Abstract
The title compound, C16H20O4, was obtained unintentionally as the byproduct of an attempted synthesis of methyl 3-(cyclopropylmethoxy)-4-hydroxybenzoate. In the crystal, the molecules are linked by intermolecular C—H⋯O interactions.
Related literature
For the preparation, see: Bose et al. (2005 ▶). For a similar structure, see: Hou et al. (2010 ▶).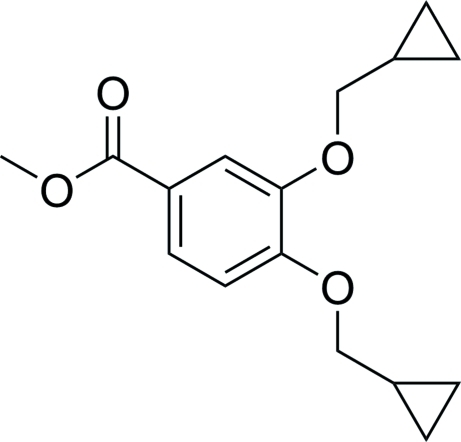
Experimental
Crystal data
C16H20O4
M r = 276.33
Orthorhombic,

a = 4.9018 (8) Å
b = 15.543 (2) Å
c = 18.846 (2) Å
V = 1435.9 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 113 K
0.22 × 0.20 × 0.18 mm
Data collection
Rigaku Saturn724 CCD diffractometer
Absorption correction: multi-scan (REQAB; Jacobson, 1998 ▶) T min = 0.891, T max = 0.984
20068 measured reflections
3852 independent reflections
3300 reflections with F 2 > 2.0σ(F 2)
R int = 0.034
Refinement
R[F 2 > 2σ(F 2)] = 0.041
wR(F 2) = 0.114
S = 1.06
3852 reflections
182 parameters
H-atom parameters constrained
Δρmax = 0.57 e Å−3
Δρmin = −0.19 e Å−3
Data collection: CrystalClear-SM Expert (Rigaku, 2009 ▶); cell refinement: CrystalClear-SM Expert; data reduction: CrystalClear-SM Expert; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: CrystalStructure (Rigaku, 2009 ▶); software used to prepare material for publication: CrystalStructure.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811013158/jh2279sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811013158/jh2279Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C12—H12B⋯O3i | 0.99 | 2.55 | 3.4073 (18) | 145 |
Symmetry code: (i) 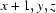 .
.
Acknowledgments
This research was supported by the Tianjin Medical University Science Foundation (2009ky16) and a China Postdoctoral Science Foundation funded project (20100480655).
supplementary crystallographic information
Comment
Roflumilast is an effective phosphodiesterase-4 inhibitor (PDE4 inhibitor), which can be used in the treatment of asthma, inflammation, bronchitis, allergy and other disorders related to immune system, heart and kidney. During the development of our own PDE4 inhibitors, roflumilast was synthesized as the positive control in the bioactivity screening, and the title compound,methyl 3,4-bis(cyclopropylmethoxy)benzoate, was a byproduct during preparation of the intermediate methyl 3-(cyclopropylmethoxy)-4-hydroxybenzoate.The crystallographic analysis of the title compound is done to confirm the chemical structure of the title compound. In the title compound, all bond lengths and angles are normal and in a good agreement with those reported previously (Hou, et al., 2010). In the crystal structure, the hydroxy groups are involved in the formation of intermolecular C—H···O hydrogen bonds(Tab 1), which link the molecules related by translation along axis b into one-dimensional chains.
Experimental
A mixture of 3,4-dihydroxy methyl benzoate (1.68 g, 10 mmol) and potassium carbonate (2.76 g, 20 mmol) in acetone (50 ml) was added with a solution of cyclopropyl methyl bromide (1.35 g, 10 mmol) in acetone (50 ml). The reaction mixture was stirred at 40 °C for 18 h, and then was filtered. The filtrate was evaporated on a rotary evaporator to get the dried solid, which was then purified by flash column chromatography to obtain methyl 3-(cyclopropylmethoxy)-4-hydroxybenzoate, methyl 4-(cyclopropylmethoxy)-3-hydroxybenzoate, and the title compound methyl 3,4-bis(cyclopropylmethoxy)benzoate(Bose,et al.,2005).
