 (15) |
| C9—C8—C7 | 122.86 (13) | O5—N3—C11 | 118.53 (15) |
| N1—C9—O1 | 112.12 (13) | C9—O1—C1 | 120.35 (12) |
| N1—C9—C8 | 127.37 (14) | C4—O6—C12 | 117.96 (14) |
| O1—C9—C8 | 120.52 (13) | ||
| C6—C1—C2—C3 | −1.5 (2) | N2—C8—C9—N1 | 7.3 (2) |
| O1—C1—C2—C3 | 177.37 (13) | C7—C8—C9—N1 | −170.06 (15) |
| C1—C2—C3—C4 | 0.5 (2) | N2—C8—C9—O1 | −173.30 (13) |
| C2—C3—C4—O6 | −179.99 (14) | C7—C8—C9—O1 | 9.4 (2) |
| C2—C3—C4—C5 | 0.8 (2) | C8—C7—C11—N3 | −55.31 (17) |
| O6—C4—C5—C6 | 179.61 (14) | C6—C7—C11—N3 | 69.86 (16) |
| C3—C4—C5—C6 | −1.1 (2) | O1—C9—N1—C10 | 3.5 (2) |
| C2—C1—C6—C5 | 1.2 (2) | C8—C9—N1—C10 | −177.05 (15) |
| O1—C1—C6—C5 | −177.60 (13) | C9—C8—N2—O3 | 179.81 (14) |
| C2—C1—C6—C7 | −178.67 (14) | C7—C8—N2—O3 | −2.7 (2) |
| O1—C1—C6—C7 | 2.5 (2) | C9—C8—N2—O2 | 0.0 (2) |
| C4—C5—C6—C1 | 0.1 (2) | C7—C8—N2—O2 | 177.50 (14) |
| C4—C5—C6—C7 | 179.97 (14) | C7—C11—N3—O4 | 89.78 (17) |
| C1—C6—C7—C8 | 11.8 (2) | C7—C11—N3—O5 | −87.28 (17) |
| C5—C6—C7—C8 | −168.03 (14) | N1—C9—O1—C1 | −173.34 (12) |
| C1—C6—C7—C11 | −114.91 (16) | C8—C9—O1—C1 | 7.2 (2) |
| C5—C6—C7—C11 | 65.23 (18) | C2—C1—O1—C9 | 167.88 (13) |
| C6—C7—C8—N2 | 164.64 (13) | C6—C1—O1—C9 | −13.3 (2) |
| C11—C7—C8—N2 | −69.08 (17) | C5—C4—O6—C12 | −178.38 (14) |
| C6—C7—C8—C9 | −17.9 (2) | C3—C4—O6—C12 | 2.3 (2) |
| C11—C7—C8—C9 | 108.34 (16) |
Hydrogen-bond geometry (Å, °)
| Cg is the centroid of the C1–C6 benzene ring. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O2 | 0.86 | 2.01 | 2.6169 (17) | 127 |
| N1—H1···O3i | 0.86 | 2.26 | 2.9808 (18) | 142 |
| C11—H11A···O2ii | 0.97 | 2.49 | 3.4366 (19) | 165 |
| C10—H10A···Cgiii | 0.96 | 2.61 | 3.548 (2) | 164 |
| C10—H10C···Cgiv | 0.96 | 2.86 | 3.706 (2) | 148 |
Symmetry codes: (i) −x+1/2, y−1/2, −z+1/2; (ii) −x+1/2, y+1/2, −z+1/2; (iii) −x, −y+1, −z; (iv) −x+1, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HQ2002).
References
- Bhaskaran, S., Velmurugan, D., Ravikumar, K., Geetha, K. & Surya Prakash Rao, H. (2006). Acta Cryst. E62, o188–o190.
- Brooks, G. T. (1998). Pestic. Sci. 22, 41–50.
- Cai, S. X. (2007). Recent Patents Anticancer Drug Discov. 2, 79–101. [DOI] [PubMed]
- Cai, S. X. (2008). Bioorg. Med. Chem. Lett. 18, 603–607.
- Cai, S. X., Drewe, J. & Kasibhatla, S. (2006). Curr. Med. Chem. 13, 2627–2644. [DOI] [PubMed]
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Desiraju, G. R. & Steiner, T. (1999). The Weak Hydrogen Bond in Structural Chemistry and Biology, pp. 11–40. New York: Oxford University Press.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Gabor, M. (1988). The Pharmacology of Benzopyrone Derivatives and Related Compounds, pp. 91–126. Budapest: Akademiai Kiado.
- Gayathri, D., Velmurugan, D., Ravikumar, K., Geetha, K. & Surya Prakash Rao, H. (2006). Acta Cryst. E62, o1961–o1963.
- Hyana, T. & Saimoto, H. (1987). Jpn Patent JP 621 812 768.
- Oxford Diffraction (2009). CrysAlis CCD, CrysAlis RED and CrysAlis PRO Oxford Diffraction Ltd, Yarnton, England.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Tang, Q.-G., Wu, W.-Y., He, W., Sun, H.-S. & Guo, C. (2007). Acta Cryst. E63, o1437–o1438.
- Valenti, P., Da Re, P., Rampa, A., Montanari, P., Carrara, M. & Cima, L. (1993). Anticancer Drug. Des. 8, 349–360. [PubMed]
- Wang, J. L., Liu, D., Zhang, Z. J., Shan, S., Han, X., Srinivasula, S. M., Croce, C. M., Alnemri, E. S. & Huang, Z. (2000). Proc. Natl Acad. Sci. USA, 97, 7124–9. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811015595/hq2002sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811015595/hq2002Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811015595/hq2002Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report