Abstract
The HMW1- and HMW2-like adhesion proteins of nontypeable Haemophilus influenzae are expressed by 75% of these strains, and antibodies directed against these proteins are protective in animal models of infection. The purpose of the present study was to define the functional activity of human antibodies specific for these proteins in an in vitro complement-dependent opsonophagocytic assay. Human promyelocytic cell line HL-60 served as the source of phagocytic cells, and a commercial preparation of intravenous immunoglobulin (IVIG) served as the source of human antibodies. High-molecular-weight (HMW) proteins were purified from four prototype nontypeable H. influenzae strains and used to prepare solid-phase affinity columns. IVIG was adsorbed on each column to remove strain-specific anti-HMW antibodies and to allow recovery of affinity-purified anti-HMW antibody fractions. Unadsorbed IVIG killed each of the prototype strains at titers of 1:80 to 1:320. HMW-adsorbed sera demonstrated fourfold decreases in opsonophagocytic titer against the homologous strains compared to unadsorbed IVIG. Affinity-purified anti-HMW antibody preparations demonstrated opsonophagocytic titers of 1:20 to 1:80 against the respective homologous strains and opsonophagocytic titers as high as 1:80 against heterologous strains. None of the affinity-purified anti-HMW antibody preparations was opsonophagocytic for a representative nontypeable H. influenzae strain that did not express HMW1- or HMW2-like proteins. These data demonstrate that human antibodies specific for the HMW1/HMW2-like adhesion proteins of nontypeable H. influenzae are opsonophagocytic and that such antibodies recognize epitopes shared by the HMW proteins of unrelated nontypeable H. influenzae strains. These results argue for continued investigation of the HMW1/HMW2-like proteins as potential vaccine candidates for prevention of disease due to nontypeable H. influenzae.
Otitis media remains a significant health problem for children in this country and elsewhere in the world (11, 44). Most children in this country have had at least one episode of otitis by the third birthday, and one-third have had three or more episodes (37, 44). In addition to the short-term morbidity and costs of this illness, the potential long-term detrimental effects upon speech and language development in children with persistent middle ear effusion remain a subject of considerable concern (36, 42, 43). The health care costs of the disease in this country are substantial. The annual cost of the medical and surgical treatment of otitis media in the United States is estimated at between $3 billion and $4 billion (39, 42). Otitis media experts strongly recommend that efforts be made to develop safe and effective vaccines for prevention of otitis media in young children (29). Although total prevention of disease will be a difficult goal to achieve, prevention of even a portion of cases would be beneficial, given the magnitude and costs of the problem.
The three major bacteria associated with otitis media are Streptococcus pneumoniae, nontypeable Haemophilus influenzae, and Moraxella catarrhalis (25, 29). Vaccine development efforts are proceeding for all three organisms and are most advanced for Streptococcus pneumoniae. Recent reports suggest that conjugate vaccines hold great promise for prevention of serious invasive pneumococcal infections such as pneumonia and meningitis (12, 20, 48). Lesser but still significant protection has been reported for the same vaccines against pneumococcal otitis media (12, 19).
Nontypeable H. influenzae vaccine development efforts have been ongoing for a number of years. A number of different Haemophilus antigens have been suggested as possible vaccine candidates (1-3, 5, 17, 21, 23, 27, 30, 32, 33, 45, 49). Several studies have suggested that nontypeable Haemophilus outer membrane proteins are the principal targets of bactericidal and protective antibody (5, 21, 27) and thus should be a focus of vaccine development efforts. Haemophilus proteins P2 and P6 have been characterized and shown to be specific targets of human bactericidal antibody (32, 33). Another vaccine candidate is the P5 fimbrin adhesion protein (2, 3). Both native P5 protein and peptide derivatives of this protein have been demonstrated to modify the course of experimental otitis media in chinchillas (2, 3). Among other proteins still under investigation as potential vaccines are lipoprotein D (1, 3), recombinant HtrA (30), transferrin receptor (45), and OMP26 (17). Even lipooligosaccharide, in the form of detoxified conjugate preparations, has been the subject of recent detailed investigations as a possible vaccine candidate (23, 49). However, despite extensive work by many different researchers, it remains unclear which if any of these vaccine candidates will ultimately be able to prevent nontypeable Haemophilus disease in the human host.
In previously reported work, we identified a family of high-molecular-weight (HMW) proteins that are major targets of antibody in serum from children who have recovered from Haemophilus otitis (6). Subsequently, we cloned and sequenced the genes encoding two such immunogenic high-molecular-weight proteins from a prototypic strain (7) and demonstrated that the proteins encoded by these genes were critical for attachment of nontypeable H. influenzae to human epithelial cells in vitro (40). The prototypic proteins were designated HMW1 and HMW2, and we demonstrated that approximately 75% of unrelated nontypeable Haemophilus organisms express these proteins (7, 41). Given the functional role of these proteins as adhesins and their highly immunogenic character, we reasoned that these high-molecular-weight proteins warranted consideration as possible vaccine candidates. Subsequently, we reported that immunization with the Haemophilus high-molecular-weight adhesion proteins was protective in the chinchilla model of otitis media (4).
In the present article, we report on the ability of human antibodies directed against these HMW1/HMW2-like proteins to mediate opsonophagocytosis of nontypeable H. influenzae in a modification of a recently described assay that employs HL-60 cells as effector cells (38). By employing guinea pig serum as a complement source, we were able to focus specifically on the ability of these antibodies to mediate opsonophagocytic killing of nontypeable H. influenzae, as opposed to complement-dependent bacteriolysis, because guinea pig serum is unable to support complement-dependent bactericidal activity with human antibodies (34).
MATERIALS AND METHODS
Bacterial strains.
The nontypeable H. influenzae strains used in these studies have been described previously (6). The five strains that are the focus of this investigation were all isolated in pure culture from middle ear fluid specimens from children with acute otitis media. Each strain was identified as H. influenzae by standard methods and was classified as nontypeable by its failure to agglutinate with a panel of typing antisera for H. influenzae types a to f (Burroughs Wellcome Co., Research Triangle Park, N.C.) and failure to show lines of precipitation with these antisera in counterimmunoelectrophoresis assays. Strains 12, 15, 16, and 17 were representative strains known to express high-molecular-weight HMW1/HMW2-like adhesion proteins (7). Strain 11 is our prototype strain that expresses the Hia high-molecular-weight adhesion protein (9) but not HMW1/HMW2-like proteins. Strains 12-4 and 15-1 are isogenic derivatives of the respective parent strains that are deficient in expression of the HMW proteins (40). All organisms were stored at −70°C in skim milk within two or three subpassages of the initial clinical isolation.