Crystals suitable for X-ray diffraction were obtained through slow evaporation of a solution of the pure title compound in ethyl acetate/n-hexane (1/10 by volume).
Refinement
H atoms were positioned geometrically (C—H = 0.95–1.00 Å) and refined as riding, with Uiso(H) = 1.2 Ueq of the parent atom.
Figures
Fig. 1.
The molecular structure of the title compound, with atom labels and 30% probability displacement ellipsoids, and H atoms are shown as small spheres of arbitrary radius.
Fig. 2.
The packing of the title compound, showing the one-dimensional structure,with intermolecular hydrogen bonds (dashed lines); for clarity H atoms have been omitted.
Crystal data
| C16H20O4 | F(000) = 592.00 |
| Mr = 276.33 | Dx = 1.278 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71075 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 6064 reflections |
| a = 4.9018 (8) Å | θ = 1.3–31.4° |
| b = 15.543 (2) Å | µ = 0.09 mm−1 |
| c = 18.846 (2) Å | T = 113 K |
| V = 1435.9 (3) Å3 | Prism, colorless |
| Z = 4 | 0.22 × 0.20 × 0.18 mm |
Data collection
| Rigaku Saturn724 CCD diffractometer | 3300 reflections with F2 > 2.0σ(F2) |
| Detector resolution: 14.222 pixels mm-1 | Rint = 0.034 |
| ω scans | θmax = 29.1° |
| Absorption correction: multi-scan (REQAB; Jacobson, 1998) | h = −6→6 |
| Tmin = 0.891, Tmax = 0.984 | k = −21→21 |
| 20068 measured reflections | l = −25→25 |
| 3852 independent reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.041 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.114 | H-atom parameters constrained |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0778P)2] where P = (Fo2 + 2Fc2)/3 |
| 3852 reflections | (Δ/σ)max = 0.001 |
| 182 parameters | Δρmax = 0.57 e Å−3 |
| 0 restraints | Δρmin = −0.19 e Å−3 |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. ENTER SPECIAL DETAILS OF THE MOLECULAR GEOMETRY |
| Refinement. Refinement was performed using all reflections. The weighted R-factor (wR) and goodness of fit (S) are based on F2. R-factor (gt) are based on F. The threshold expression of F2 > 2.0 σ(F2) is used only for calculating R-factor (gt). |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O(1) | 0.3872 (2) | 0.69719 (7) | 0.66936 (5) | 0.0321 | |
| O(2) | 0.6968 (2) | 0.59026 (7) | 0.66908 (5) | 0.0306 | |
| O(3) | 0.7825 (2) | 0.48335 (5) | 0.42141 (5) | 0.0209 | |
| O(4) | 0.4320 (2) | 0.56811 (6) | 0.34625 (4) | 0.0250 | |
| C(1) | 0.4862 (3) | 0.62225 (8) | 0.56094 (7) | 0.0192 | |
