Abstract
Pili of pathogenic Neisseria are major virulence factors associated with adhesion, cytotoxicity, twitching motility, autoaggregation, and DNA transformation. Pili are modified posttranslationally by the addition of phosphorylcholine. However, no genes involved in either the biosynthesis or the transfer of phosphorylcholine in Neisseria meningitidis have been identified. In this study, we identified five candidate open reading frames (ORFs) potentially involved in the biosynthesis or transfer of phosphorylcholine to pilin in N. meningitidis. Insertional mutants were constructed for each ORF in N. meningitidis strain C311#3 to determine their effect on phosphorylcholine expression. The effect of the mutant ORFs on the modification by phosphorylcholine was analyzed by Western analysis with phosphorylcholine-specific monoclonal antibody TEPC-15. Analysis of the mutants showed that ORF NMB0415, now defined as pptA (pilin phosphorylcholine transferase A), is involved in the addition of phosphorylcholine to pilin in N. meningitidis. Additionally, the phase variation (high frequency on-off switching of expression) of phosphorylcholine on pilin is due to changes in a homopolymeric guanosine tract in pptA.
The genus Neisseria consists of gram-negative pathogenic and nonpathogenic bacteria (generally diplococci) that colonize mucosal surfaces. The two major pathogenic species are Neisseria gonorrhoeae and N. meningitidis, which are closely related and colonize only humans. Pili of pathogenic Neisseria are long filamentous structures of the type IV fimbria family that extend from the bacterial surface and consist primarily of the monomer pilin (27). Pili play a major role in disease and have been associated with adhesion, cytotoxicity, twitching motility, autoaggregation, and DNA transformation (15, 17, 22, 39). Phosphorylcholine (ChoP) is involved in the posttranslational modification of pili of pathogenic Neisseria (14, 42). ChoP is expressed by a range of organisms of the respiratory tract, including Streptococcus pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae (11). In S. pneumoniae, ChoP is attached to teichoic acid and lipoteichoic acid (5, 10, 20). In H. influenzae and commensal Neisseria, ChoP is attached to lipopolysaccharide (LPS) (25, 33, 46). ChoP increases the adherence of pneumococci and nontypeable H. influenzae (7, 35) and aids in the colonization of the nasopharynx by H. influenzae (35). Thus, it seems that ChoP is of general importance in the colonization of the respiratory tract (35). ChoP expression is phase variable in S. pneumoniae, H. influenzae, N. meningitidis, and commensal Neisseria, suggesting the existence of functional and/or immunological selection for the switching expression of ChoP (33, 42, 44, 46).
In both nontypeable H. influenzae and commensal Neisseria, ChoP is important in receptor binding and signal cascades, resulting in increased adherence to and invasion of epithelial cells via the platelet-activating factor receptor (32, 35). C-reactive protein binds to the ChoP epitope on teichoic acid of S. pneumoniae (36) and on LPS of H. influenzae and commensal Neisseria (33, 45). This binding results in decreased attachment via binding to the platelet-activating factor receptor (12). Also, C-reactive protein binding acts as an opsonin, activating complement via the classical pathway and resulting in a bactericidal effect (45). Therefore the phase-variable expression of ChoP seems to be a critical mechanism in respiratory bacterial colonization and pathogenesis. Variants expressing ChoP may be better adapted to colonization of the nasopharynx, while ChoP-negative variants may be able to avoid complement-mediated killing in serum (45).
In S. pneumoniae, H. influenzae, and commensal Neisseria, ChoP is attached to sugar moieties (teichoic acid, lipoteichoic acid, and LPS) (5, 10, 20, 25, 33, 46). In pathogenic Neisseria, ChoP is attached to the pilus subunit protein, pilin (42). To determine the gene(s) responsible for the biosynthesis, transfer, and phase-variable expression of ChoP on pilin, we analyzed homologues of known biosynthetic and transferase genes in both the ChoP-positive strain N. meningitidis C311#3 (42) and its ChoP-negative variant 26A (24a). This study describes the characterization of a gene involved in the addition of ChoP to pili in N. meningitidis and the mechanism responsible for the phase variation of ChoP expression.
MATERIALS AND METHODS
Bacterial strains and media.
The meningococcal strains used in this study were N. meningitidis C311#3 (40) and its phase variant 26A (which lacks ChoP) (Power et al., submitted) or clinical isolates (6). Meningococcal strains were grown on brain heart infusion agar (Oxoid) at 37°C with 5% CO2 for 16 to 18 h. Brain heart infusion agar plates were made with 1% agar and supplemented with 10% Levinthal base (1). All recombinant plasmids were replicated in Escherichia coli DH5α and grown on Luria-Bertani media (29). Luria-Bertani agar plates were supplemented with 1.5% agar (Difco). Ampicillin and kanamycin were used in media at a final concentration of 100 μg/ml each.
DNA manipulation and analysis.