Growth conditions of bacteria for opsonophagocytosis assay.
Bacteria were recovered from skim milk stocks by transfer of a loopful of thawed organisms to a chocolate agar plate and incubation for 16 h at 37°C in a 5% CO2 atmosphere. The next day, 5 to 10 colonies were isolated with a sterile loop and used to inoculate 50 ml of brain heart infusion broth supplemented with NAD and hemin, each at 10 μg per ml. Growth proceeded for 4 to 6 h at 37°C in 250-ml Erlenmeyer flasks with a shaker-incubator (model number G25; New Brunswick Scientific Co., Inc., Edison, N.J.). Bacteria in mid-log phase with an A600 of 0.5 to 0.6 were harvested by centrifugation at 12,000 × g at 4°C and washed twice with Veronal-buffered saline with 0.5% bovine serum albumin (BSA) and CaCl2 and MgCl2 at final concentrations of 0.15 mM and 0.5 mM, respectively. In preliminary experiments, bacteria demonstrated no loss of viability when maintained in this buffer for up to 4 h at 0 or 37°C. The washed bacterial cells were maintained at 0°C for less than 1 h before being used in the opsonophagocytosis assay described below.
Purification of HMW adhesion proteins.
The native high-molecular-weight adhesion proteins HMW1 and HMW2 were purified from prototype nontypeable Haemophilus strains as previously described (4). The frozen bacterial stock culture was streaked onto a chocolate plate and allowed to grow overnight at 37°C in an atmosphere of 5% CO2. The following day a 50-ml starter culture of brain heart infusion (BHI) broth supplemented with hemin and NAD was inoculated with 5 to 10 colonies. The starter culture was shaken at 37°C in a rotary incubator at 250 rpm until the culture reached an A600 of 0.6 to 0.8. Six 500-ml flasks of supplemented BHI broth were then inoculated with 8 to 10 ml of the bacterial suspension from the starter culture and allowed to grow to an optical density of 1.2 to 1.5. Bacterial cells from the six flasks were pelleted by centrifugation at 12,000 × g for 10 min at 4°C and frozen overnight at −20°C in preparation for purification of the proteins.
The following day, the bacterial pellets from the six flasks were resuspended uniformly and combined in 250 ml of extraction solution, consisting of 0.5 M NaCl, 0.01 M disodium EDTA, 0.01 M Tris, and 50 μM 1,10-phenanthroline, pH 7.5. The bacterial cells were not sonicated or otherwise mechanically disrupted, but simply resuspended and allowed to incubate at 0°C for 60 min. The bacterial suspensions were then centrifuged at 12,000 × g for 10 min at 4°C to remove the majority of intact cells and cellular debris. The supernatant, containing the water-soluble high-molecular-weight adhesion proteins, was then subjected to centrifugation at 100,000 × g for 60 min at 4°C to remove membrane fragments and additional debris. The supernatant from this ultracentrifugation step, containing the high-molecular-weight proteins, was dialyzed overnight at 4°C against 0.01 M sodium phosphate, pH 6.0.
The next day, the sample was centrifuged at 12,000 × g for 10 min at 4°C to remove insoluble debris, which sometimes precipitated from the solution during overnight dialysis. The supernatant was then applied to a 10-ml carboxymethyl-Sepharose column (Sigma Chemical Co., St. Louis, Mo.) which had been preequilibrated with 0.01 M sodium phosphate, pH 6.0. Following application of the protein-containing sample, the column was washed with 2 column volumes of 0.01 M sodium phosphate, and the proteins were eluted with a 0 to 0.5 M KCl gradient. Column fractions were analyzed on Coomassie gels to identify fractions containing high-molecular-weight proteins. Column fractions containing the high-molecular-weight proteins were pooled, concentrated to a volume of 1 to 3 ml, and maintained at 0°C overnight.
The next day the sample was applied to a Sepharose CL-6B (Sigma) gel filtration column equilibrated with phosphate-buffered saline (PBS), pH 7.5. Column fractions containing high-molecular-weight proteins free of contamination by lower-molecular-weight species were identified by analysis on Coomassie gels. The relevant fractions were pooled and stored at −70°C in preparation for coupling to the affinity matrix.
Preparation of HMW protein affinity columns.
Each of the purified protein preparations was coupled to cyanogen bromide-activated Sepharose 4B (Sigma) with standard techniques to generate solid-phase adsorbent columns. In brief, 15 to 20 mg of each purified protein was dissolved in 5 ml of coupling buffer (0.1 M sodium bicarbonate, 0.5 M NaCl, pH 8.3) and reacted overnight at 4°C with gentle rocking with 0.75 g of activated Sepharose 4B. Residual reactive groups were then blocked by the addition to the gel suspensions of 1 ml of 3 M ethanolamine and incubation until the following day at 4°C. The gel-protein matrices were then washed extensively with alternating volumes of coupling buffer and acetate buffer (0.1 M sodium acetate, 0.5 M NaCl, pH 4.0) to remove noncovalently bound HMW proteins. Columns were then equilibrated with PBS-0.5% BSA prior to use for affinity chromatography. Total swollen gel volume was approximately 2 ml for each column. A control column was prepared with BSA as the coupled protein. The protocol used was as outlined above for the HMW proteins.
Affinity chromatography.
The intravenous immune globulin (IVIG) product used for preparation of the affinity-purified anti-HMW fractions was commercially available Immune Globulin Intravenous (Human) Gammagard S/D, 0.5 g (Baxter Healthcare Corporation, Glendale, Calif.). This preparation is a solvent- and detergent-treated sterile, freeze-dried preparation of highly purified immunoglobulin G (IgG) derived from large pools of human plasma (package insert, Baxter Healthcare Corporation, Glendale, Calif.). When reconstituted with sterile water, this preparation contains approximately 50 mg of protein per ml, of which at least 90% is gamma globulin. For reference, this final concentration is approximately five times the concentration of IgG present in normal human adult serum (10). Exactly 5 ml of the IVIG solution was passed over each of the four HMW protein affinity columns to generate the respective adsorbed fractions and allow recovery of affinity-purified anti-HMW antibody fractions. Strain-specific anti-HMW antibody, as measured by enzyme-linked immunosorbent assay (ELISA; see below), was removed with greater that 95% efficiency by each of the columns. The columns were next washed with 10 column volumes of PBS-0.5% albumin, and affinity-purified anti-HMW antibodies were then eluted with 2 column volumes of 0.1 M glycine, pH 2.5. The anti-HMW antibody fractions were immediately neutralized following elution with 1 M Tris, pH 8.0. Both the IVIG-adsorbed fractions and the affinity-purified anti-HMW antibody fractions were dialyzed extensively against PBS at 4°C before further use. Prior to use in the opsonophagocytic assay, the affinity-purified strain-specific anti-HMW fractions were concentrated so that their anti-HMW titers as determined by ELISA were equivalent to those of the original starting IVIG solution.