| C(2) | 0.6558 (3) | 0.56094 (8) | 0.52884 (7) | 0.0184 | |
| C(3) | 0.6319 (3) | 0.54351 (8) | 0.45719 (6) | 0.0174 | |
| C(4) | 0.4378 (3) | 0.58918 (8) | 0.41612 (7) | 0.0193 | |
| C(5) | 0.2695 (3) | 0.64967 (8) | 0.44863 (7) | 0.0213 | |
| C(6) | 0.2930 (3) | 0.66574 (8) | 0.52102 (7) | 0.0211 | |
| C(7) | 0.5139 (3) | 0.64194 (8) | 0.63788 (7) | 0.0216 | |
| C(8) | 0.7487 (4) | 0.60502 (11) | 0.74361 (7) | 0.0406 | |
| C(9) | 0.9395 (3) | 0.42507 (8) | 0.46452 (6) | 0.0199 | |
| C(10) | 1.0118 (3) | 0.34763 (8) | 0.42075 (7) | 0.0205 | |
| C(11) | 1.2662 (3) | 0.29938 (9) | 0.44212 (8) | 0.0262 | |
| C(12) | 1.2614 (3) | 0.35005 (9) | 0.37423 (7) | 0.0246 | |
| C(13) | 0.2283 (3) | 0.61039 (9) | 0.30217 (7) | 0.0302 | |
| C(14) | 0.2718 (4) | 0.57919 (10) | 0.22787 (7) | 0.0330 | |
| C(15) | 0.5052 (4) | 0.61632 (12) | 0.18752 (10) | 0.0442 | |
| C(16) | 0.2225 (4) | 0.64137 (13) | 0.16830 (8) | 0.0428 | |
| H(2) | 0.7878 | 0.5312 | 0.5565 | 0.022* | |
| H(5) | 0.1381 | 0.6800 | 0.4213 | 0.026* | |
| H(6) | 0.1765 | 0.7066 | 0.5431 | 0.025* | |
| H(8A) | 0.5750 | 0.6078 | 0.7693 | 0.049* | |
| H(8B) | 0.8469 | 0.6595 | 0.7495 | 0.049* | |
| H(8C) | 0.8593 | 0.5578 | 0.7626 | 0.049* | |
| H(9A) | 0.8319 | 0.4073 | 0.5065 | 0.024* | |
| H(9B) | 1.1078 | 0.4538 | 0.4813 | 0.024* | |
| H(10) | 0.8558 | 0.3120 | 0.4033 | 0.025* | |
| H(11A) | 1.3747 | 0.3225 | 0.4821 | 0.031* | |
| H(11B) | 1.2633 | 0.2358 | 0.4386 | 0.031* | |
| H(12A) | 1.2558 | 0.3176 | 0.3291 | 0.030* | |
| H(12B) | 1.3671 | 0.4043 | 0.3725 | 0.030* | |
| H(13A) | 0.2504 | 0.6736 | 0.3045 | 0.036* | |
| H(13B) | 0.0423 | 0.5955 | 0.3186 | 0.036* | |
| H(14) | 0.2271 | 0.5175 | 0.2183 | 0.040* | |
| H(15A) | 0.6201 | 0.6597 | 0.2118 | 0.053* | |
| H(15B) | 0.6047 | 0.5779 | 0.1546 | 0.053* | |
| H(16A) | 0.1462 | 0.6184 | 0.1235 | 0.051* | |
| H(16B) | 0.1615 | 0.7002 | 0.1807 | 0.051* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O(1) | 0.0398 | 0.0311 | 0.0253 | 0.0080 | 0.0001 | −0.0084 |
| O(2) | 0.0391 | 0.0348 | 0.0179 | 0.0103 | −0.0046 | −0.0070 |
| O(3) | 0.0251 | 0.0207 | 0.0170 | 0.0073 | −0.0011 | 0.0001 |
| O(4) | 0.0346 | 0.0232 | 0.0170 | 0.0084 | −0.0041 | 0.0008 |
| C(1) | 0.0205 | 0.0176 | 0.0194 | −0.0020 | 0.0025 | −0.0008 |
| C(2) | 0.0171 | 0.0187 | 0.0194 | −0.0004 | −0.0002 | −0.0003 |
| C(3) | 0.0192 | 0.0152 | 0.0178 | 0.0006 | 0.0017 | −0.0000 |
| C(4) | 0.0228 | 0.0182 | 0.0170 | −0.0013 | −0.0017 | 0.0020 |
| C(5) | 0.0222 | 0.0188 | 0.0229 | 0.0010 | −0.0014 | 0.0024 |
