Abstract
The asymmetric unit of the title compound, C14H17N3O3, contains two independent molecules with different conformations of the ethyl groups. In the crystal, intermolecular N—H⋯O hydrogen bonds link the molecules into ribbons extending along the a axis.
Related literature
For the bioactivity and chemiluminescence of coumarin derivatives, see: Munasinghe et al. (2007 ▶). For a related structure, see: Yu et al. (2009 ▶). For details of the synthesis, see: Ma et al. (2010 ▶).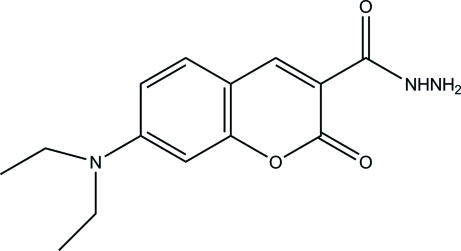
Experimental
Crystal data
C14H17N3O3
M r = 275.31
Triclinic,

a = 9.3438 (19) Å
b = 12.771 (3) Å
c = 12.978 (3) Å
α = 95.17 (3)°
β = 110.13 (3)°
γ = 106.18 (3)°
V = 1366.4 (5) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 290 K
0.14 × 0.12 × 0.11 mm
Data collection
Rigaku R-AXIS RAPID diffractometer
Absorption correction: multi-scan (ABSCOR; Higashi, 1995 ▶) T min = 0.987, T max = 0.990
13506 measured reflections
6186 independent reflections
3402 reflections with I > 2σ(I)
R int = 0.030
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.117
S = 1.01
6186 reflections
383 parameters
6 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.18 e Å−3
Δρmin = −0.19 e Å−3
Data collection: RAPID-AUTO (Rigaku Corporation, 1998 ▶); cell refinement: RAPID-AUTO; data reduction: CrystalStructure (Rigaku/MSC & Rigaku Corporation, 2002 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811010944/cv5061sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811010944/cv5061Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2⋯O2 | 0.86 (3) | 2.01 (2) | 2.7098 (18) | 137 (2) |
| N5—H5⋯O5 | 0.87 (3) | 2.03 (2) | 2.733 (2) | 138 (2) |
| N6—H6B⋯O3 | 0.86 (3) | 2.30 (1) | 3.131 (2) | 164 (2) |
| N3—H3A⋯O3i | 0.87 (3) | 2.20 (1) | 3.002 (2) | 155 (2) |
| N3—H3B⋯O6ii | 0.87 (3) | 2.23 (1) | 3.039 (2) | 154 (2) |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
The authors acknowledge financial support from the National Natural Science Foundation of China (grant No. 21062022) and the Open Project of the State Key Laboratory of Supramolecular Structure and Materials, Jilin University.
supplementary crystallographic information
Comment
Coumarin derivatives have received considerable attention since their diverse bioactivities and chemiluminescence (Munasinghe et al. 2007). Herein, we report the crystal structure of the title compound, an important organic intermediate and a fluorescent tagging agent for chemosensors.
In the title compound, all bond lengths and angles are normal and comparable to those observed in the related struture (Yu et al., 2009). There are two independent molecules in the asymmetric unit with different conformation of the ethyl groups (Fig. 1). Except for four terminal carbon atoms, the other non-hydrogen atoms are nearly coplanar for two components [mean deviations from the mean planes are 0.065 (1) and 0.07 (1), respectively] and form an angle of 19.39 (4) °. Intermolecuar N—H···O hydrogen bonds (Table 1) link the molecules into ribbons extended along axis a.
Experimental
The title compound was prepared according to the literature (Ma et al., 2010). Single crystals suitable for X-ray diffraction were prepared by slow evaporation a mixture of dichloromethane and petroleum (60–90 °C) at room temperature.