ELISA methodology.
Antibody directed against the native HMW1 and HMW2 proteins was determined by ELISA in an assay modified slightly from that described previously (4). In brief, 96-well flat-bottomed enzyme immunoassay microtitration plates (Linbro/Titertek; Flow Laboratories, Inc, McClean, Va.) were coated overnight at 4°C with a purified HMW1/HMW2 mixture (10 μg of total protein per ml) in NaCO3 buffer, pH 9.6. The following day, the plates were blocked with PBS-0.5% BSA at room temperature for 1 h. The plates were then washed with PBS-0.5% BSA-0.05% Tween 20 prior to addition of the gamma globulin preparation or derived fractions. The test samples were serially diluted in PBS-0.5% BSA-0.05% Tween 20 with a starting dilution of 1:100. Samples were incubated for 1 h at room temperature. Following additional washes, the wells were incubated with a 1:3,000 dilution of goat anti-human IgG antibody (Life Technologies, Gaithersburg, Md.) and incubation was also carried out for 1 h at room temperature. Following additional washes, the wells were incubated with 200 μl of a 1-mg/ml solution of p-nitrophenylphosphate in 10% diethanolamine buffer (pH 9.8). Absorbance was monitored at 405 nm with a Titertek multiscan spectrophotometer (Flow Laboratories).
Adsorption of affinity-purified anti-HMW antibody preparations with parent or HMW-deficient mutant nontypeable H. influenzae.
Nontypeable H. influenzae strains 12 and 15 and their isogenic HMW-deficient mutants were grown as described above. Following buffer washes, each of the bacterial strains was resuspended in Veronal-buffered saline to a final optical density of approximately 2.0. A 300-μl volume of each of these suspensions was mixed in a microcentrifuge tube with 300 μl of affinity-purified antibodies prepared against either strain 12 or strain 15 HMW proteins. The mixtures were incubated for 18 h overnight with constant rocking at 4°C. Following incubation, bacterial cells and bound antibodies were removed by centrifugation, and the supernatants were recovered in preparation for analysis in the opsonophagocytic assay.
Growth and differentiation of HL-60 cells.
The methods used for growth and differentiation of the HL-60 cells were based upon those described previously by Romero-Steiner and coworkers (38). Cells of the tissue culture cell line HL-60 (promyelocytic leukemia cells; CCL 240; American Type Culture Collection, Rockville, Md.), which is of human origin, were used as the effector cells. A frozen stock passage obtained from the American Type Culture Collection was diluted 1:20 and expanded in tissue culture flasks (Costar T-75; Corning, Corning, N.Y.) to a cell density of approximately 6 × 105 cells per ml in 85% RPMI 1640 medium containing 1% l-glutamine (Life Technologies, Grand Island, N.Y.) supplemented with 15% fetal bovine serum (Life Technologies) and antibiotics (1× penicillin-streptomycin solution; Life Technologies). Cells were grown in suspension to approximately 106 cells per ml at 37°C in a 5% CO2 atmosphere.
Undifferentiated cells were divided two to three times per week (starting inoculum, 2 × 105 cells per ml) to maintain cell densities in the desired range. Undifferentiated cells grown to a cell density of approximately 5 × 105 per ml were used for differentiation. Differentiation was carried out in cultures with 180-ml volumes (T-150) of RPMI 1640 medium containing 1% l-glutamine, 15% fetal bovine serum, 100 mM N,N-dimethylformamide (99.8% purity; Sigma), and no antibiotics. A sufficient volume of the undifferentiated cell culture suspension was used to provide 2 × 105 cell per ml in 180 ml in the differentiated cell culture flask. Undifferentiated cells were pelleted by centrifugation (150 × g for 8 min at room temperature) and resuspended in 150 ml of medium in a T-150 flask. N,N-Dimethylformamide (1.5 ml) was added to a 30-ml aliquot of culture medium, mixed thoroughly, and then added to the 150-ml cell suspension. Cultures were incubated in a horizontal position at 37°C in a 5% CO2 atmosphere for 5 to 7 days. Differentiation medium was not replaced during the incubation period. Granulocytic differentiation was determined by visual examination of the tissue culture flasks with an inverted microscope for characteristic morphological changes of differentiation and by microscopic examination of Giemsa-stained smears.
Opsonophagocytic assay with HL-60 cells.
Differentiated HL-60 cells were used in the opsonophagocytic assay at an effector-to-target cell ratio of approximately 100:1. Differentiated cells were harvested by centrifugation (150 × g for 8 min at room temperature). The volume of differentiated cell culture required per microtiter plate was determined by the viable cell count of the culture (always ≥90% for the cells to be considered acceptable for use) and by the number of microtiter wells being used in a given day's assay. The appropriate volume was centrifuged as described above, and the supernatant was discarded, removing any excess medium. The cell pellet was resuspended in Hanks' buffer without Ca2+ and Mg2+ (Life Technologies) at 5 ml per 50 ml of centrifuged cell culture. The resuspended cells were kept at 37°C in a 5% CO2 atmosphere until immediately before use in the functional assay. At this point, the cell suspension was centrifuged as described above, and the supernatant was discarded. The cell pellet was gently resuspended in opsonophagocytosis buffer (3 ml of Veronal-buffered saline with Ca2+ and Mg2+ and 0.5% BSA) (6) and used immediately in the functional assay.
For the functional assay, 20 μl of each antibody preparation was aliquoted into a well (round-bottomed) in the first row of the microtiter plate (Costar, Cambridge, Mass.). Samples were serially diluted (twofold) in 10 μl of opsonophagocytosis buffer for from four to eight dilutions, depending upon the assay. Once all serum samples were diluted, 20 μl of the bacterial suspension appropriately diluted in opsonophagocytosis buffer (approximately 5 × 103 CFU) was added to each well. The bacterial suspension was prepared by dilution of freshly grown and harvested log-phase bacteria prepared as described above. The assay plate was allowed to incubate at 37°C in a 5% CO2 atmosphere for 15 min. Following this incubation period, 15 μl of complement source (sterile guinea pig serum; Invitrogen Life Technologies, Carlsbad, Calif., or Rockland Inc., Gilbertsville, Pa.) was added to each well. Guinea pig serum was kept frozen at −70°C in 0.5-ml aliquots until used.