Routine DNA manipulations were carried out essentially as described by Sambrook et al. (29). Nucleotide sequencing was carried out by using a Big Dye Terminator kit (29). The homopolymeric tract of NMB0415 was sequenced with primers NMB0415PolyGFor and NMB0415PolyGRev; the primer sequences are shown in Table 1. PCR was done essentially as previously described (28). Nucleotide analysis was performed by using the MacVector program (Oxford Molecular).
TABLE 1.
List of primers
| Primer name | Sequence (5′-3′) |
|---|---|
| NMB0415For | GAAATCGTCGGACAGGCTTTGTTAACTCG |
| NMB0415Rev | CACAGATTATTTTCCCATTCTCATTCGGC |
| NMB1638For | CCAACCCTTATCCGACACATTCCA |
| NMB1638Rev | ATGTCCATCAGCCCCAATACCGTGCTG |
| PmtAFor | TTGCGGGCAATAGTGCCTTCA |
| PmtARev | TGAGGGGTGCTTTCGTTGTAA |
| BetTFor | GGTTTTAACCGTGCCGGATCA |
| BetTRev | CTCTGCCAATTCCACCTGCTC |
| NMB0939For | CCGTGCAGTATATCGAAGTCAA |
| NMB0939Rev | CTGTCTTACCCGACGCGGTAAA |
| KanUpOut | TCCCGTTGAATATGGCTCA |
| NMB0415PolyGFor | TTACGTCGGCACAACCGCCCTA |
| NMB0415PolyGRev | CAACAACAGGCCGGCATCAGGT |
Southern blotting and hybridization.
Restriction endonuclease (StyI)-digested DNA was separated on a 1% agarose gel and blotted onto a GeneScreen polyvinylidene difluoride membrane (Perkin-Elmer) essentially as previously described (29). Primers NMB0415For and NMB0415Rev (primer sequences are shown in Table 1) were used to amplify the DNA fragment, which was then purified by using a QIAquick gel extraction kit (Qiagen). This product was labeled by using a DIG-High Prime DNA labeling kit (Roche), and hybridization was performed according to the manufacturer's instructions.
Construction of knockout mutants of the various ChoP biosynthetic and transferase homologues.
Candidate ChoP biosynthetic genes were PCR amplified with the primers shown in Table 2 (primer sequences are shown in Table 1) and cloned into vector pGEM-T-Easy (Promega). Plasmids containing the cloned genes were digested with a restriction endonuclease (Table 2) that cut only once within the open reading frame (ORF) and not in the cloning vector. A kanamycin resistance cassette was inserted into the various ORFs by ligation of the HincII 1.3-kb restriction fragment from plasmid pUC4Kan (Amersham Biosciences). The constructs of the knockout alleles were linearized with restriction enzyme NotI and transformed into N. meningitidis C311#3 as previously described (24). Kanamycin-resistant colonies were screened for the insertion of the kanamycin resistance cassette by PCR with various forward primers and KanUpOut (relevant primers and primer sequences are shown in Tables 1 and 2).
TABLE 2.
Genes knocked out and methods used
| System | ORF name | Homologue | %
|
Proposed function of homologue | Effect of mutant on TEPC-15 reactivity with pilin | Forward primer | Reverse primer | Insertion site | Construct name | |
|---|---|---|---|---|---|---|---|---|---|---|
| Identitya | Similaritya | |||||||||
| Biosynthesis or uptake | NMB1270 | pmtA | 32 | 47 | Methyltransferase of PEA of R. sphaeroides | − | PmtAFor | PmtARev | ClaI | pGEM-PmtAKan |
| NMB1277 | betT | 35 | 62 | Choline uptake of E. coli | − | BetTFor | BetTRev | BsmI | pGEM-BetTKan | |
| NMB0939 | pmtA | 28 | 42 | Methyltransferase of PEA of S. meliloti | − | NMB0939For | NMB0939Rev | BsmI | pGEM-NMB0939Kan | |
| Transferase | NMB0415 | lpt-3 | 27 | 44 | PEA transferase of N. meningitidis | + | NMB0415For | NMB0415Rev | SmaI | pGEM-NMB0415Kan |
| NMB1638 | lpt-3 | 26 | 44 | PEA transferase of N. meningitidis | − | NMB1638For | NMB1638Rev | BssHII | pGEM-NMB1638Kan | |
Western immunoblotting.
Pilin from parent strain C311#3, 26A, and the various C311#3 mutants constructed in this study was isolated as previously described (24). These samples were analyzed by Western blotting with monoclonal antibody (MAb) SM1 (specific for pilin) (41) and MAb TEPC-15 (specific for ChoP) (Sigma Chemical Co.) (42).
RESULTS
ChoP is not linked to the pilin-linked trisaccharide.
