Abstract
Moraxella catarrhalis is a major mucosal pathogen of the human respiratory tract, but the mucosal immune response directed against surface components of this organism has not been characterized in detail. The aim of this study was to investigate the salivary immunoglobulin A (IgA) response toward outer membrane proteins (OMP) of M. catarrhalis in healthy adults, the group of individuals least likely to be colonized and thus most likely to display mucosal immunity. Unstimulated saliva samples collected from 14 healthy adult volunteers were subjected to IgA immunoblot analysis with OMP preparations of M. catarrhalis strain O35E. Immunoblot analysis revealed a consistent pattern of IgA reactivity, with the appearance of five major bands located at >250, 200, 120, 80, and 60 kDa. Eleven (79%) of 14 saliva samples elicited reactivity to all five bands. Immunoblot analysis with a set of isogenic knockout mutants lacking the expression of individual OMP was used to determine the identities of OMP giving rise to IgA bands. Human saliva was shown consistently to exhibit IgA-binding activity for oligomeric UspA2 (>250 kDa), hemagglutinin (200 kDa), monomeric UspA1 (120 kDa), transferrin-binding protein B (TbpB), monomeric UspA2, CopB, and presumably OMP CD. TbpB, oligomeric UspA2, and CopB formed a cluster of bands at about 80 kDa. These data indicate that the human salivary IgA response is directed consistently against a small number of major OMP, some of which are presently considered vaccine candidates. The functional properties of these mucosal antibodies remain to be elucidated.
Moraxella catarrhalis is a major mucosal pathogen of the human respiratory tract (24). This gram-negative organism is the third most common cause of otitis media in children (15, 25) and is associated with acute exacerbations of chronic obstructive pulmonary disease in adults (30). The interaction between M. catarrhalis and the human host is incompletely understood. Colonization and infection with M. catarrhalis induce a systemic humoral immune response (4, 21, 29, 42, 43). Serum immunoglobulin G (IgG) concentrations increase with age and are functionally active through the induction of complement-mediated bacterial killing (5, 6). Target antigens of serum antibodies include various outer membrane proteins (OMP) and lipooligosaccharide (4, 6, 7, 10, 13, 31). In contrast, little is known about the mucosal immune response against surface determinants of M. catarrhalis. While one study failed to detect OMP-specific IgA antibodies in nasopharyngeal secretions from 15 infants (42), a more recent study detected salivary IgA (sIgA) antibodies in 62 (94%) of 66 young children (32). IgA antibodies could be detected in sputum samples from adult patients exhibiting exacerbations of chronic obstructive pulmonary disease (4). These antibodies reacted with both OMP and lipooligosaccharide of M. catarrhalis (4). However, the occurrence and specificity of mucosal antibodies against surface determinants of M. catarrhalis in healthy adults have not been investigated comprehensively.
The aim of the present study was to characterize sIgA antibodies against OMP of M. catarrhalis in saliva samples from healthy adults. The identities of specific OMP targeted by IgA antibodies were determined by constructing isogenic knockout mutants for various candidate OMP.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
M. catarrhalis O35E and its isogenic knockout mutants used in this study are described in Table 1. All M. catarrhalis strains were routinely cultured at 37°C and 200 rpm in brain heart infusion (BHI) broth or on BHI agar plates in an atmosphere of 5% CO2. Media were supplemented with kanamycin (20 mg/liter), chloramphenicol (0.5 mg/liter), or erythromycin (1 mg/liter) for culturing of isogenic mutants (Table 1). Escherichia coli DH5α, the host strain for the plasmid constructs in this study, was grown in Luria-Bertani broth or on Luria-Bertani agar plates supplemented with ampicillin (100 mg/liter), kanamycin (100 mg/liter), or erythromycin (500 mg/liter). All antibiotics were purchased from Sigma Chemical Co., St. Louis, Mo.
TABLE 1.
Strains used in this study
| Strain | Description | Reference or source |
|---|---|---|
| O35E | Parent strain | 18 |
| O35E.1 | Isogenic uspA1 mutant; kanamycin resistant | 1, 2 |
| O35E.2 | Isogenic uspA2 mutant; kanamycin resistant | 1 |
| O35E.12 | Isogenic uspA1 uspA2 double mutant; kanamycin and chloramphenicol resistant | 1 |
| O35E.copB | Isogenic copB mutant; kanamycin resistant | 32 |
| O35E.tbpB | Isogenic tbpB mutant; kanamycin resistant | 32 |
| O35E.hag | Isogenic hag mutant; kanamycin resistant | 32 |
| O35E.12-hag | Isogenic uspA1 uspA2 hag triple mutant; resistant to kanamycin, chloramphenicol, and erythromycin | This study |
| DH5α (E. coli) | Host strain for plasmid constructs | 41 |
DNA methods.