Immediately after the addition of complement, 60 μl of washed differentiated HL-60 cells (approximately 5 × 105 cells) was added to each well. The assay plate was then incubated at 37°C for 90 min with horizontal shaking (220 rpm) in room air to promote the phagocytic process. At the end of the incubation period, a 10-μl aliquot from each well was plated onto a chocolate agar plate and spread for subsequent counting. Culture plates were incubated overnight at 37°C, and viable colony counts were performed the following day. Complement control wells included all of the test reagents except the antibody preparation and were included in the analysis of each strain under study. In addition, each antibody sample tested had a heat-inactivated complement control included on the same plate. Each antibody sample was run in duplicate on the same plate.
Means and standard deviations for measured killing at each antibody dilution were calculated with the heat-inactivated control sample in each dilution series as the reference. Statistical analyses were performed with the NCSS 2000 software package (NCSS, Kaysville, Utah). Opsonophagocytosis titers were defined as the reciprocal of the serum or antibody dilution that resulted in ≥50% killing of the bacterial inoculum compared to growth in the heat-inactivated complement control wells.
RESULTS
Opsonophagocytosis assay for measurement of functional antibody against nontypeable H. influenzae.
Our principal goal in this investigation was to assess the contribution of antibodies directed against the HMW1/HMW2-like proteins of nontypeable H. influenzae to the opsonophagocytic activity present in the natural human antibody repertoire. As a source of antibodies, we used a commercially available intravenous immunoglobulin preparation, Gammagard S/D. This preparation is a freeze-dried preparation of highly purified IgG derived from large pools of human plasma. The manufacturing process isolates IgG without additional chemical or enzymatic modification, and the Fc portion of the molecule remains intact. The antibody preparation contains all of the IgG antibody activities that are present in the donor population, and the IgG subclasses are present in a distribution similar to that in normal plasma (package insert, Baxter Healthcare Corporation, Glendale, Calif.). The nontypeable H. influenzae antibody specificities of such a preparation would be predicted to be representative of the normal antibody repertoire of adults. Of note, Romero-Steiner and coworkers used a similar commercially available antibody preparation in standardization of their opsonophagocytic assay (38).
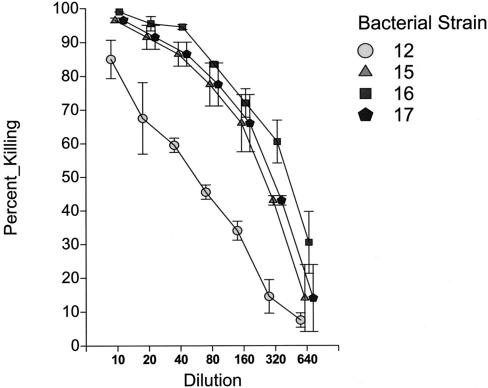
The IVIG preparation was first monitored for its ability to mediate killing activity in the opsonophagocytic assay with a panel of four prototype nontypeable H. influenzae strains. These four strains were all known to express HMW1/HMW2-like adhesion proteins. As shown in Table 1, measured opsonophagocytic titers ranged from 1:80 to 1:320 for the four strains tested. Figure 1 demonstrates a composite set of killing curves for these four strains. As can be seen, each of the strains demonstrated a concentration-dependent decrease in killing as the IVIG preparation was serially diluted. On repeated measurements, the titer at which 50% or greater killing was demonstrable was always within one tube dilution of the listed titers. It should be noted that the range of IVIG dilutions tested, 1:10 to 1:1,280, corresponds to IgG concentrations of 500 to 1.8 mg per dl, concentrations well within the normal concentration range of IgG antibody in adult serum (normal adult values, 640 to 1,350 mg of IgG per dl) (10). None of the bacterial strains was killed by the IVIG preparation if guinea pig complement alone (without HL-60 cells) was added to the reaction wells, consistent with the published literature (34).
TABLE 1.
Opsonophagocytic activity of intravenous immunoglobulin (Gammagard) pre- and post-adsorption with HMW adhesion proteins against a panel of nontypable H. influenzae strainsa
| Strain | Opsonophagocytic titer
|
|
|---|---|---|
| Preadsorption | Postadsorption | |
| 12 | 80 | 20 |
| 15 | 320 | 80 |
| 16 | 320 | 80 |
| 17 | 320 | 80 |
Each strain was tested with either unadsorbed intravenous immunoglobulin or intravenous immunoglobulin adsorbed with HMW proteins purified from the homologous strain.
FIG. 1.
Opsonophagocytic activity of a commercial preparation of intravenous immunoglobulin (Gammagard) assayed against a panel of nontypeable H. influenzae strains.
Preparation and characterization of HMW adsorbed fractions and affinity-purified anti-HMW antibodies.
We next examined the effect of removal of antibodies directed against the HMW1/HMW2-like adhesion proteins on the opsonophagocytic activity measurable in the IVIG preparation. Solid phase affinity columns were prepared with purified HMW1/HMW2-like proteins from each of the prototype strains and the IVIG preparation was adsorbed on each of the columns to remove antibodies specific for the proteins. As a control, we also prepared an affinity column with bovine serum albumin coupled to the affinity matrix. Strain-specific anti-HMW antibodies were subsequently eluted from each column for further study. The efficiency of column adsorption and recovery of affinity-purified antibodies were monitored both by ELISA and by Western blot.
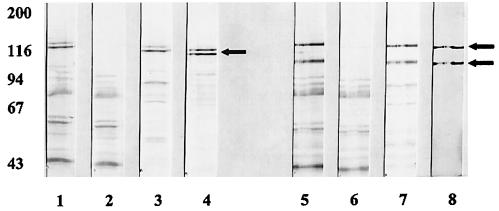
Shown in Fig. 2 is a Western blot with representative serum fractions assayed against cell sonicates of two of our prototype strains, strain 12 sonicates (lanes 1 to 4) and strain 15 sonicates (lanes 5 to 8). Lanes 1 and 5 demonstrate the reactivity of the unadsorbed IVIG preparation with sonicates of strain 12 and of strain 15, respectively. As can be seen, the preparation had significant reactivity with a number of protein species in each strain. Lanes 2 and 6 demonstrate the reactivity of the IVIG preparation after adsorption on the strain 12 HMW and strain 15 HMW affinity columns, respectively. As can be seen, such adsorption resulted in loss of reactivity with two prominent high-molecular-weight bands for each strain, as indicated by the arrows. These bands correspond to the HMW1 and HMW2 proteins, in the case of strain 12, and the corresponding HMW1- and HMW2-like proteins, in the case of strain 15. Reactivity with the remainder of the bands remained virtually unchanged.
FIG. 2.