Refinement
C-bound H-atoms were placed in calculated positions with C—H = 0.93, 096 or 0.97 Å and were included in the refinement in the riding model with Uiso(H) = 1.2 or 1.5 Ueq(C). The H of nitrogen atom were located from differecne Fourier Map and refined with N—H bond lengths restrained to 0.87 (3) Å, and with Uiso(H) = 1.5 Ueq(N).
Figures
Fig. 1.
Two independent molecules of the title compound, with the atom numbering. Displacement ellipsoids of non-H atoms are drawn at the 30% probalility level.
Crystal data
| C14H17N3O3 | Z = 4 |
| Mr = 275.31 | F(000) = 584 |
| Triclinic, P1 | Dx = 1.338 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.3438 (19) Å | Cell parameters from 8202 reflections |
| b = 12.771 (3) Å | θ = 3.2–27.5° |
| c = 12.978 (3) Å | µ = 0.10 mm−1 |
| α = 95.17 (3)° | T = 290 K |
| β = 110.13 (3)° | Block, yellow |
| γ = 106.18 (3)° | 0.14 × 0.12 × 0.11 mm |
| V = 1366.4 (5) Å3 |
Data collection
| Rigaku R-AXIS RAPID diffractometer | 6186 independent reflections |
| Radiation source: fine-focus sealed tube | 3402 reflections with I > 2σ(I) |
| graphite | Rint = 0.030 |
| ω scans | θmax = 27.5°, θmin = 3.2° |
| Absorption correction: multi-scan (ABSCOR; Higashi, 1995) | h = −12→10 |
| Tmin = 0.987, Tmax = 0.990 | k = −16→16 |
| 13506 measured reflections | l = −16→16 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.045 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.117 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.0543P)2] where P = (Fo2 + 2Fc2)/3 |
| 6186 reflections | (Δ/σ)max = 0.005 |
| 383 parameters | Δρmax = 0.18 e Å−3 |
| 6 restraints | Δρmin = −0.19 e Å−3 |
Special details
| Experimental. (See detailed section in the paper) |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.21139 (18) | 0.57501 (13) | 1.19989 (14) | 0.0463 (4) | |
| C2 | 0.13466 (18) | 0.48762 (12) | 1.24574 (13) | 0.0436 (4) | |
| C3 | 0.13127 (19) | 0.50961 (13) | 1.35921 (14) | 0.0492 (4) | |
| C4 | 0.06725 (18) | 0.38172 (12) | 1.18533 (13) | 0.0463 (4) | |
| H4 | 0.0150 | 0.3265 | 1.2146 | 0.056* | |
| C5 | 0.07300 (18) | 0.35191 (12) | 1.08046 (13) | 0.0443 (4) | |
| C6 | 0.15295 (17) | 0.43610 (12) | 1.03866 (13) | 0.0426 (4) | |
| C7 | 0.17389 (18) | 0.41745 (13) | 0.93993 (14) | 0.0494 (4) | |