In respiratory pathogens, ChoP is usually attached to sugar moieties. In S. pneumoniae, ChoP is attached to teichoic acid and lipoteichoic acid (5, 10, 20), and in H. influenzae and commensal Neisseria, it is attached to LPS (25, 33, 46). As the pilin in N. meningitidis is modified by the pilin-linked trisaccharide, it is possible that ChoP could be attached to meningococcal pilin via this glycan. However, Western analysis of previously described pilin glycosylation mutants (pglA to pglE) (13, 24, 24a) showed there was no difference in the binding of MAb TEPC-15 to these mutants and to the wild type (results not shown).
Identification and analysis of ORFs potentially involved in ChoP biosynthesis in N. meningitidis.
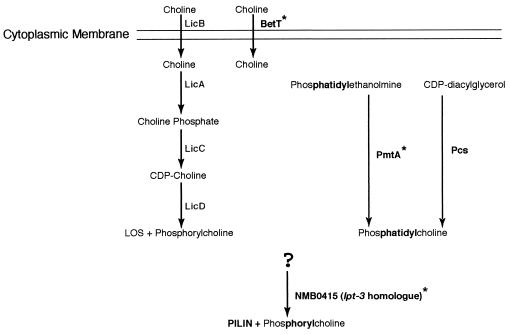
The mechanisms responsible for the phase variation of ChoP expression on pilin, the biosynthesis or acquisition of ChoP, and the transfer of ChoP to pili of Neisseria have not been described. In other bacteria, there are three known pathways for the biosynthesis of ChoP and phosphatidylcholine. These pathways are the lic-1 pathway (found in H. influenzae, S. pneumoniae, and commensal Neisseria) (33, 43, 48), the pmtA pathway (found in Rhodobacter sphaeroides, Sinorhizobium meliloti, Bradyrhizobium japonicum, and P. aeruginosa) (4, 8, 19, 47), and the pcs pathway (found in S. meliloti and P. aeruginosa) (8, 47) (Fig. 1 shows the details of these pathways). In commensal Neisseria, ChoP is synthesized by a pathway homologous to the lic-1 pathway of H. influenzae; however, the genes for this pathway are absent in pathogenic Neisseria (32, 33). The pmtA gene is responsible for three sequential methylations of phosphatidylethanolamine, resulting in phosphatidylcholine in P. aeruginosa, B. japonicum, R. sphaeroides, and S. meliloti (4, 8, 19, 47). Phosphatidylcholine could be a substrate for phosphorylcholine transfer.
FIG. 1.
Phosphatidylcholine and phosphorylcholine biosynthetic pathways showing biosynthetic genes and intermediates. The three described pathways are the lic-1 pathway (in grey), found in commensal Neisseria and H. influenzae (33, 43)—previous work showed that there are no homologues of this pathway in pathogenic Neisseria (32, 33); the pmtA pathway, found in Pseudomonas, Bradyrhizobium, Rhodobacter, and Sinorhizobium (4, 8, 19, 47); and the pcs pathway, found in Sinorhizobium and Pseudomonas (8, 47). Genes and proteins identified and characterized in this study are marked by asterisks. LOS, lipooligosaccharide.
TBLAST-X (2) homology searches were used to identify ORFs in the N. meningitidis MC58 genome (37) that were homologous to candidate ChoP biosynthetic genes. The homologues identified in this study are listed in Table 2. In addition to ChoP biosynthetic gene homologues, we investigated ORFs potentially involved in ChoP uptake. A previous study with 14C-labeled choline suggested that ChoP is not taken up from the medium and attached to pilin in pathogenic Neisseria (33); however, we identified an ORF that is homologous to choline transporter gene betT in the N. meningitidis genome (Table 2). In E. coli, the betT gene product is a choline transporter responsible for the uptake of choline used for glycine betaine synthesis (3).
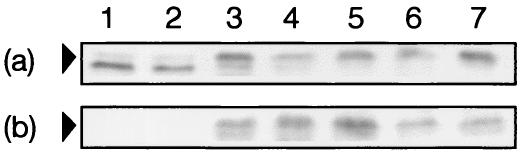
To determine whether the ORFs identified were involved in ChoP expression, each of the candidate genes was inactivated by the insertion of a kanamycin resistance cassette into the unique restriction endonuclease site as described in Table 2. These constructs were transformed into N. meningitidis strain C311#3 to recombine the inactive allele into the chromosome. The presence of the inactivated allele was confirmed by PCR or Southern hybridization. The phenotypes of the mutants were investigated by Western immunoblotting; MAb TEPC-15 was used to determine whether ChoP was present on pilin expressed by the various mutants. MAb SM1 was used to confirm that there were similar concentrations of pilin in all samples and to detect any apparent changes in migration. This analysis showed there was no change in the migration or reactivity of pilin from the NMB0939, NMB1270 (pmtA homologue), and NMB1277 (betT homologue) mutant strains compared to that from the parent strain with MAbs SM1 and TEPC-15 (Fig. 2). As there was no change in the binding of MAb TEPC-15, which is specific for ChoP (42), no role for these ORFs in ChoP biosynthesis or addition could be assigned.
FIG. 2.