Western immunoblot assay with a commercial preparation of intravenous immunoglobulin (Gammagard) assayed against cell sonicates of nontypeable H. influenzae isolates strain 12 (lanes 1 to 4) and strain 15 (lanes 5 to 8). Antibody preparations assayed in each are as follows: lane 1, IVIG; lane 2, IVIG adsorbed on strain 12 HMW protein affinity column; lane 3, affinity-purified anti-strain 12 HMW protein antibodies; lane 4, monoclonal antibody 4B2, an antibody that recognizes both the HMW1 and HMW2 proteins; lane 5, IVIG; lane 6, IVIG adsorbed on strain 15 HMW protein affinity column; lane 7, affinity-purified anti-strain 15 HMW protein antibodies; lane 8, monoclonal antibody 1D5.
Evidence that the bands against which reactivity was removed are in fact members of the HMW family of proteins is demonstrated in lanes 4 and 8. These are lanes in which monoclonal antibodies that recognize common epitopes on the HMW1- and HMW2-like proteins were reacted with the respective sonicates, monoclonal antibody 4B2 in lane 4 and monoclonal antibody 1D5 in lane 8. Lanes 3 and lane 7 demonstrate the reactivity of the affinity-purified antibodies recovered from the strain 12 HMW and the strain 15 HMW affinity columns, respectively. As can be seen in these lanes, the affinity-purified antibodies had predominant activity directed against the same bands against which reactivity was removed in the adsorbed fractions. Lesser reactivity with lower-molecular-weight bands is also noted. This latter reactivity likely represents reaction with breakdown products of the mature HMW proteins. We have observed that the HMW proteins are very susceptible to proteolysis. Of note, in this regard, the monoclonal antibodies also recognized a number of lower-molecular-weight species. This is perhaps best appreciated in careful examination of the reactivity of monoclonal antibody 4B2 with the strain 12 sonicate (lane 4).
Effect of adsorption with HMW adhesion proteins on the opsonophagocytic activity of the intravenous immunoglobulin preparation.
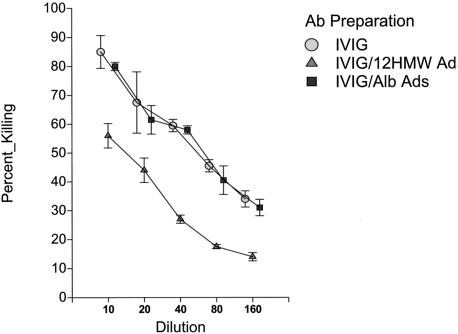
Having prepared the various HMW-adsorbed fractions noted above, we next examined the functional activity of these fractions in the opsonophagocytic assay. Shown in Fig. 3 are results of a representative opsonophagocytic assay when samples before and after removal of antibodies specific for the HMW proteins were examined. As can be seen, removal of anti-HMW antibodies led to a substantial decrease in opsonophagocytic killing activity. In contrast, no evidence of decreased killing was observed for the IVIG sample adsorbed on the control albumin affinity column.
FIG. 3.
Opsonophagocytic activity of a preparation of intravenous immunoglobulin (Gammagard) pre- and postadsorption on either a strain 12 HMW protein affinity column or an albumin control affinity column assayed against nontypeable H. influenzae strain 12.
Shown in the Table 1 are the measured titers of the IVIG preparation against each of the four strains before and after removal of strain-specific HMW antibodies. As can be seen, removal of anti-HMW antibodies resulted in fourfold decreases in the measured opsonophagocytic titer for each of the strains examined. It should be emphasized that each of these experiments tested the effect of removal of antibodies against the homologous strain. Little to no decrease in titer was demonstrable if the HMW-adsorbed IVIG preparations were tested against heterologous strains. Furthermore, as inferred above, no decrease in titer was demonstrable with any of the strains when the IVIG preparation adsorbed on the albumin control column was compared to the unadsorbed preparation.
Opsonophagocytic activity of affinity-purified anti-HMW antibodies against homologous and heterologous nontypeable H. influenzae strains.
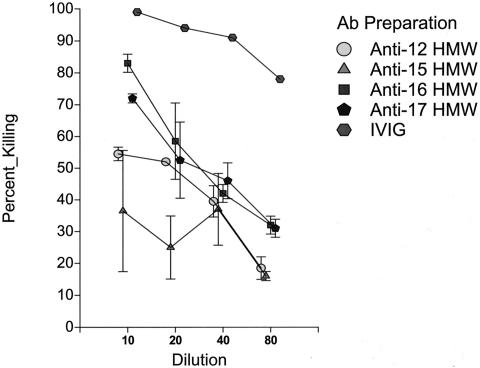
Finally, having prepared the affinity-purified anti-HMW antibody preparations as described above, we examined their functional activity in the opsonophagocytic assay against both homologous and heterologous strains. Shown in Fig. 4 are representative results of the opsonophagocytic assay with one of our prototype bacterial strains when assayed with the entire panel of affinity-purified anti-HMW preparations. As can be seen, each of the affinity-purified anti-HMW preparations was able to mediate opsonophagocytic killing at some level against strain 16. In contrast, the affinity-purified antialbumin control preparation had no demonstrable activity (data not shown). .
FIG. 4.
Opsonophagocytic activity of a preparation of intravenous immunoglobulin (Gammagard) and affinity-purified anti-HMW antibodies derived from that preparation assayed against nontypeable H. influenzae strain 16.
Table 2 summarizes the opsonophagocytic titers of each of the affinity-purified antibody preparations against the entire panel of prototype strains. Although the measured titers are clearly much lower than those of the IVIG starting preparation, the data demonstrate opsonophagocytic activity of the affinity-purified anti-HMW preparations against both homologous and heterologous nontypeable H. influenzae strains that express the HMW1/HMW2-like proteins. None of the anti-HMW preparations had demonstrable opsonophagocytic killing activity against nontypeable Haemophilus strain 11, our prototype strain that expresses the Hia adhesion protein but does not express an HMW1/HMW2-like protein (data not shown). However, it should be noted that strain 11 was efficiently killed in the opsonophagocytic assay at a titer of 1:80 by the intravenous immunoglobulin preparation
TABLE 2.
Opsonophagocytic activity of affinity-purified anti-HMW antibodies purified from intravenous immunoglobulin against a panel of nontypable H. influenzae strainsa
| Anti-HMW Prepn | Opsonophagocytic titer for strain:
|
||||
|---|---|---|---|---|---|
| 12 | 15 | 16 | 17 | 11 | |
| Anti-12 | 40 | 20 | 20 | 80 | 0 |
| Anti-15 | 20 | 80 | <10 | 80 | 0 |
| Anti-16 | 40 | 40 | 20 | 80 | 0 |
| Anti-17 | 20 | 40 | 20 | 80 | 0 |
Strains 12, 15, 16, and 17 all express proteins of the HMW1/HMW2 family. Strain 11 does not express proteins of the HMW1/HMW2 family.