Plasmids were isolated by using a Wizard Plus SV Minipreps DNA purification system (Promega Corp., Madison, Wis.). E. coli DH5α was transformed as described previously (17). Restriction enzymes were purchased from New England Biolabs, Inc., Beverly, Mass. Electrocompetent M. catarrhalis was prepared and DNA was electroporated into these bacteria as described previously (20). DNA sequencing was performed by using an ABI PRISM 310 genetic analyzer (PE Biosystems, Rotkreuz, Switzerland) with a Big Dye Terminator cycle sequencing ready reaction kit (PE Biosystems). Sequences were analyzed and aligned by using the Lasergene software package (DNASTAR Inc., Madison, Wis.).
Construction of the isogenic triple knockout mutant O35E.12-hag.
A part of the hemagglutinin (hag) gene of M. catarrhalis O35E was amplified with forward primer hagF3 (5′-CAGGGCAAGTTGGCAGTGTATG-3′) and reverse primer hagB3 (5′-TGGAGACAAAGTCAACCGCTTC-3′). The PCR product was ligated into plasmid pGEM-T-Easy (Promega). An erythromycin resistance cassette amplified by PCR from plasmid pJDC9 (9) was ligated into the BbsI restriction sites of hag. The construct Δhag::ermB was used for electroporation of competent M. catarrhalis O35E.12, the uspA1 uspA2 double knockout mutant (Table 1). Transformants were selected on BHI agar plates containing 1 mg of erythromycin/liter. Insertional inactivation of hag was confirmed by PCR analysis, sequencing, Southern blot analysis, and a phenotypic autoagglutination test (37).
Saliva sampling.
The study was approved by the local ethics committee, and informed consent was given by the study participants. Unstimulated saliva samples were collected from 14 healthy adult volunteers by using a Salivette collection system (Sarstedt, Nümbrecht, Germany). Saliva-soaked cotton tampons were centrifuged for 10 min at 2,200 × g. Samples were divided into aliquots and stored at −20°C until use.
Immunoblotting.
OMP were prepared by the EDTA buffer method (36), resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) analysis with 7.5% polyacrylamide gels, and electrotransferred to polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore Corp., Bedford, Mass.). Membranes containing OMP from individual strains were cut into 5-mm strips. Membranes were blocked for 1 h in Tris-buffered saline (TBS) containing 4% nonfat dry milk and incubated for 2 h with saliva samples diluted 1:20 in TBS-Tween (0.05%) containing 1% nonfat dry milk. Membranes were washed three times with TBS-Tween and incubated for 1 h with a secondary antibody (horseradish peroxidase-conjugated goat anti-human IgA; Sigma) diluted 1:4,000 in TBS-Tween containing 1% nonfat dry milk. Some of the membranes were incubated with a mouse monoclonal antibody (17C7) recognizing UspA1 and UspA2 (2) and diluted 1:4 and with horseradish peroxidase-conjugated goat anti-mouse IgG diluted 1:4,000. Super Signal West Pico chemiluminescence substrate (Pierce Chemical Co., Rockford, Ill.) was used for the detection of antibody binding.
Flow cytometry.
Bacteria in stationary growth phase were analyzed. Cells were harvested, and the optical density at 600 nm was adjusted to 0.2. Aliquots of 200 μl were centrifuged, resuspended, and incubated for 1 h in 200 μl of saliva diluted 1:20 in phosphate-buffered saline. Bacteria were harvested, resuspended in 200 μl of fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgA (Roche Molecular Biochemicals, Rotkreuz, Switzerland), transferred to 2 ml of phosphate-buffered saline with 1% paraformaldehyde, and analyzed by flow cytometry with a FACScan cytometer and CellQuest software (BD Biosciences, San Jose, Calif.).
RESULTS
Study population.