| H7 | 0.2289 | 0.4764 | 0.9159 | 0.059* | |
| C8 | 0.11153 (18) | 0.30841 (13) | 0.87521 (13) | 0.0484 (4) | |
| C9 | 0.02840 (19) | 0.22172 (13) | 0.91645 (14) | 0.0524 (4) | |
| H9 | −0.0144 | 0.1489 | 0.8754 | 0.063* | |
| C10 | 0.01086 (19) | 0.24390 (13) | 1.01450 (14) | 0.0516 (4) | |
| H10 | −0.0443 | 0.1855 | 1.0391 | 0.062* | |
| C11 | 0.0769 (2) | 0.17291 (15) | 0.71424 (16) | 0.0645 (5) | |
| H11A | 0.0960 | 0.1239 | 0.7666 | 0.077* | |
| H11B | 0.1411 | 0.1699 | 0.6698 | 0.077* | |
| C12 | −0.0988 (2) | 0.13259 (17) | 0.63824 (16) | 0.0768 (6) | |
| H12A | −0.1625 | 0.1375 | 0.6817 | 0.115* | |
| H12B | −0.1295 | 0.0565 | 0.6013 | 0.115* | |
| H12C | −0.1168 | 0.1780 | 0.5832 | 0.115* | |
| C13 | 0.2090 (2) | 0.37670 (15) | 0.72996 (15) | 0.0640 (5) | |
| H13A | 0.1828 | 0.4430 | 0.7462 | 0.077* | |
| H13B | 0.1687 | 0.3532 | 0.6491 | 0.077* | |
| C14 | 0.3890 (2) | 0.40375 (17) | 0.77862 (19) | 0.0777 (6) | |
| H14A | 0.4301 | 0.4333 | 0.8577 | 0.116* | |
| H14B | 0.4371 | 0.4580 | 0.7430 | 0.116* | |
| H14C | 0.4149 | 0.3373 | 0.7662 | 0.116* | |
| C15 | 0.2466 (2) | 0.11381 (14) | 1.16812 (14) | 0.0526 (4) | |
| C16 | 0.36577 (19) | 0.22392 (14) | 1.21170 (14) | 0.0506 (4) | |
| C17 | 0.3901 (2) | 0.29587 (15) | 1.31736 (15) | 0.0556 (4) | |
| C18 | 0.4607 (2) | 0.26342 (14) | 1.15444 (14) | 0.0544 (4) | |
| H18 | 0.5359 | 0.3349 | 1.1819 | 0.065* | |
| C19 | 0.44932 (19) | 0.20030 (13) | 1.05563 (14) | 0.0493 (4) | |
| C20 | 0.33527 (18) | 0.09381 (13) | 1.01525 (13) | 0.0466 (4) | |
| C21 | 0.31367 (19) | 0.02453 (13) | 0.92049 (14) | 0.0512 (4) | |
| H21 | 0.2345 | −0.0454 | 0.8962 | 0.061* | |
| C22 | 0.41151 (19) | 0.05924 (13) | 0.85967 (14) | 0.0494 (4) | |
| C23 | 0.5282 (2) | 0.16798 (14) | 0.90010 (14) | 0.0547 (4) | |
| H23 | 0.5936 | 0.1939 | 0.8614 | 0.066* | |
| C24 | 0.5454 (2) | 0.23412 (14) | 0.99384 (15) | 0.0571 (4) | |
| H24 | 0.6236 | 0.3045 | 1.0184 | 0.069* | |
| C25 | 0.4975 (2) | 0.02687 (16) | 0.70252 (16) | 0.0687 (5) | |
| H25A | 0.5055 | −0.0386 | 0.6639 | 0.082* | |
| H25B | 0.6054 | 0.0717 | 0.7547 | 0.082* | |
| C26 | 0.4354 (3) | 0.09271 (17) | 0.61840 (16) | 0.0806 (6) | |
| H26A | 0.3285 | 0.0490 | 0.5663 | 0.121* | |
| H26B | 0.5055 | 0.1117 | 0.5786 | 0.121* | |
| H26C | 0.4322 | 0.1596 | 0.6563 | 0.121* | |