Adsorption of affinity-purified anti-HMW antibody preparations with nontypeable H. influenzae parent strains and isogenic HMW-deficient mutants.
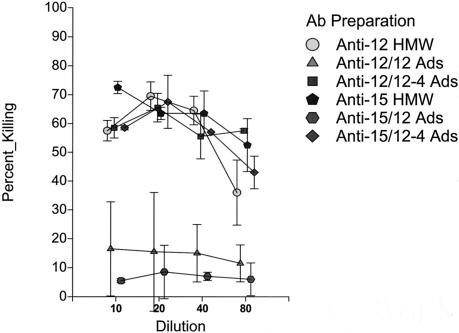
To confirm the specificity of the observed opsonophagocytic killing just described for the HMW1/HMW2-like proteins, we adsorbed representative anti-HMW antibody preparations with nontypeable H. influenzae parent strains and their respective isogenic HMW-deficient mutants. Shown in Fig. 5 are the results of such an experiment when anti-12 HMW and anti-15 HMW antibody preparations were adsorbed either with nontypeable H. influenzae strain 12 or with the isogenic HMW-deficient strain 12-4 (40). As can be seen, adsorption of either the anti-12 HMW or the anti-15 HMW preparation with strain 12, a strain that expresses high levels of the HMW proteins, resulted in marked decreases in the opsonophagocytic killing activity. In contrast, adsorption with the isogenic HMW-deficient strain was not associated with any notable change in opsonophagocytic activity.
FIG. 5.
Opsonophagocytic activity of affinity-purified anti-HMW strain 12 or anti-HMW strain 15 antibodies pre- and postadsorption with nontypeable H. influenzae strain 12 or nontypeable H. influenzae strain 12-4, the isogenic mutant deficient in expression of HMW proteins. Each antibody preparation was assayed against strain 12.
In a second experiment, the same two anti-HMW preparations were adsorbed with nontypeable Haemophilus strain 15 and the isogenic HMW-deficient strain 15-1. Comparable results were obtained in that adsorption of either affinity-purified antibody preparation with the parent strain 15 that expresses HMW proteins was associated with a marked decrease in opsonophagocytic activity while adsorption with the HMW-deficient strain led to no demonstrable decrease in killing activity (data not shown).
DISCUSSION
Host immunity against nontypeable H. influenzae is mediated both by the relatively nonspecific components of the innate immune system (22, 31, 46, 47) and by specific antibodies that induce bacteriolysis of the infecting organisms (6, 32, 33) or that facilitate opsonophagocytosis in concert with host leukocytes and complement (34). Although several laboratories have published reports on the opsonophagocytic activity of antibodies directed against selected H. influenzae antigens (14, 24, 34), the details of the assays have varied and the assays in general have been hampered by nonuniformity of the phagocytic cell preparations used from day to day. These problems have precluded development of a standardized opsonophagocytic assay. Recently, Romero-Steiner and coworkers described the development of an opsonophagocytic assay for measurement of functional antibody activity against Streptococcus pneumoniae with differentiated HL-60 cells (38). The HL-60 cell line is a human promyelocytic continuous cell line that can be grown in suspension and differentiated into polymorphonuclear-like cells with polar organic compounds such as N,N-dimethylformamide (15). The induced cells possess the cell receptors necessary for an effective phagocytic function, primarily FcγII, CR1, and CR3 (38). The opsonophagocytic assay has been quite valuable for monitoring the functional activities of antibodies directed against Streptococcus pneumoniae in several different investigations (26, 28, 35), and the assay may ultimately be able to serve as an in vitro surrogate of protection against pneumococcal disease (38).
In the work described in this paper, the opsonophagocytic assay was adapted to allow the measurement of antibody with opsonophagocytic activity against nontypeable H. influenzae. Minor modifications in the assay were made, including changes in the preparation of the bacteria, changes in the buffer used for bacterial washes and the reaction mixture, and a change in the complement source. However, the basic protocol was as originally described. One small but important change was the substitution of guinea pig serum for rabbit serum as the source of complement. Because nontypeable H. influenzae is susceptible not only to opsonophagocytic killing but also to complement-dependent bactericidal killing, if the complement source is capable of mediating both sorts of activities, it would be difficult in an assay such as this to distinguish between the two. It is well known that rabbit complement can support complement-dependent bacteriolysis of nontypeable H. influenzae and, in fact, often has naturally occurring bactericidal activity for these organisms (18). In contrast, guinea pig serum as a complement source is unable to support complement-dependent bactericidal activity with human antibodies (34). Thus, in our modified assay we were able to focus specifically on the ability of the antibodies to mediate opsonophagocytic killing of nontypeable H. influenzae.
Removal of anti-HMW antibodies from the intravenous immunoglobulin preparation resulted in strain-specific decreases in opsonophagocytic activity of the resulting adsorbed fractions. In contrast, the affinity-purified anti-HMW antibodies recovered from affinity columns demonstrated opsonophagocytic activity, not only against the homologous strain, but also against heterologous strains. The explanation for these seemingly disparate findings remains speculative at this point. We know from DNA sequence analyses and examination of the molecular masses of the HWM proteins from unrelated strains that the HMW proteins demonstrate significant strain heterogeneity (7; authors' unpublished observations). However, these proteins have also been demonstrated to share a number of surface-accessible B-cell epitopes (8). Our speculation is that adsorption of anti-HMW antibodies from the IVIG preparation by affinity chromatography removes both strain-specific antibodies and other antibodies recognizing “common” epitopes on the HMW proteins. However, even after adsorption, many antibodies capable of mediating opsonophagocytic activity would be predicted to remain in the adsorbed fractions, including anti-HMW antibodies that recognize strain-specific epitopes on the HMW proteins of heterologous strains. Thus, it is not surprising that the opsonophagocytic activity of the adsorbed fractions against heterologous strains is little changed by adsorption. The affinity-purified antibody fractions, containing antibodies against both strain-specific and common epitopes on the HMW proteins, would be predicted to be capable of mediating opsonophagocytic activity against both homologous and heterologous strains, as we demonstrated experimentally. Additional characterization of the immunogenic epitopes present on the HMW proteins should help to further clarify these issues.