Fourteen healthy adult volunteers without clinical evidence of acute or chronic respiratory tract disease were included in the study population. The median age was 31.5 years (range, 22 to 63 years). Nine volunteers were women. Four volunteers were married and had children.
Detection of sIgA antibodies directed against OMP of wild-type M. catarrhalis O35E.
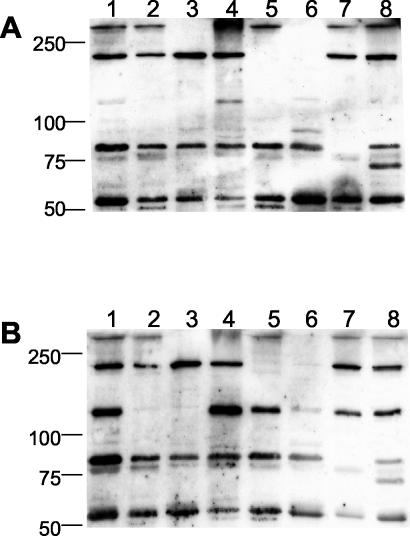
OMP of strain M. catarrhalis O35E were resolved by SDS-PAGE and transferred to PVDF membranes. OMP strips were incubated with saliva samples from 14 healthy adult volunteers. All saliva samples contained sIgA directed against OMP of M. catarrhalis, although one sample (from individual 3) revealed only faint bands in Fig. 1. The pattern of targeted OMP was consistent from individual to individual. Typical patterns are shown in Fig. 1. Comparative analysis allowed the identification of five distinct bands with molecular masses of >250, 200, 120, 80 (multiple band), and 60 kDa. All five bands were present in 11 (79%) of 14 individuals. Semiquantitative analysis of the bands revealed the presence of two major bands, one with an apparent molecular mass exceeding 250 kDa and one with a molecular mass of about 80 kDa (multiple bands). The intensity of the 250-kDa band was highly variable (Fig. 1).
FIG. 1.
Representative selection of immunoblots for detection of sIgA directed against OMP of M. catarrhalis O35E. OMP were separated by SDS-7.5% PAGE, transferred to PVDF membranes, and incubated with saliva samples at a 1:20 dilution or with monoclonal antibody 17C7 hybridoma culture supernatant (recognizes both UspA1 and UspA2) at a 1:4 dilution. Numbers below lanes refer to individuals as listed in Table 2. Molecular masses (in kilodaltons) are indicated on the left.
Identification of OMP targets for sIgA by mutant analysis.
For molecular identificaton of each OMP band reacting with sIgA, isogenic knockout mutants of parent strain O35E were constructed (Table 1). With the exception of triple mutant O35E.12-hag, which lacks surface expression of UspA1, UspA2, and Hag, these mutants have been described elsewhere (1, 2, 18, 32, 44). The triple mutant was constructed by allelic replacement of the hag wild-type gene in the uspA1 uspA2 double mutant by a hag construct containing an erythromycin resistance cassette within the open reading frame. This set of mutants was used to assign IgA immunoblot bands to individual OMP. The results obtained with two representative saliva samples are shown in Fig. 2. The high-molecular-mass band (>250 kDa) was identified as the oligomeric form of UspA2 (Fig. 2, lanes 3 and 6). In some individuals (7 and 13; Fig. 1), UspA2 was responsible for broad IgA reactivity extending from >250 kDa to approximately 120 kDa. This pattern of reactivity was known from immunoblot analysis with UspA-specific monoclonal antibody 17C7 (2), as shown in Fig. 1. The 200-kDa band was identified as Hag (Fig. 2, lanes 5 and 6), which is similar or identical to the Moraxella IgD-binding protein (14, 37). Monomeric UspA1 was found to account for the 120-kDa band (Fig. 2B, lanes 2, 3, and 6) (1).
FIG. 2.
Immunoblot analysis of two representative saliva samples for detection of sIgA antibodies directed against wild-type M. catarrhalis O35E and seven isogenic knockout mutants. OMP were separated by SDS-7.5% PAGE, transferred to PVDF membranes, and incubated with saliva samples at a 1:20 dilution. Lane 1, O35E; lane 2, O35E.1 (uspA1 mutant); lane 3, O35E.12 (uspA1 uspA2 double mutant); lane 4, O35E.2 (uspA2 mutant); lane 5, O35E.hag (hag mutant); lane 6, O35E.12-hag (uspA1 uspA2 hag triple mutant); lane 7, O35E.tbpB (tbpB mutant); lane 8, O35E.copB (copB mutant). Numbers to the left of each panel indicate molecular mass standards (in kilodaltons).