| C27 | 0.2699 (2) | −0.11834 (14) | 0.72177 (16) | 0.0646 (5) | |
| H27A | 0.2679 | −0.1551 | 0.7837 | 0.078* | |
| H27B | 0.2989 | −0.1627 | 0.6727 | 0.078* | |
| C28 | 0.1023 (2) | −0.11651 (16) | 0.65768 (16) | 0.0717 (5) | |
| H28A | 0.0664 | −0.0811 | 0.7077 | 0.108* | |
| H28B | 0.0299 | −0.1915 | 0.6253 | 0.108* | |
| H28C | 0.1040 | −0.0758 | 0.5992 | 0.108* | |
| N1 | 0.12851 (17) | 0.28688 (11) | 0.77682 (12) | 0.0596 (4) | |
| N2 | 0.19388 (19) | 0.61432 (12) | 1.41508 (12) | 0.0581 (4) | |
| H2 | 0.231 (2) | 0.6657 (13) | 1.3829 (16) | 0.087* | |
| N3 | 0.1989 (2) | 0.64809 (13) | 1.52280 (14) | 0.0672 (4) | |
| H3B | 0.268 (2) | 0.6211 (18) | 1.5658 (15) | 0.101* | |
| H3A | 0.1032 (15) | 0.6189 (17) | 1.5226 (19) | 0.101* | |
| N4 | 0.39535 (17) | −0.00890 (12) | 0.76634 (12) | 0.0579 (4) | |
| N5 | 0.3063 (2) | 0.25209 (14) | 1.37695 (14) | 0.0663 (4) | |
| H5 | 0.241 (2) | 0.1841 (10) | 1.3507 (18) | 0.099* | |
| N6 | 0.3254 (2) | 0.30988 (17) | 1.48147 (15) | 0.0782 (5) | |
| H6B | 0.270 (3) | 0.3544 (18) | 1.470 (2) | 0.117* | |
| H6A | 0.4263 (14) | 0.3504 (18) | 1.5136 (19) | 0.117* | |
| O1 | 0.21779 (13) | 0.54484 (8) | 1.09751 (9) | 0.0493 (3) | |
| O2 | 0.27280 (15) | 0.67378 (9) | 1.24139 (10) | 0.0638 (3) | |
| O3 | 0.07200 (17) | 0.43205 (10) | 1.39865 (11) | 0.0697 (4) | |
| O4 | 0.23680 (13) | 0.05352 (9) | 1.07136 (9) | 0.0542 (3) | |
| O5 | 0.15179 (15) | 0.06693 (10) | 1.20795 (11) | 0.0694 (4) | |
| O6 | 0.48723 (17) | 0.39132 (11) | 1.34921 (11) | 0.0751 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0444 (9) | 0.0409 (9) | 0.0559 (10) | 0.0098 (7) | 0.0261 (8) | 0.0083 (8) |
| C2 | 0.0416 (8) | 0.0405 (8) | 0.0540 (10) | 0.0136 (7) | 0.0244 (8) | 0.0110 (7) |
| C3 | 0.0499 (9) | 0.0471 (9) | 0.0602 (11) | 0.0185 (8) | 0.0302 (8) | 0.0131 (8) |
| C4 | 0.0442 (9) | 0.0412 (9) | 0.0556 (10) | 0.0098 (7) | 0.0238 (8) | 0.0152 (8) |
| C5 | 0.0424 (8) | 0.0398 (8) | 0.0497 (9) | 0.0092 (7) | 0.0195 (7) | 0.0114 (7) |
| C6 | 0.0362 (8) | 0.0362 (8) | 0.0508 (10) | 0.0065 (7) | 0.0163 (7) | 0.0063 (7) |
| C7 | 0.0488 (9) | 0.0415 (9) | 0.0561 (10) | 0.0046 (7) | 0.0268 (8) | 0.0080 (8) |
| C8 | 0.0442 (9) | 0.0478 (9) | 0.0495 (10) | 0.0091 (8) | 0.0199 (8) | 0.0051 (8) |