Analysis of pilin from N. meningitidis C311#3 ChoP biosynthetic mutants. Western blotting of pilin was carried out to investigate the effects of mutations on the presence of ChoP. Pilin was isolated from C311#3 phase variant 5 (lane 1), C311#3 phase variant 6 (lane 2), C311#3 (lane 3), C311#3 betT (lane 4), C311#3 NMB0939 (lane 5), C311#3 pmtA (lane 6), and C311#3 NMB1638 (lane 7). Arrowheads represent the 20.5-kDa molecular mass marker. (a) Western blot with MAb SM1 (specific for class I pili of Neisseria) (41) showing the presence of pilin in all samples. (b) Western blot with MAb TEPC-15 (specific for ChoP) (42) showing the presence of ChoP on pilin in lanes 3 to 7.
A homologue of the lpt-3 gene product is involved in the addition of ChoP to pilin.
The lpt-3 gene was recently identified as encoding the transferase responsible for the addition of phosphoethanolamine (PEA) to position 3 of the β-chain heptose of the inner core of LPS in N. meningitidis (16). As PEA is similar to ChoP, we hypothesized that homologues of the lpt-3 gene product could be transferases involved in the addition of ChoP to pilin. TBLAST-X (2) homology searches showed there were two homologues of this gene in N. meningitidis MC58 (37), ORFs NMB1638 and NMB0415.
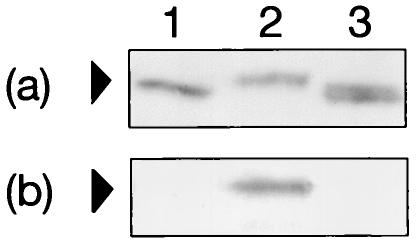
Pilin was isolated from the putative NMB1638 and NMB0415 ChoP transferase mutants and analyzed as described above. These experiments showed that pilin from the NMB1638 mutant strain had the same migration and reactivity with MAb TEPC-15 as the parent strain, indicating that this ORF is not involved in the transfer of ChoP to pilin in N. meningitidis (Fig. 2). Conversely, inactivation of NMB0415 resulted in an increased migration of pilin (similar to that seen with ChoP-negative strain 26A), and the pilin no longer bound MAb TEPC-15 (Fig. 3). As TEPC-15 is specific for ChoP (42) and ChoP is absent from pilin in the NMB0415 mutant strain, we conclude that NMB0415, now called pptA (pilin phosphorylcholine transferase A), is involved in the addition of ChoP to pilin.
FIG. 3.
Analysis of pilin from N. meningitidis C311#3 ChoP transferase mutants. Western blotting of pilin was carried out to investigate the effects of mutations on the presence of ChoP. Pilin was isolated from C311#3 26A (lane 1), C311#3 (lane 2), and C311#3 pptA (lane 3). Arrowheads represents the 20.5-kDa molecular mass marker. (a) Western blot with MAb SM1 (specific for class I pili of Neisseria) (41) showing the presence of pilin in all samples. (b) Western blot with MAb TEPC-15 (specific for ChoP) (42) showing the presence of ChoP on pilin in the parent strain only.
Changes in the homopolymeric tract of pptA mediate the phase variation of ChoP.
Phase variation of surface-expressed epitopes is a common feature of host-adapted pathogens (9, 18, 21). ChoP is a phase-variable modification of pilin in N. meningitidis (42). The pptA gene contains a homopolymeric guanosine tract located approximately 420 bp from the start of the ORF. Previous studies showed that this homopolymeric tract is of variable lengths in different strains, suggesting that the ORF is potentially phase variable (34).
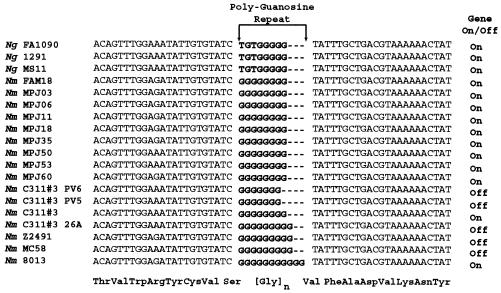
In this study, we expanded the survey of the homopolymeric tract of pptA to include N. meningitidis isolates from patients, additional N. meningitidis strains, and three independently identified ChoP phase variants. The three independently identified ChoP phase variants are the previously identified 26A (24a) and two spontaneous ChoP phase variants identified in this study, C311#3 phase variant 5 and C311#3 phase variant 6.
Our survey revealed that pptA was present in all 13 N. meningitidis strains analyzed. Sequencing of the homopolymeric repeat from these strains showed that the guanosine repeat length varied from 8 to 11 bp in the analyzed strains (Fig. 4). In the three independently isolated ChoP phase variants, 26A, phase variant 5, and phase variant 6, either the gain or the loss of a repeat in the homopolymeric tract of pptA resulted in the ORF being out of frame (Fig. 4). Therefore, the phase variation of ChoP on pilin in N. meningitidis is controlled by a frameshift of the homopolymeric repeat of pptA.