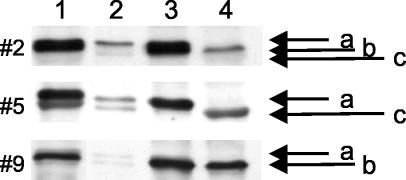
Elucidation of the identities of OMP migrating in the vicinity of 80 kDa and giving rise to a cluster of bands required combined analysis of band patterns generated from the uspA2, copB, and tbpB mutants. Figure 3 displays the findings obtained with saliva samples from individuals 2, 5, and 9. TbpB, monomeric UspA2, and CopB appeared to be responsible for the upper, middle, and lower bands on Fig. 3, respectively. For individual 5, it appeared that monomeric UspA2 comigrated with whichever of the other two OMP was present (Fig. 3). The M. catarrhalis OMP migrating with an apparent molecular mass of 60 kDa most likely was OMP CD (35). A detailed analysis of this band was not performed.
FIG. 3.
Identification of OMP responsible for bands near 80 kDa. A composite of the wild type (lane 1), uspA2 mutant (lane 2), copB mutant (lane 3), and tbpB mutant (lane 4) is shown. Bacteria were incubated with saliva samples from individuals 2, 5, and 9, as indicated on the left. Saliva samples were used at a 1:20 dilution for all individuals. The positions of TbpB (a), monomeric UspA2 (b), and CopB (c) are indicated.
Frequency of sIgA antibodies to individual OMP of M. catarrhalis.
As shown in Table 2, sIgA reactivity was highly consistent despite minor differences in band patterns and intensities when saliva samples were probed with OMP from the parent strain, M. catarrhalis O35E. To corroborate the assignment of bands to individual OMP as detailed above, eight individual saliva samples were immunoblotted against the complete set of isogenic mutants. Whereas sIgA directed against oligomeric UspA2, Hag, and TbpB could be detected in all eight samples, antibodies against UspA1 and CopB were detected in seven and six samples, respectively.
TABLE 2.
sIgA antibodies directed against M. catarrhalis OMP antigens
| Individual | Reactivitya with sIgA of OMP antigen at the following kDa:
|
||||
|---|---|---|---|---|---|
| >250 | 200 | 120 | 80 | 60 | |
| 1 | +++ | ++ | ++ | +++ | ++ |
| 2 | ++ | ++ | + | +++ | + |
| 3 | + | − | − | + | + |
| 4 | + | ++ | + | +++ | + |
| 5 | + | ++ | + | +++ | + |
| 6 | + | ++ | − | ++ | + |
| 7 | +++ | ++ | − | ++ | + |
| 8 | ++ | ++ | + | ++ | + |
| 9 | +++ | ++ | + | +++ | + |
| 10 | +++ | ++ | + | ++ | + |
| 11 | + | ++ | +++ | +++ | + |
| 12 | +++ | ++ | ++ | +++ | + |
| 13 | +++ | ++ | + | ++ | + |
| 14 | +++ | ++ | + | +++ | ++ |
| Total no. (%) of individuals | 14 (100) | 13 (93) | 11 (79) | 14 (100) | 14 (100) |
The intensity of the bands on IgA immunoblots was rated semiquantitatively from − to +++.
Detection of surface-exposed epitopes reacting with sIgA.
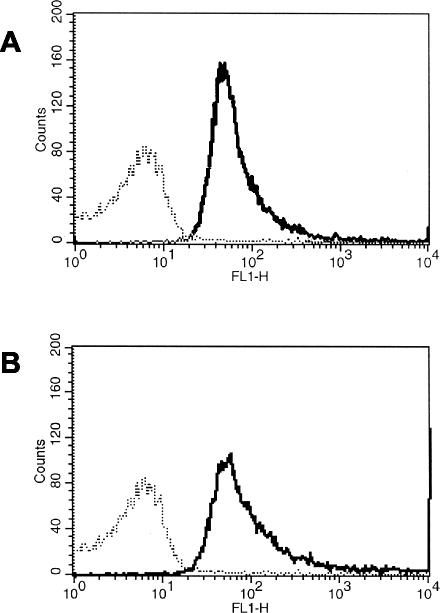
One limitation of Western blot analysis is that it cannot distinguish between surface-exposed and non-surface-exposed antigenic determinants. We therefore analyzed saliva samples from 10 individuals for the presence of surface-exposed epitopes by flow cytometry. All samples contained sIgA reacting with intact M. catarrhalis cells, as indicated by the presence of a fluorescence shift in comparison with the results for the negative control. Figure 4 shows the results obtained with two representative saliva samples.