| C9 | 0.0530 (10) | 0.0374 (8) | 0.0553 (11) | 0.0043 (8) | 0.0177 (8) | 0.0028 (8) |
| C10 | 0.0530 (10) | 0.0392 (8) | 0.0568 (11) | 0.0031 (8) | 0.0236 (8) | 0.0107 (8) |
| C11 | 0.0749 (13) | 0.0558 (11) | 0.0622 (12) | 0.0167 (10) | 0.0326 (10) | 0.0004 (9) |
| C12 | 0.0884 (15) | 0.0648 (12) | 0.0565 (12) | 0.0097 (11) | 0.0179 (11) | 0.0050 (10) |
| C13 | 0.0696 (12) | 0.0645 (12) | 0.0595 (12) | 0.0189 (10) | 0.0313 (10) | 0.0050 (9) |
| C14 | 0.0777 (14) | 0.0684 (13) | 0.1055 (17) | 0.0265 (11) | 0.0537 (13) | 0.0260 (12) |
| C15 | 0.0563 (10) | 0.0527 (10) | 0.0567 (11) | 0.0170 (9) | 0.0300 (9) | 0.0196 (9) |
| C16 | 0.0521 (10) | 0.0534 (10) | 0.0534 (10) | 0.0198 (8) | 0.0252 (8) | 0.0191 (8) |
| C17 | 0.0559 (10) | 0.0583 (11) | 0.0579 (11) | 0.0219 (10) | 0.0240 (9) | 0.0202 (9) |
| C18 | 0.0549 (10) | 0.0469 (9) | 0.0607 (11) | 0.0109 (8) | 0.0250 (9) | 0.0161 (9) |
| C19 | 0.0505 (9) | 0.0470 (9) | 0.0515 (10) | 0.0100 (8) | 0.0245 (8) | 0.0168 (8) |
| C20 | 0.0456 (9) | 0.0469 (9) | 0.0530 (10) | 0.0114 (8) | 0.0265 (8) | 0.0208 (8) |
| C21 | 0.0521 (10) | 0.0441 (9) | 0.0586 (11) | 0.0078 (8) | 0.0276 (9) | 0.0168 (8) |
| C22 | 0.0505 (9) | 0.0503 (9) | 0.0535 (10) | 0.0155 (8) | 0.0261 (8) | 0.0192 (8) |
| C23 | 0.0531 (10) | 0.0576 (10) | 0.0560 (11) | 0.0089 (8) | 0.0296 (9) | 0.0208 (9) |
| C24 | 0.0576 (10) | 0.0482 (10) | 0.0611 (11) | 0.0010 (8) | 0.0297 (9) | 0.0161 (9) |
| C25 | 0.0768 (13) | 0.0694 (12) | 0.0750 (13) | 0.0238 (11) | 0.0470 (11) | 0.0165 (11) |
| C26 | 0.0798 (14) | 0.0811 (14) | 0.0630 (13) | 0.0053 (12) | 0.0223 (11) | 0.0185 (11) |
| C27 | 0.0742 (13) | 0.0515 (10) | 0.0740 (13) | 0.0163 (10) | 0.0409 (11) | 0.0064 (9) |
| C28 | 0.0660 (12) | 0.0690 (12) | 0.0765 (14) | 0.0100 (10) | 0.0366 (11) | 0.0004 (10) |
| N1 | 0.0636 (9) | 0.0501 (8) | 0.0602 (9) | 0.0039 (7) | 0.0324 (8) | 0.0005 (7) |
| N2 | 0.0738 (10) | 0.0494 (9) | 0.0568 (10) | 0.0158 (8) | 0.0362 (8) | 0.0081 (7) |
| N3 | 0.0833 (12) | 0.0614 (10) | 0.0638 (11) | 0.0183 (9) | 0.0431 (10) | 0.0042 (8) |
| N4 | 0.0605 (9) | 0.0560 (9) | 0.0631 (10) | 0.0128 (7) | 0.0359 (8) | 0.0124 (8) |
| N5 | 0.0763 (11) | 0.0713 (10) | 0.0619 (10) | 0.0225 (9) | 0.0404 (9) | 0.0155 (9) |
| N6 | 0.0926 (13) | 0.0951 (14) | 0.0573 (11) | 0.0356 (11) | 0.0380 (10) | 0.0145 (10) |