FIG. 4.
Sequencing of the poly(G) tracts in various pathogenic Neisseria strains. Ng, N. gonorrhoeae; Nm, N. meningitidis. MPJ laboratory strains are patient isolates (see reference 6 for details). Only one sequence is displayed for each of the patient isolate sets, as no difference was observed in the homopolymeric tracts within each set.
There have been reports of phase variation of ChoP in N. gonorrhoeae (42). However, sequencing of the homopolymeric guanosine repeat of pptA in several N. gonorrhoeae strains showed that the tract was shortened to 5 bp and had an additional 3 nucleotides, TGT, in the repeat region. As repeats of less than 6 bp are not considered phase variable (30), it would appear that ChoP phase variation in N. gonorrhoeae is not due to the homopolymeric tract of pptA.
In this study, eight sets of clinical isolates from different body compartments within an individual patient were also analyzed. Typically, these sets included isolates from the blood and cerebrospinal fluid of the patient. This analysis showed that pptA was present in all patient isolates and that isolates from different body compartments in a patient all had the same number of repeats (Fig. 4).
DISCUSSION
Phosphorylcholine is a covalently linked posttranslational modification found on several surface-exposed moieties of many different pathogens of the respiratory tract (11). In H. influenzae and commensal Neisseria, ChoP is attached to LPS; in S. pneumoniae, ChoP is attached to teichoic and lipoteichoic acids, and the biosynthetic and attachment pathways are well understood (33, 43, 48). The biosynthetic pathway, mechanism of attachment, phase variation, phenotypic effects, and location of attachment for commensal Neisseria and H. influenzae are very similar (32, 33). The addition of ChoP to LPS in commensal Neisseria is mediated by homologues of the lic-1 pathway (including the potential ChoP transferase gene licD), which is absent in pathogenic Neisseria (32, 33). In pathogenic Neisseria, ChoP is covalently attached to surface-exposed pilin but is not found attached to LPS (42). The expression of ChoP on pathogenic Neisseria is phase variable (42). Prior to this study, the mechanisms of ChoP biosynthesis, attachment, and phase variation were not understood.
ChoP is linked via a sugar moiety in H. influenzae, S. pneumoniae, and commensal Neisseria (33, 43, 48). N. meningitidis has a pilin-linked glycan, suggesting that ChoP could be attached to pilin via this pilin-linked trisaccharide. However, Western analysis of previously described pilin glycosylation mutant strains showed that they all have reactivities with MAb TEPC-15 similar to that of the parent strain. This finding demonstrates that ChoP is not linked to pilin via this glycan. However, it does not eliminate the possibility that ChoP is attached to pilin via another, as-yet-uncharacterized pilin-linked glycan. Therefore, the site of ChoP addition to pilin in N. meningitidis is still unclear.
The precursors and intermediates involved in the biosynthesis of ChoP in N. meningitidis are unknown. A previous study suggested that there is no uptake of ChoP from the environment in N. meningitidis. That study showed that 14C-labeled choline added to defined medium could not be detected on pilin by autoradiography, suggesting that N. meningitidis endogenously produces ChoP and its precursors rather than acquiring them from the environment (33).
The inactivation of several potential ChoP biosynthetic ORFs and one potential choline uptake system in this study resulted in no obvious change in the presence of ChoP on pilin in N. meningitidis. Thus, the mechanism by which N. meningitidis makes ChoP is still unclear. However, there could be multiple pathways for the biosynthesis of ChoP in N. meningitidis. This redundancy would allow ChoP to still be made if one pathway were inactivated. Alternatively, ChoP biosynthesis could occur either by an unknown pathway or by a pathway similar to the pcs pathway of Sinorhizobium and Pseudomonas, or the uptake of choline from the medium could be important (8, 47). The mechanism of ChoP biosynthesis in N. meningitidis remains to be determined.
Analysis of lpt-3 suggested that its product is a transferase responsible for the addition of PEA to position 3 of the β-chain heptose of the inner core of LPS in N. meningitidis (16). This assignment of function is based on the phenotype of the lpt-3 mutant, which showed a loss of PEA from only one site of the LPS molecule, suggesting that lpt-3 is responsible for the transfer of PEA to this particular position and not for PEA biosynthesis(16). The results presented here demonstrate that the inactivation of pptA results in the loss of ChoP from pilin in N. meningitidis. These results, combined with the homology of this ORF to lpt-3, suggest that the pptA gene product is the ChoP transferase responsible for the addition of ChoP to pilin in N. meningitidis. pptA is found in a region of the chromosome that is specific for pathogenic Neisseria (23), within the cell wall biosynthesis cluster (34).