FIG. 4.
Flow cytometry results obtained with two representative saliva samples for detection of sIgA against surface-exposed epitopes of M. catarrhalis O35E. Bacterial suspensions were incubated with saliva samples at a 1:20 dilution. Saliva samples were from individuals 7 (A) and 10 (B). The broken line represents the negative control (bacteria incubated with secondary antibodies only). The solid line indicates the fluorescence observed when bacteria were incubated with saliva samples.
DISCUSSION
Humans are frequently exposed to M. catarrhalis. Although the prevalence of colonization is low in healthy adults (16, 23, 45, 45), high rates of mucosal colonization and infection in children (12, 27, 32) and in the elderly (45) imply that exposure is common in all age groups. This concept is supported by our finding of a conserved and diverse sIgA response directed against multiple M. catarrhalis OMP in healthy adults. Reactivity was prominent in all but one sample, suggesting that M. catarrhalis-specific sIgA antibodies are continuously synthesized. Considering that IgA-producing plasma cells have an average half-life of 5 days (40), this finding implies recurrent or continuous exposure to conserved antigens. It is conceivable that occasional boosting of the immune response in adults results from brief episodes of M. catarrhalis colonization, e.g., during viral respiratory tract infections. It has been shown that M. catarrhalis colonization rates are approximately twofold higher during episodes of upper respiratory tract infections (5, 11). Also, in vitro studies with related organisms (e.g., Haemophilus influenzae and Neisseria meningitidis) have revealed enhanced adherence to respiratory epithelial cells previously infected with respiratory viruses (38, 39). Similar data are not available for M. catarrhalis.
The band patterns found in the immunoblot analysis of saliva samples from different individuals were highly consistent. Five bands were regularly detectable. This finding was compatible with that of a similar study analyzing the sIgA response to M. catarrhalis OMP in young children (32). However, while adults consistently showed an expanded antibody response, the number of bands was more restricted in young children. These observations suggest that the spectrum of antibody specificities expands in response to the duration or frequency of exposure, a phenomenon frequently observed in chronic infections. The conserved nature of the immune response is remarkable given that the experimental approach used in this study underestimates the true diversity of sIgA specificities against M. catarrhalis OMP. Antigens used for immunoblotting were derived from a single clinical isolate and therefore are unlikely to represent the entire spectrum of M. catarrhalis OMP antigenic determinants. Also, OMP expressed at low levels in vitro and therefore present in small quantities on the blotting membranes may not have elicited a visually detectable sIgA band (e.g., lactoferrin-binding proteins A and B). Control of this shortcoming would require the use of recombinant immunoblot technology. At least some of the detected sIgA antibodies were directed against surface-exposed antigens, as revealed by flow cytometry. The technologies used here did not allow us to assign surface exposure to individual targets of IgA or to identify conformational epitopes.
Using immunoblot analysis of isogenic mutants of five individual OMP, we characterized major target OMP of sIgA antibodies in adults. These targets were UspA1, UspA2, hemagglutinin, TbpB, CopB, and OMP CD. Both UspA1 and UspA2 were previously recognized as vaccine candidates due to their consistent expression of conserved, surface-exposed epitopes (2, 33), which are capable of inducing a bactericidal serum IgG response (8, 19). Anti-UspA1 and anti-UspA2 sIgA antibodies are already detectable in infancy (32). In SDS-PAGE, both UspA1 and UspA2 migrate as a high-molecular-mass oligomeric form and a monomeric form. On the bacterial surface, both proteins are expressed as oligomers forming lollipop-shaped structures (22, 37). We here demonstrate that UspA1 and UspA2 are recognized by sIgA. This observation, however, does not prove the in vivo expression of both proteins, because they have identical immunoreactive domains (2). The function of specific sIgA antibodies is unclear. In general, IgA antibodies have neutralizing capacity, e.g., by inhibiting the binding of surface structures to ligands on the host cell surface. UspA1 is an adhesin (1, 26), and anti-UspA1 IgA antibodies may have the potential to block the attachment of M. catarrhalis to human epithelial cells.