| O1 | 0.0545 (6) | 0.0380 (6) | 0.0568 (7) | 0.0050 (5) | 0.0322 (6) | 0.0062 (5) |
| O2 | 0.0802 (8) | 0.0391 (6) | 0.0750 (8) | 0.0036 (6) | 0.0481 (7) | 0.0033 (6) |
| O3 | 0.0996 (10) | 0.0517 (7) | 0.0758 (9) | 0.0195 (7) | 0.0587 (8) | 0.0168 (7) |
| O4 | 0.0581 (7) | 0.0490 (6) | 0.0606 (7) | 0.0078 (5) | 0.0360 (6) | 0.0146 (6) |
| O5 | 0.0763 (9) | 0.0634 (8) | 0.0779 (9) | 0.0078 (7) | 0.0526 (8) | 0.0166 (7) |
| O6 | 0.0871 (10) | 0.0642 (9) | 0.0697 (9) | 0.0104 (8) | 0.0380 (8) | 0.0079 (7) |
Geometric parameters (Å, °)
| C1—O2 | 1.2131 (19) | C16—C17 | 1.489 (2) |
| C1—O1 | 1.3757 (18) | C17—O6 | 1.231 (2) |
| C1—C2 | 1.443 (2) | C17—N5 | 1.332 (2) |
| C2—C4 | 1.354 (2) | C18—C19 | 1.406 (2) |
| C2—C3 | 1.487 (2) | C18—H18 | 0.9300 |
| C3—O3 | 1.2372 (19) | C19—C20 | 1.393 (2) |
| C3—N2 | 1.324 (2) | C19—C24 | 1.406 (2) |
| C4—C5 | 1.403 (2) | C20—C21 | 1.367 (2) |
| C4—H4 | 0.9300 | C20—O4 | 1.3808 (17) |
| C5—C6 | 1.395 (2) | C21—C22 | 1.410 (2) |
| C5—C10 | 1.404 (2) | C21—H21 | 0.9300 |
| C6—C7 | 1.371 (2) | C22—N4 | 1.363 (2) |
| C6—O1 | 1.3785 (18) | C22—C23 | 1.422 (2) |
| C7—C8 | 1.409 (2) | C23—C24 | 1.350 (2) |
| C7—H7 | 0.9300 | C23—H23 | 0.9300 |
| C8—N1 | 1.3534 (19) | C24—H24 | 0.9300 |
| C8—C9 | 1.425 (2) | C25—N4 | 1.475 (2) |
| C9—C10 | 1.353 (2) | C25—C26 | 1.496 (3) |
| C9—H9 | 0.9300 | C25—H25A | 0.9700 |
| C10—H10 | 0.9300 | C25—H25B | 0.9700 |
| C11—N1 | 1.462 (2) | C26—H26A | 0.9600 |
| C11—C12 | 1.502 (3) | C26—H26B | 0.9600 |
| C11—H11A | 0.9700 | C26—H26C | 0.9600 |
| C11—H11B | 0.9700 | C27—N4 | 1.462 (2) |
| C12—H12A | 0.9600 | C27—C28 | 1.507 (3) |
| C12—H12B | 0.9600 | C27—H27A | 0.9700 |
| C12—H12C | 0.9600 | C27—H27B | 0.9700 |
| C13—N1 | 1.487 (2) | C28—H28A | 0.9600 |
| C13—C14 | 1.501 (3) | C28—H28B | 0.9600 |
| C13—H13A | 0.9700 | C28—H28C | 0.9600 |
| C13—H13B | 0.9700 | N2—N3 | 1.406 (2) |
| C14—H14A | 0.9600 | N2—H2 | 0.86 (3) |
| C14—H14B | 0.9600 | N3—H3B | 0.87 (3) |
| C14—H14C | 0.9600 | N3—H3A | 0.87 (3) |
| C15—O5 | 1.2187 (18) | N5—N6 | 1.413 (2) |
| C15—O4 | 1.3732 (19) | N5—H5 | 0.87 (3) |
| C15—C16 | 1.445 (3) | N6—H6B | 0.86 (3) |
| C16—C18 | 1.366 (2) | N6—H6A | 0.87 (3) |
| O2—C1—O1 | 115.01 (14) | C16—C18—H18 | 118.7 |