Clear differences were observed among our four prototype strains in their susceptibility to opsonophagocytic killing. For example, the opsonophagocytic activity of the IVIG preparation against strain 12 was fourfold lower than that demonstrated against the other three strains. Differences were also seen in the susceptibility of strains to killing by the affinity-purified antibody preparations. The explanation for these differences can only be speculated upon at this point. The bacterial surface of nontypeable H. influenzae is a very dynamic structure and a number of surface molecules, some of which may undergo antigenic and phase variation, are known to influence the interactions of bacteria with the host immune system (13, 16, 18, 46, 47). In the case of the HMW proteins, variation in expression level may directly influence the ability of the immune system to clear infection in vivo (5) and similar differences in levels of HMW protein expression could influence the ability of antibodies directed against these proteins to mediate opsonophagocytic activity in vitro. At present, we have no information on any differences that may exist in the lipooligosaccharide phenotypes or in the relative levels of expression of the HWM proteins in our panel of prototype strains, but these may be fruitful areas to pursue in future investigations.
Antibodies specific for the HMW1/HMW2-like proteins are not capable of mediating bacteriolysis in a standard bactericidal assay for nontypeable H. influenzae (6, Authors' unpublished observations). However, with our modification of the Romero-Steiner assay, we demonstrated that antibodies directed against the HMW1/HMW2-like proteins are major mediators of the opsonophagocytic activity present in the natural human antibody repertoire. These data suggest that antibodies specific for the HMW1/HMW2-like proteins may have an important role in human host immunity. We also demonstrated that antibodies directed against the HMW1/HMW2-like proteins could mediate opsonophagocytic activity not only against homologous, but also against heterologous nontypeable H. influenzae strains. These data suggest that common epitopes recognized by opsonophagocytic human antibodies are present on the HMW1/HMW2-like proteins of nontypeable H. influenzae. Demonstration that the HMW1/HMW2-like adhesion proteins of nontypeable H. influenzae are targets of naturally acquired opsonophagocytic human antibodies and that shared epitopes recognized by such antibodies exist argue for the continued investigation of these proteins as potential vaccine candidates for prevention of nontypeable H. influenzae disease.
Acknowledgments
We thank Sandra Romero-Steiner for helpful discussions in development of the opsonophagocytic assay for nontypeable H. influenzae.
The work was supported by Public Health Service grant AI 48066 and by funds provided by Aventis Pasteur Canada.
Editor: J. N. Weiser
REFERENCES
- 1.Akkoyunlu, M., H. Janson, M. Ruan, and A. Forsgren. 1996. Biological activity of serum antibodies to a nonacylated form of lipoprotein D of Haemophilus influenzae. Infect. Immun. 64:4586-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakaletz, L. O., B.-J. Kennedy, L. A. Novotny, G. Duquesne, J. Cohen, and Y. Lobet. 1999. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect. Immun. 67:1745-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakaletz, L. O., E. R. Leake, J. M. Billy, and P. T. Kaumaya. 1997. Relative immunogenicity and efficacy of two synthetic chimeric peptides of fimbrin as vaccinogens against nasopharyngeal colonization by nontypeable Haemophilus influenzae in the chinchilla. Vaccine 15:955-961. [DOI] [PubMed] [Google Scholar]
- 4.Barenkamp, S. J. 1996. Immunization with high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae modifies experimental otitis media in chinchillas. Infect. Immun. 64:1246-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barenkamp, S. J. 1986. Protection by serum antibodies in experimental nontypeable Haemophilus influenzae otitis media. Infect. Immun. 52:572-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barenkamp, S. J., and F. F. Bodor. 1990. Development of serum bactericidal activity following nontypeable Haemophilus influenzae acute otitis media. Pediatr. Infect. Dis. J. 9:333-339. [DOI] [PubMed] [Google Scholar]
- 7.Barenkamp, S. J., and E. Leininger. 1992. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect. Immun. 60:1302-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barenkamp, S. J., and J. W. St Geme, 3rd. 1996. Identification of surface-exposed B-cell epitopes on high molecular-weight adhesion proteins of nontypeable Haemophilus influenzae. Infect. Immun. 64:3032-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barenkamp, S. J., and J. W. St. Geme, 3rd. 1996. Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typable Haemophilus influenzae. Mol. Microbiol. 19:1215-1223. [DOI] [PubMed] [Google Scholar]
- 10.Behrman, R. 1996. Laboratory medicine and reference tables, p. 2031-2084. In R. Behrman, R. Kliegman, and A. Arvin (ed.), Nelson textbook of pediatrics. W. B. Saunders Company, Philadelphia, Pa.
- 11.Berman, S. 1995. Otitis media in developing countries. Pediatrics 96:126-131. [PubMed] [Google Scholar]
- 12.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, and K. Edwards. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 13.Bouchet, V., D. W. Hood, J. Li, J. R. Brisson, G. A. Randle, A. Martin, Z. Li, R. Goldstein, E. K. Schweda, S. I. Pelton, J. C. Richards, and E. R. Moxon. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. USA 100:8898-8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cates, K. L., K. H. Marsh, and D. M. Granoff. 1985. Serum opsonic activity after immunization of adults with Haemophilus influenzae type b-diphtheria toxoid conjugate vaccine. Infect. Immun. 48:183-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins, S. J., F. W. Ruscetti, R. E. Gallagher, and R. C. Gallo. 1978. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc. Natl. Acad. Sci. USA 75:2458-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawid, S., S. J. Barenkamp, and J. W. St Geme, 3rd. 1999. Variation in expression of the Haemophilus influenzae HMW adhesins: a prokaryotic system reminiscent of eukaryotes. Proc. Natl. Acad. Sci. USA 96:1077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Adhami, W., J. M. Kyd, D. A. Bastin, and A. W. Cripps. 1999. Characterization of the gene encoding a 26-kilodalton protein (OMP26) from nontypeable Haemophilus influenzae and immune responses to the recombinant protein. Infect. Immun. 67:1935-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erwin, A. L., Y. A. Brewah, D. A. Couchenour, P. R. Barren, S. J. Burke, G. H. Choi, R. Lathigra, M. S. Hanson, and J. N. Weiser. 2000. Role of lipopolysaccharide phase variation in susceptibility of Haemophilus influenzae to bactericidal immunoglobulin M antibodies in rabbit sera. Infect. Immun. 68:2804-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, and P. H. Makela. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 20.Giebink, G. S. 2001. The prevention of pneumococcal disease in children. N. Engl. J. Med. 345:1177-1183. [DOI] [PubMed] [Google Scholar]