Furthermore, we consistently detected antibodies against hemagglutinin, a putative virulence factor of M. catarrhalis whose gene was identified and sequenced only recently (37). Antibodies to hemagglutinin were detected in almost all saliva samples, indicating a high degree of conservation of at least some immunoreactive epitopes. Although sequence comparisons of the hemagglutinin of strain O35E and other strains as well as the Moraxella IgD-binding protein revealed a sequence identity of between 60 and 85% only (34), these data strongly indicate the presence of conserved epitopes between individual strains. Other targets of sIgA were TbpB and CopB, two OMP involved in the acquisition of iron from human transferrin (3, 28). In contrast to anti-TbpB antibodies, anti-CopB antibodies were not uniformely present, although CopB is constitutively expressed in M. catarrhalis. However, CopB is known to display antigenic variations of its surface-exposed domains (44), and anti-CopB antibodies thus may have been missed, as we used OMP from a single isolate only.
In summary, this study describes a consistent and highly conserved salivary immune response directed against a limited number of M. catarrhalis OMP in healthy adults. Among the major targets of sIgA are OMP which are presently considered major vaccine candidates for M. catarrhalis, i.e., UspA1, UspA2, TbpB, and hemagglutinin.
Acknowledgments
This work was supported by grant no. 32-52901.97 (to C.A.) from the Swiss National Science Foundation.
We thank Kristian Riesbeck, University of Malmö, Malmö, Sweden, for providing the sequence of the mid gene.
Editor: D. L. Burns
REFERENCES
- 1.Aebi, C., E. R. Lafontaine, L. D. Cope, J. L. Latimer, S. L. Lumbley, G. H. McCracken, Jr., and E. J. Hansen. 1998. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis O35E. Infect. Immun. 66:3113-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi, C., I. Maciver, J. L. Latimer, L. D. Cope, M. K. Stevens, S. E. Thomas, G. H. McCracken, Jr., and E. J. Hansen. 1997. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect. Immun. 65:4367-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aebi, C., B. Stone, M. Beucher, L. D. Cope, I. Maciver, S. E. Thomas, G. H. McCracken, Jr., P. F. Sparling, and E. J. Hansen. 1996. Expression of the CopB outer membrane protein by Moraxella catarrhalis is regulated by iron and affects iron acquisition from transferrin and lactoferrin. Infect. Immun. 64:2024-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakri, F., A. L. Brauer, S. Sethi, and T. F. Murphy. 2002. Systemic and mucosal antibody response to Moraxella catarrhalis after exacerbation of chronic obstructive pulomary disease. J. Infect. Dis. 185:632-640. [DOI] [PubMed] [Google Scholar]
- 5.Berner, R., R. F. Schumacher, M. Brandis, and J. Forster. 1996. Colonization and infection with Moraxella catarrhalis in childhood. Eur. J. Clin. Microbiol. Infect. Dis. 15:506-509. [DOI] [PubMed] [Google Scholar]
- 6.Black, A., and T. Wilson. 1988. Immunoglobulin G (IgG) serological response to Branhamella catarrhalis in patients with acute bronchopulmonary infections. J. Clin. Pathol. 41:329-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman, A. J., D. Musher, S. Jonsson, J. Clarridge, and R. J. Wallace. 1985. Development of bactericidal antibody during Branhamella catarrhalis infection. J. Infect. Dis. 151:878-882. [DOI] [PubMed] [Google Scholar]
- 8.Chen, D., V. Barniak, K. R. Vandermeid, and J. C. McMichael. 1999. The levels and bactericidal capacity of antibodies directed against the UspA1 and UspA2 outer membrane proteins of Moraxella (Branhamella) catarrhalis in adults and children. Infect. Immun. 67:1310-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, J., and D. Morrison. 1988. Construction and properties of a new insertion vector, pJDC9, that is protected by transcriptional terminators and useful for cloning of DNA from Streptococcus pneumoniae. Gene 64:155-164. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, J. 1999. Moraxella (Branhamella) catarrhalis: clinical, microbiological and immunological features in lower respiratory tract infections. APMIS Suppl. 88:1-36. [PubMed] [Google Scholar]