| O2—C1—C2 | 127.53 (15) | C19—C18—H18 | 118.7 |
| O1—C1—C2 | 117.45 (14) | C20—C19—C18 | 117.92 (15) |
| C4—C2—C1 | 119.34 (14) | C20—C19—C24 | 116.24 (16) |
| C4—C2—C3 | 118.69 (14) | C18—C19—C24 | 125.83 (16) |
| C1—C2—C3 | 121.94 (14) | C21—C20—O4 | 116.70 (14) |
| O3—C3—N2 | 122.13 (15) | C21—C20—C19 | 123.23 (14) |
| O3—C3—C2 | 120.46 (15) | O4—C20—C19 | 120.07 (14) |
| N2—C3—C2 | 117.41 (14) | C20—C21—C22 | 119.95 (16) |
| C2—C4—C5 | 122.75 (14) | C20—C21—H21 | 120.0 |
| C2—C4—H4 | 118.6 | C22—C21—H21 | 120.0 |
| C5—C4—H4 | 118.6 | N4—C22—C21 | 121.31 (16) |
| C6—C5—C4 | 117.57 (14) | N4—C22—C23 | 121.43 (15) |
| C6—C5—C10 | 116.19 (14) | C21—C22—C23 | 117.25 (16) |
| C4—C5—C10 | 126.19 (14) | C24—C23—C22 | 121.02 (15) |
| C7—C6—O1 | 116.17 (13) | C24—C23—H23 | 119.5 |
| C7—C6—C5 | 123.47 (14) | C22—C23—H23 | 119.5 |
| O1—C6—C5 | 120.35 (13) | C23—C24—C19 | 122.30 (16) |
| C6—C7—C8 | 119.52 (15) | C23—C24—H24 | 118.9 |
| C6—C7—H7 | 120.2 | C19—C24—H24 | 118.9 |
| C8—C7—H7 | 120.2 | N4—C25—C26 | 113.65 (15) |
| N1—C8—C7 | 121.23 (15) | N4—C25—H25A | 108.8 |
| N1—C8—C9 | 121.16 (15) | C26—C25—H25A | 108.8 |
| C7—C8—C9 | 117.60 (14) | N4—C25—H25B | 108.8 |
| C10—C9—C8 | 120.88 (15) | C26—C25—H25B | 108.8 |
| C10—C9—H9 | 119.6 | H25A—C25—H25B | 107.7 |
| C8—C9—H9 | 119.6 | C25—C26—H26A | 109.5 |
| C9—C10—C5 | 122.33 (15) | C25—C26—H26B | 109.5 |
| C9—C10—H10 | 118.8 | H26A—C26—H26B | 109.5 |
| C5—C10—H10 | 118.8 | C25—C26—H26C | 109.5 |
| N1—C11—C12 | 111.83 (15) | H26A—C26—H26C | 109.5 |
| N1—C11—H11A | 109.3 | H26B—C26—H26C | 109.5 |
| C12—C11—H11A | 109.3 | N4—C27—C28 | 114.99 (15) |
| N1—C11—H11B | 109.3 | N4—C27—H27A | 108.5 |
| C12—C11—H11B | 109.3 | C28—C27—H27A | 108.5 |
| H11A—C11—H11B | 107.9 | N4—C27—H27B | 108.5 |
| C11—C12—H12A | 109.5 | C28—C27—H27B | 108.5 |
| C11—C12—H12B | 109.5 | H27A—C27—H27B | 107.5 |
| H12A—C12—H12B | 109.5 | C27—C28—H28A | 109.5 |
| C11—C12—H12C | 109.5 | C27—C28—H28B | 109.5 |
| H12A—C12—H12C | 109.5 | H28A—C28—H28B | 109.5 |
| H12B—C12—H12C | 109.5 | C27—C28—H28C | 109.5 |
| N1—C13—C14 | 111.26 (15) | H28A—C28—H28C | 109.5 |
| N1—C13—H13A | 109.4 | H28B—C28—H28C | 109.5 |
| C14—C13—H13A | 109.4 | C8—N1—C11 | 121.48 (14) |
| N1—C13—H13B | 109.4 | C8—N1—C13 | 122.05 (14) |