This study also demonstrates that all three independently identified ChoP phase variants resulted from changes in the homopolymeric repeat of pptA. Phase-variable expression of ChoP is important in the colonization of different microniches and contributes to the pathogenicity of nontypeable H. influenzae (38, 45). These data suggest that the phase variation of pptA also may be important in the pathogenesis of N. meningitidis. In contrast, our work suggests that this gene may not be phase variable in N. gonorrhoeae due to the short homopolymeric tract. It is interesting that there have been reports of N. gonorrhoeae strains that exhibit phase variation of ChoP expression (42). As these same strains do not contain long homopolymeric tracts, it seems that an alternative mechanism of phase variation must be operating. Changes at the site of ChoP addition, mediated by changes in the amino acid sequence at this site, may occur at a high frequency (9, 18, 26, 31). Alternatively, the phase variation seen in N. gonorrhoeae could occur through alterations in as-yet-uncharacterized pilin-linked glycans or through the phase variation of other ORFs involved in either the biosynthesis or the uptake of ChoP in N. gonorrhoeae.
The results presented in this communication demonstrate that pptA is a phase-variable ORF involved in the addition of ChoP to pilin in N. meningitidis. It is interesting that there are homologues of this ORF in many other bacteria (Table 3). As the expression of ChoP on surface oligosaccharides has been described for many of these bacteria, the presence of pptA homologues suggests that the modification of proteins by ChoP also may occur in these organisms.
TABLE 3.
Homologues of pptA found in other pathogens
| Organism | ORF name | %
|
|
|---|---|---|---|
| Identitya | Similaritya | ||
| H. influenzae | HI 1005 | 38 | 53 |
| E. coli (O157:H7) | NP308920 | 32 | 47 |
| Shigella flexneri | NP706693 | 31 | 46 |
| Salmonella enterica serovar Typhimurium | NP459811 | 31 | 45 |
| P. aeruginosa | NC002156 | 26 | 44 |
Relative to N. meningitidis pptA.
Acknowledgments
This work was supported by NHMRC project grant 210310 to M.P.J. M.J.W. is supported by a University of Queensland midyear scholarship.
Editor: J. N. Weiser
REFERENCES
- 1.Alexander, H. E. 1965. The Haemophilus group, p. 724-741. In J. Dabos and J. G. Hirsch (ed.), Bacterial and mycotic infections of man. Pitman Medical Publishing Co., Ltd., London, England.
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andresen, P. A., I. Kaasen, O. B. Styrvold, G. Boulnois, and A. R. Strom. 1988. Molecular cloning, physical mapping and expression of the bet genes governing the osmoregulatory choline-glycine betaine pathway of Escherichia coli. J. Gen. Microbiol. 134:1737-1746. [DOI] [PubMed] [Google Scholar]
- 4.Arondel, V., C. Benning, and C. R. Somerville. 1993. Isolation and functional expression in Escherichia coli of a gene encoding phosphatidylethanolamine methyltransferase (EC 2.1.1.17) from Rhodobacter sphaeroides. J. Biol. Chem. 268:16002-16008. [PubMed] [Google Scholar]
- 5.Behr, T., W. Fischer, J. Peter-Katalinic, and H. Egge. 1992. The structure of pneumococcal lipoteichoic acid. Improved preparation, chemical and mass spectrometric studies. Eur. J. Biochem. 207:1063-1075. [DOI] [PubMed] [Google Scholar]
- 6.Berrington, A. W., Y.-C. Tan, Y. Srikhanta, B. Kuipers, P. van der Ley, I. R. A. Peak, and M. P. Jennings. 2002. Phase variation in meningococcal lipooligosaccharide biosynthesis genes. FEMS Immunol. Med. Microbiol. 34:267-275. [DOI] [PubMed]
- 7.Cundell, D. R., N. P. Gerard, C. Gerard, I. Idanpaan-Heikkila, and E. I. Tuomanen. 1995. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435-438. [DOI] [PubMed] [Google Scholar]
- 8.de Rudder, K. E., I. M. Lopez-Lara, and O. Geiger. 2000. Inactivation of the gene for phospholipid N-methyltransferase in Sinorhizobium meliloti: phosphatidylcholine is required for normal growth. Mol. Microbiol. 37:763-772. [DOI] [PubMed] [Google Scholar]
- 9.Dybvig, K. 1993. DNA rearrangements and phenotypic switching in prokaryotes. Mol. Microbiol. 10:465-471. [DOI] [PubMed] [Google Scholar]
- 10.Fischer, W., T. Behr, R. Hartmann, J. Peter-Katalinic, and H. Egge. 1993. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoid acid (C polysaccharide). Eur. J. Biochem. 215:851-857. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie, S. H., S. Ainscough, A. Dickens, and J. Lewin. 1996. Phosphorylcholine-containing antigens in bacteria from the mouth and respiratory tract. J. Med. Microbiol. 44:35-40. [DOI] [PubMed] [Google Scholar]
- 12.Gould, J. M., and J. N. Weiser. 2002. The inhibitory effect of C-reactive protein on bacterial phosphorylcholine platelet-activating factor receptor-mediated adherence is blocked by surfactant. J. Infect. Dis. 186:361-371. [DOI] [PubMed] [Google Scholar]
- 13.Jennings, M. P., M. Virji, D. Evans, V. Foster, Y. N. Srikhanta, L. Steeghs, P. van der Ley, and E. R. Moxon. 1998. Identification of a novel gene involved in pilin glycosylation in Neisseria meningitidis. Mol. Microbiol. 29:975-984. [DOI] [PubMed] [Google Scholar]
- 14.Kolberg, J., E. A. Hoiby, and E. Jantzen. 1997. Detection of the phosphorylcholine epitope in streptococci, Haemophilus and pathogenic Neisseriae by immunoblotting. Microb. Pathog. 22:321-329. [DOI] [PubMed] [Google Scholar]
- 15.Koomey, M. 1994. Mechanisms of pilus antigenic variation in Neisseria gonorrhoeae, p. 113-126. In V. L. Miller, J. B. Kaper, D. A. Kaper, and R. R. Isberg (ed.), Molecular genetics of bacterial pathogenesis. American Society for Microbiology, Washington, D.C.