- 11.Ejlertsen, T., E. Thisted, F. Ebbesen, B. Olesen, and J. Renneberg. 1994. A study of prevalence, time of colonisation, and association with upper and lower respiratory tract infections. J. Infect. 29:23-31. [DOI] [PubMed] [Google Scholar]
- 12.Faden, H., Y. Harabuchi, and J. J. Hong. 1994. Epidemiology of Moraxella catarrhalis in children during the first 2 years of life: relationship to otitis media. J. Infect. Dis. 169:1312-1317. [DOI] [PubMed] [Google Scholar]
- 13.Faden, H., J. Hong, and T. F. Murphy. 1992. Immune response to outer membrane antigens of Moraxella catarrhalis in children with otitis media. Infect. Immun. 60:3824-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsgren, A., M. Brant, A. Möllenkvist, A. Muyombwe, H. Janson, N. Woin, and K. Riesbeck. 2001. Isolation and characterization of a novel IgD-binding protein from Moraxella catarrhalis. J. Immunol. 167:2112-2120. [DOI] [PubMed] [Google Scholar]
- 15.Gehanno, P., A. Panajotopoulos, B. Barry, L. Nguyen, D. Levy, E. Bingen, and P. Berche. 2001. Microbiology of otitis media in the Paris, France, area from 1987 to 1997. Pediatr. Infect. Dis. J. 20:570-573. [DOI] [PubMed] [Google Scholar]
- 16.Gunnarsson, R. K., S. E. Holm, and M. Soderstrom. 1998. The prevalence of potential pathogenic bacteria in nasopharyngeal samples from healthy children and adults. Scand. J. Prim. Health Care 16:13-17. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 18.Helminen, M. E., I. Maciver, J. L. Latimer, L. D. Cope, G. H. McCracken, Jr., and E. J. Hansen. 1993. A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect. Immun. 61:2003-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helminen, M. E., I. Maciver, J. L. Latimer, J. Klesney-Tait, L. D. Cope, M. Paris, G. H. McCracken, Jr., and E. J. Hansen. 1994. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J. Infect. Dis. 170:867-872. [DOI] [PubMed] [Google Scholar]
- 20.Helminen, M. E., I. Maciver, J. L. Latimer, S. L. Lumbley, L. D. Cope, and G. H. McCracken, Jr. 1993. A mutation affecting expression of a major outer membrane protein of Moraxella catarrhalis alters serum resistance and survival of this organism in vivo. J. Infect. Dis. 168:1201. [DOI] [PubMed] [Google Scholar]
- 21.Helminen, M. E., R. Beach, I. Maciver, G. Jarosik, E. J. Hansen, and M. Leinonen. 1995. Hum. immune response against outer membrane proteins of Moraxella (Branhamella) catarrhalis determined by immunoblotting and enzyme immunoassay. Clin. Diagn. Lab. Immunol. 2:35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoiczyk, E., A. Roggenkamp, M. Reichenbecher, A. Lupas, and J. Heesemann. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 19:5989-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jousimies-Somer, H. R., S. Savolainen, and J. S. Ylikoski. 1989. Comparison of the nasal bacterial floras in two groups of healthy subjects and in patients with acute maxillary sinusitis. J. Clin. Microbiol. 27:2736-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karalus, R., and A. A. Campagnari. 2000. Moraxella catarrhalis: a review of an important human mucosal pathogen. Microbes Infect. 2:547-559. [DOI] [PubMed] [Google Scholar]
- 25.Kilpi, T., E. Herva, T. Kaijalainen, R. Syrjanen, and A. K. Takala. 2001. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 20:654-662. [DOI] [PubMed] [Google Scholar]
- 26.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 182:1364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leach, A. J., J. B. Boswell, V. Asche, T. G. Nienhuys, and J. D. Mathews. 1994. Bacterial colonization of the nasopharynx predicts very early onset and pesistence of otitis media in Australian aboriginal infants. Pediatr. Infect. Dis. J. 13:983-989. [DOI] [PubMed] [Google Scholar]