| C14—C13—H13B | 109.4 | C11—N1—C13 | 116.37 (13) |
| H13A—C13—H13B | 108.0 | C3—N2—N3 | 123.96 (15) |
| C13—C14—H14A | 109.5 | C3—N2—H2 | 118.5 (14) |
| C13—C14—H14B | 109.5 | N3—N2—H2 | 117.5 (14) |
| H14A—C14—H14B | 109.5 | N2—N3—H3B | 103.8 (14) |
| C13—C14—H14C | 109.5 | N2—N3—H3A | 108.3 (16) |
| H14A—C14—H14C | 109.5 | H3B—N3—H3A | 112 (2) |
| H14B—C14—H14C | 109.5 | C22—N4—C27 | 121.26 (14) |
| O5—C15—O4 | 115.15 (15) | C22—N4—C25 | 121.55 (15) |
| O5—C15—C16 | 127.16 (16) | C27—N4—C25 | 117.10 (15) |
| O4—C15—C16 | 117.69 (14) | C17—N5—N6 | 122.83 (18) |
| C18—C16—C15 | 118.90 (16) | C17—N5—H5 | 117.4 (15) |
| C18—C16—C17 | 118.62 (16) | N6—N5—H5 | 119.7 (15) |
| C15—C16—C17 | 122.48 (15) | N5—N6—H6B | 108.7 (17) |
| O6—C17—N5 | 121.62 (18) | N5—N6—H6A | 106.1 (17) |
| O6—C17—C16 | 120.77 (16) | H6B—N6—H6A | 107 (2) |
| N5—C17—C16 | 117.59 (17) | C1—O1—C6 | 122.45 (12) |
| C16—C18—C19 | 122.60 (17) | C15—O4—C20 | 122.81 (13) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2···O2 | 0.86 (3) | 2.01 (2) | 2.7098 (18) | 137.(2) |
| N5—H5···O5 | 0.87 (3) | 2.03 (2) | 2.733 (2) | 138 (2) |
| N6—H6B···O3 | 0.86 (3) | 2.30 (1) | 3.131 (2) | 164 (2) |
| N3—H3A···O3i | 0.87 (3) | 2.20 (1) | 3.002 (2) | 155 (2) |
| N3—H3B···O6ii | 0.87 (3) | 2.23 (1) | 3.039 (2) | 154 (2) |
Symmetry codes: (i) −x, −y+1, −z+3; (ii) −x+1, −y+1, −z+3.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: CV5061).
References
- Higashi, T. (1995). ABSCOR Rigaku Corporation, Tokyo, Japan.
- Ma, W. H., Xu, Q., Du, J. J., Song, B., Peng, X. J., Wang, Z., Li, G. D. & Wang, X. F. (2010). Spectrochim. Acta Part A, 76, 248–252. [DOI] [PubMed]
- Munasinghe, V. R. N., Corrie, J. E. T., Geoff, K. G. & Martin, S. R. (2007). Bioconjugate Chem. 18, 231–237. [DOI] [PubMed]
- Rigaku Corporation (1998). RAPID-AUTO Rigaku Corporation, Tokyo, Japan.
- Rigaku/MSC & Rigaku Corporation (2002). CrystalStructure Rigaku/MSC Inc., The Woodlands, Texas, USA.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Yu, T. Z., Zhang, P., Zhao, Y. L., Zhang, H., Meng, J. & Fan, D. W. (2009). Org. Electron. 10, 653–660.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811010944/cv5061sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811010944/cv5061Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