- 21.Gnehm, H. E., S. I. Pelton, S. Gulati, and P. A. Rice. 1985. Characterization of antigens from nontypeable Haemophilus influenzae recognized by human bactericidal antibodies. Role of Haemophilus outer membrane proteins. J. Clin. Investig. 75:1645-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gould, J. M., and J. N. Weiser. 2002. The inhibitory effect of C-reactive protein on bacterial phosphorylcholine platelet-activating factor receptor-mediated adherence is blocked by surfactant. J. Infect. Dis. 186:361-371. [DOI] [PubMed] [Google Scholar]
- 23.Gu, X. X., J. Sun, S. Jin, S. J. Barenkamp, D. J. Lim, J. B. Robbins, and J. Battey. 1997. Detoxified lipooligosaccharide from nontypeable Haemophilus influenzae conjugated to proteins confers protection against otitis media in chinchillas. Infect. Immun. 65:4488-4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen, E. J., D. A. Hart, J. L. McGehee, and G. B. Toews. 1988. Immune enhancement of pulmonary clearance of nontypeable Haemophilus influenzae. Infect. Immun. 56:182-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson, F. W., A. M. Collier, M. A. Sanyal, J. M. Watkins, D. L. Fairclough, W. A. Clyde, Jr., and F. W. Denny. 1982. A longitudinal study of respiratory viruses and bacteria in the etiology of acute otitis media with effusion. N. Engl. J. Med. 306:1377-1383. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, S. E., L. Rubin, S. Romero-Steiner, J. K. Dykes, L. B. Pais, A. Rizvi, E. Ades, and G. M. Carlone. 1999. Correlation of opsonophagocytosis and passive protection assays with human anticapsular antibodies in an infant mouse model of bacteremia for Streptococcus pneumoniae. J. Infect. Dis. 180:133-140. [DOI] [PubMed] [Google Scholar]
- 27.Karasic, R. B., C. E. Trumpp, H. E. Gnehm, P. A. Rice, and S. I. Pelton. 1985. Modification of otitis media in chinchillas rechallenged with nontypeable Haemophilus influenzae and serological response to outer membrane antigens. J. Infect. Dis. 151:273-279. [DOI] [PubMed] [Google Scholar]
- 28.Kim, J. O., S. Romero-Steiner, U. B. Sorensen, J. Blom, M. Carvalho, S. Barnard, G. Carlone, and J. N. Weiser. 1999. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 67:2327-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein, J. O. 1994. Otitis media. Clin. Infect. Dis. 19:823-833. [DOI] [PubMed] [Google Scholar]
- 30.Loosmore, S. M., Y. P. Yang, R. Oomen, J. M. Shortreed, D. C. Coleman, and M. H. Klein. 1998. The Haemophilus influenzae HtrA protein is a protective antigen. Infect. Immun. 66:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lysenko, E. S., J. Gould, R. Bals, J. M. Wilson, and J. N. Weiser. 2000. Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract. Infect. Immun. 68:1664-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy, T. F., and L. C. Bartos. 1988. Human bactericidal antibody response to outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect. Immun. 56:2673-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy, T. F., L. C. Bartos, P. A. Rice, M. B. Nelson, K. C. Dudas, and M. A. Apicella. 1986. Identification of a 16,600-dalton outer membrane protein on nontypeable Haemophilus influenzae as a target for human serum bactericidal antibody. J. Clin. Investig. 78:1020-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musher, D. M., M. Hague-Park, R. E. Baughn, R. J. Wallace, Jr., and B. Cowley. 1983. Opsonizing and bactericidal effects of normal human serum on nontypeable Haemophilus influenzae. Infect. Immun. 39:297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nahm, M. H., J. V. Olander, and M. Magyarlaki. 1997. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae. J. Infect. Dis. 176:698-703. [DOI] [PubMed] [Google Scholar]
- 36.Paradise, J. L., C. A. Dollaghan, T. F. Campbell, H. M. Feldman, B. S. Bernard, D. K. Colborn, H. E. Rockette, J. E. Janosky, D. L. Pitcairn, D. L. Sabo, M. Kurs-Lasky, and C. G. Smith. 2000. Language, speech sound production, and cognition in three-year-old children in relation to otitis media in their first three years of life. Pediatrics 105:1119-1130. [DOI] [PubMed] [Google Scholar]
- 37.Paradise, J. L., H. E. Rockette, D. K. Colborn, B. S. Bernard, C. G. Smith, M. Kurs-Lasky, and J. E. Janosky. 1997. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics 99:318-333. [DOI] [PubMed] [Google Scholar]
- 38.Romero-Steiner, S., D. Libutti, L. B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae with differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schappert, S. 1992. National Center for Health Statistics: office visits for otitis media: United States, 1975-90. Advance data from vital and health statistics. No. 214. DHHS publication no. (PHS) 92-1250. Public Health Service, Atlanta, Ga. [PubMed]
- 40.St. Geme, J. W., 3rd, S. Falkow, and S. J. Barenkamp. 1993. High-molecular-weight proteins of nontypeable Haemophilus influenzae mediate attachment to human epithelial cells. Proc. Natl. Acad. Sci. USA 90:2875-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.St. Geme, J. W., 3rd, V. V. Kumar, D. Cutter, and S. J. Barenkamp. 1998. Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect. Immun. 66:364-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stool, S., A. Berg, S. Berman, et. al. 1994. Otitis media with effusion in young children. No. 12 of Clinical practice guideline. DHHS publication no. (AHCPR) 94-0622. Department of Health and Human Services, Washington, D.C.
- 43.Teele, D. W., J. O. Klein, C. Chase, P. Menyuk, and B. A. Rosner. 1990. Otitis media in infancy and intellectual ability, school achievement, speech, and language at age 7 years. Greater Boston Otitis Media Study Group. J. Infect. Dis. 162:685-694. [DOI] [PubMed] [Google Scholar]
- 44.Teele, D. W., J. O. Klein, and B. Rosner. 1989. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J. Infect. Dis. 160:83-94. [DOI] [PubMed] [Google Scholar]
- 45.Webb, D. C., and A. W. Cripps. 1999. Immunization with recombinant transferrin binding protein B enhances clearance of nontypeable Haemophilus influenzae from the rat lung. Infect. Immun. 67:2138-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiser, J. N., and N. Pan. 1998. Adaptation of Haemophilus influenzae to acquired and innate humoral immunity based on phase variation of lipopolysaccharide. Mol. Microbiol. 30:767-775. [DOI] [PubMed] [Google Scholar]
- 47.Weiser, J. N., N. Pan, K. L. McGowan, D. Musher, A. Martin, and J. Richards. 1998. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J. Exp. Med. 187:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]
- 49.Wu, T.-H., and X.-X. Gu. 1999. Outer membrane proteins as a carrier for detoxified lipooligosaccharide conjugate vaccines for nontypeable Haemophilus influenzae. Infect. Immun. 67:2118-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]