- 28.Luke, N. R., and A. A. Campagnari. 1999. Construction and characterization of Moraxella catarrhalis mutants defective in expression of transferrin receptors. Infect. Immun. 67:5815-5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathers, K., M. Leinonen, and D. Goldblatt. 1999. Antibody response to outer membrane proteins of Moraxella catarrhalis in children with otitis media. Pediatr. Infect. Dis. J. 18:982-988. [DOI] [PubMed] [Google Scholar]
- 30.McLeod, D., F. Ahmad, S. Capewell, M. Croughan, M. Calder, and A. Seaton. 1986. Increase in bronchopulmonary infections due to Branhamella catarrhalis. Br. Med. J. 292:1103-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMichael, J. C. 2000. Vaccines for Moraxella catarrhalis. Vaccine 19:S101-S107. [DOI] [PubMed] [Google Scholar]
- 32.Meier, P. S., S. Freiburghaus, A. Martin, N. Heiniger, R. Troller, and C. Aebi. 2003. Mucosal immune response to specific outer membrane proteins of Moraxella catarrhalis in young children. Pediatr. Infect. Dis. J. 22:256-262. [DOI] [PubMed] [Google Scholar]
- 33.Meier, P. S., R. Troller, I. N. Grivea, G. A. Syrogiannopoulos, and C. Aebi. 2002. The outer membrane proteins UspA1 and UspA2 of Moraxella catarrhalis are highly conserved in nasopharyngeal isolates from young children. Vaccine 20:1754-1760. [DOI] [PubMed] [Google Scholar]
- 34.Mollenkvist, A., T. Nordstrom, C. Hallden, J. J. Christensen, A. Forsgren, and K. Riesbeck. 2003. The Moraxella catarrhalis immunoglobulin D-binding protein MID has conserved sequences and is regulated by a mechanism corresponding to phase variation. J. Bacteriol. 185:2285-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy, T. F., C. Kirkham, and A. Lesse. 1993. The major heat-modifiable outer membrane protein CD is highly conserved among strains of Branhamella catarrhalis. Mol. Microbiol. 10:87-97. [DOI] [PubMed] [Google Scholar]
- 36.Murphy, T. F., and M. R. Loeb. 1989. Isolation of the outer membrane of Branhamella catarrhalis. Microb. Pathog. 6:159-174. [DOI] [PubMed] [Google Scholar]
- 37.Pearson, M. M., E. R. Lafontaine, N. J. Wagner, J. W. Geme III, and E. J. Hansen. 2002. A hag mutant of Moraxella catarrhalis strain O35E is deficient in hemagglutination, autoagglutination, and immunoglobulin D-binding activities. Infect. Immun. 70:4523-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raza, M., C. Blackwell, M. Ogilive, A. Saadi, J. Stewart, R. Elton, and D. Weir. 1994. Evidence for the role of glycoprotein G of respiratory syncytial virus in binding of Neisseria meningitidis to HEp-2. FEMS Immunol. Med. Microbiol. 10:25-30. [DOI] [PubMed] [Google Scholar]
- 39.Raza, M., M. Ogilive, C. Blackwell, J. Stewart, R. Elton, and D. Weir. 1993. Effect of respiratory syncytial virus infection on binding of Neisseria meningitidis and Haemophilus influenzae type b to human epithelial cell line (HEp-2). Epidemiol. Infect. 110:339-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salvi, S., and S. T. Holgate. 1999. Could the airway epithelium play an important role in mucosal immunoglobulin A production? Clin. Exp. Allergy 29:1597-1605. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Samukawa, T., N. Ymanaka, S. Hollingshead, K. Klingman, and H. Faden. 2000. Immune responses to specific antigens of Streptococcus pneumoniae and Moraxella catarrhalis in the respiratory tract. Infect. Immun. 68:1569-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samukawa, T., N. Ymanaka, S. Hollingshead, T. F. Murphy, and H. Faden. 2000. Immune response to surface protein A of Streptococcus pneumoniae and to high-molecular-weight outer membrane protein A of Moraxella catarrhalis in children with acute otitis media. J. Infect. Dis. 181:1842-1845. [DOI] [PubMed] [Google Scholar]
- 44.Sethi, S., J. M. Surface, and T. F. Murphy. 1997. Antigenic heterogeneity and molecular analysis of CopB of Moraxella (Branhamella) catarrhalis. Infect. Immun. 65:3666-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaneechoutte, M., G. Verschraegen, G. Claeys, B. Weise, and A. van den Abeele. 1990. Respiratory tract carrier rates of Moraxella (Branhamella) catarrhalis in adults and children and interpretation of the isolation of M. catarrhalis from sputum. J. Clin. Microbiol. 28:2674-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]