- 16.Mackinnon, F. G., A. D. Cox, J. S. Plested, C. M. Tang, K. Makepeace, P. A. Coull, J. C. Wright, R. Chalmers, D. W. Hood, J. C. Richards, and E. R. Moxon. 2002. Identification of a gene (lpt-3) required for the addition of phosphoethanolamine to the lipopolysaccharide inner core of Neisseria meningitidis and its role in mediating susceptibility to bactericidal killing and opsonophagocytosis. Mol. Microbiol. 43:931-943. [DOI] [PubMed] [Google Scholar]
- 17.Merz, A. J., C. A. Enns, and M. So. 1999. Type IV pili of pathogenic Neisseriae elicit cortical plaque formation in epithelial cells. Mol. Microbiol. 32:1316-1332. [DOI] [PubMed] [Google Scholar]
- 18.Meyer, T. F., and J. P. van Putten. 1989. Genetic mechanisms and biological implications of phase variation in pathogenic neisseriae. Clin. Microbiol. Rev. 2(Suppl.):S139-S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minder, A. C., K. E. de Rudder, F. Narberhaus, H. M. Fischer, H. Hennecke, and O. Geiger. 2001. Phosphatidylcholine levels in Bradyrhizobium japonicum membranes are critical for an efficient symbiosis with the soybean host plant. Mol. Microbiol. 39:1186-1198. [PubMed] [Google Scholar]
- 20.Mosser, J. L., and A. Tomasz. 1970. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J. Biol. Chem. 245:287-298. [PubMed] [Google Scholar]
- 21.Moxon, E. R., P. B. Rainey, M. A. Nowak, and R. E. Lenski. 1994. Adaptive evolution of highly mutable loci in pathogenic bacteria. Curr. Biol. 4:24-33. [DOI] [PubMed] [Google Scholar]
- 22.Parge, H. E., K. T. Forest, M. J. Hickey, D. A. Christensen, E. D. Getzoff, and J. A. Tainer. 1995. Structure of the fibre-forming protein pilin at 2.2 Å resolution. Nature 378:32-38. [DOI] [PubMed] [Google Scholar]
- 23.Perrin, A., X. Nassif, and C. Tinsley. 1999. Identification of regions of the chromosome of Neisseria meningitidis and Neisseria gonorrhoeae which are specific to the pathogenic Neisseria species. Infect. Immun. 67:6119-6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Power, P. M., L. F. Roddam, M. Dieckelmann, Y. N. Srikhanta, Y. C. Tan, A. W. Berrington, and M. P. Jennings. 2000. Genetic characterisation of pilin glycosylation in Neisseria meningitidis. Microbiology 146:967-979. [DOI] [PubMed] [Google Scholar]
- 24a.Power, P. M., L. F. Roddam, K. Rutter, S. Z. Fitzpatrick, Y. N. Srikhanta, and M. P. Jennings. 2003. Genetic characterization of pilin glycosylation and phase variation in Neisseria meningitidis Mol. Microbiol. 49:833-847. [DOI] [PubMed] [Google Scholar]
- 25.Risberg, A., E. K. Schweda, and P. E. Jansson. 1997. Structural studies of the cell-envelope oligosaccharide from the lipopolysaccharide of Haemophilus influenzae strain RM.118-28. Eur. J. Biochem. 243:701-707. [DOI] [PubMed] [Google Scholar]
- 26.Robertson, B. D., and T. F. Meyer. 1992. Genetic variation in pathogenic bacteria. Trends Genet. 8:422-427. [DOI] [PubMed] [Google Scholar]
- 27.Rudel, T., J. P. van Putten, C. P. Gibbs, R. Haas, and T. F. Meyer. 1992. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol. Microbiol. 6:3439-3450. [DOI] [PubMed] [Google Scholar]
- 28.Saiki, R. K., D. H. Gelfand, S. Stoffel, S. J. Scharf, R. Higuchi, G. T. Horn, K. B. Mullis, and H. A. Erlich. 1988. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487-491. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Saunders, N. J., A. C. Jeffries, J. F. Peden, D. W. Hood, H. Tettelin, R. Rappuoli, and E. R. Moxon. 2000. Repeat-associated phase variable genes in the complete genome sequence of Neisseria meningitidis strain MC58. Mol. Microbiol. 37:207-215. [DOI] [PubMed] [Google Scholar]
- 31.Seifert, H. S. 1996. Questions about gonococcal pilus phase- and antigenic-variation. Mol. Microbiol. 21:433-440. [DOI] [PubMed] [Google Scholar]
- 32.Serino, L., and M. Virji. 2002. Genetic and functional analysis of the phosphorylcholine moiety of commensal Neisseria lipopolysaccharide. Mol. Microbiol. 43:437-448. [DOI] [PubMed] [Google Scholar]
- 33.Serino, L., and M. Virji. 2000. Phosphorylcholine decoration of lipopolysaccharide differentiates commensal Neisseriae from pathogenic strains: identification of licA-type genes in commensal Neisseriae. Mol. Microbiol. 35:1550-1559. [DOI] [PubMed] [Google Scholar]
- 34.Snyder, L. A., N. J. Saunders, and W. M. Shafer. 2001. A putatively phase variable gene (dca) required for natural competence in Neisseria gonorrhoeae but not Neisseria meningitidis is located within the division cell wall (dcw) gene cluster. J. Bacteriol. 183:1233-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swords, W. E., B. A. Buscher, K. Ver Steeg Ii, A. Preston, W. A. Nichols, J. N. Weiser, B. W. Gibson, and M. A. Apicella. 2000. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol. Microbiol. 37:13-27. [DOI] [PubMed] [Google Scholar]
- 36.Szalai, A. J., D. E. Briles, and J. E. Volanakis. 1995. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in transgenic mice. J. Immunol. 155:2557-2563. [PubMed] [Google Scholar]
- 37.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 38.Tong, H. H., L. E. Blue, M. A. James, Y. P. Chen, and T. F. DeMaria. 2000. Evaluation of phase variation of nontypeable Haemophilus influenzae lipooligosaccharide during nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect. Immun. 68:4593-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tonjum, T., and M. Koomey. 1997. The pilus colonization factor of pathogenic neisserial species: organelle biogenesis and structure/function relationships—a review. Gene 192:155-163. [DOI] [PubMed] [Google Scholar]
- 40.Virji, M., H. Kathty, D. J. P. Ferguson, C. Alexandrescu, J. E. Heckels, and E. R. Moxon. 1991. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol. Microbiol. 5:1831-1841. [DOI] [PubMed] [Google Scholar]
- 41.Virji, M., J. R. Saunders, G. Sims, K. Makepeace, D. Maskell, and D. J. Ferguson. 1993. Pilus-facilitated adherence of Neisseria meningitidis to human epithelial and endothelial cells: modulation of adherence phenotype occurs concurrently with changes in primary amino acid sequence and the glycosylation status of pilin. Mol. Microbiol. 10:1013-1028. [DOI] [PubMed] [Google Scholar]
- 42.Weiser, J. N., J. B. Goldberg, N. Pan, L. Wilson, and M. Virji. 1998. The phosphorylcholine epitope undergoes phase variation on a 43-kilodalton protein in Pseudomonas aeruginosa and on pili of Neisseria meningitidis and Neisseria gonorrhoeae. Infect. Immun. 66:4263-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiser, J. N., A. A. Lindberg, E. J. Manning, E. J. Hansen, and E. R. Moxon. 1989. Identification of a chromosomal locus for expression of lipopolysaccharide epitopes in Haemophilus influenzae. Infect. Immun. 57:3045-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiser, J. N., Z. Markiewicz, E. I. Tuomanen, and J. H. Wani. 1996. Relationship between phase variation in colony morphology, intrastrain variation in cell wall physiology, and nasopharyngeal colonization by Streptococcus pneumoniae. Infect. Immun. 64:2240-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiser, J. N., N. Pan, K. L. McGowan, D. Musher, A. Martin, and J. Richards. 1998. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J. Exp. Med. 187:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiser, J. N., M. Shchepetov, and S. T. H. Chong. 1997. Decoration of lipopolysaccharide with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect. Immun. 65:943-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilderman, P. J., A. I. Vasil, W. E. Martin, R. C. Murphy, and M. L. Vasil. 2002. Pseudomonas aeruginosa synthesizes phosphatidylcholine by use of the phosphatidylcholine synthase pathway. J. Bacteriol. 184:4792-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, J. R., I. Idanpaan-Heikkila, W. Fischer, and E. I. Tuomanen. 1999. Pneumococcal licD2 gene is involved in phosphorylcholine metabolism. Mol. Microbiol. 31:1477-1488. [DOI] [PubMed] [Google Scholar]