| C(6) | 0.0213 | 0.0185 | 0.0235 | 0.0012 | 0.0042 | −0.0012 |
| C(7) | 0.0243 | 0.0197 | 0.0208 | −0.0031 | 0.0009 | −0.0025 |
| C(8) | 0.0575 | 0.0441 | 0.0202 | 0.0155 | −0.0122 | −0.0113 |
| C(9) | 0.0213 | 0.0205 | 0.0179 | 0.0038 | −0.0004 | 0.0030 |
| C(10) | 0.0189 | 0.0203 | 0.0222 | 0.0011 | 0.0009 | 0.0013 |
| C(11) | 0.0251 | 0.0244 | 0.0291 | 0.0071 | 0.0026 | 0.0046 |
| C(12) | 0.0247 | 0.0255 | 0.0236 | 0.0017 | 0.0060 | 0.0002 |
| C(13) | 0.0337 | 0.0335 | 0.0235 | 0.0069 | −0.0052 | 0.0038 |
| C(14) | 0.0389 | 0.0350 | 0.0251 | 0.0013 | −0.0049 | 0.0053 |
| C(15) | 0.0457 | 0.0498 | 0.0371 | 0.0028 | 0.0038 | 0.0098 |
| C(16) | 0.0510 | 0.0542 | 0.0230 | 0.0148 | −0.0043 | 0.0114 |
Geometric parameters (Å, °)
| O(1)—C(7) | 1.2143 (17) | C(15)—C(16) | 1.484 (3) |
| O(2)—C(7) | 1.3397 (11) | C(2)—H(2) | 0.950 |
| O(2)—C(8) | 1.4458 (19) | C(5)—H(5) | 0.950 |
| O(3)—C(3) | 1.3692 (14) | C(6)—H(6) | 0.950 |
| O(3)—C(9) | 1.4397 (10) | C(8)—H(8A) | 0.980 |
| O(4)—C(4) | 1.3572 (17) | C(8)—H(8B) | 0.980 |
| O(4)—C(13) | 1.4558 (13) | C(8)—H(8C) | 0.980 |
| C(1)—C(2) | 1.4017 (18) | C(9)—H(9A) | 0.990 |
| C(1)—C(6) | 1.3856 (15) | C(9)—H(9B) | 0.990 |
| C(1)—C(7) | 1.488 (3) | C(10)—H(10) | 1.000 |
| C(2)—C(3) | 1.382 (3) | C(11)—H(11A) | 0.990 |
| C(3)—C(4) | 1.4169 (14) | C(11)—H(11B) | 0.990 |
| C(4)—C(5) | 1.3930 (18) | C(12)—H(12A) | 0.990 |
| C(5)—C(6) | 1.392 (3) | C(12)—H(12B) | 0.990 |
| C(9)—C(10) | 1.502 (2) | C(13)—H(13A) | 0.990 |
| C(10)—C(11) | 1.5098 (14) | C(13)—H(13B) | 0.990 |
| C(10)—C(12) | 1.506 (2) | C(14)—H(14) | 1.000 |
| C(11)—C(12) | 1.5026 (17) | C(15)—H(15A) | 0.990 |
| C(13)—C(14) | 1.497 (3) | C(15)—H(15B) | 0.990 |
| C(14)—C(15) | 1.490 (3) | C(16)—H(16A) | 0.990 |
| C(14)—C(16) | 1.5009 (15) | C(16)—H(16B) | 0.990 |
| O(1)···C(6) | 2.875 (2) | H(6)···C(1)viii | 3.4340 |
| O(1)···C(8) | 2.6738 (14) | H(6)···C(2)iv | 3.4221 |
| O(2)···C(2) | 2.6895 (19) | H(6)···C(4)viii | 3.4694 |
| O(3)···O(4) | 2.5873 (12) | H(6)···C(5)viii | 2.9995 |
| O(3)···C(12) | 3.2546 (14) | H(6)···C(6)viii | 2.9886 |
| O(4)···C(15) | 3.1046 (16) | H(6)···H(2)iv | 3.3350 |
| C(1)···C(4) | 2.787 (3) | H(6)···H(5)viii | 3.2433 |
| C(2)···C(5) | 2.7880 (15) | H(6)···H(5)i | 2.9456 |
| C(2)···C(9) | 2.804 (2) | H(6)···H(6)viii | 3.2354 |
| C(3)···C(6) | 2.7955 (18) | H(6)···H(6)i | 3.2354 |
| C(5)···C(13) | 2.834 (3) | H(8A)···O(3)ii | 3.2735 |
| O(1)···C(13)i | 3.468 (4) | H(8A)···C(10)ii | 2.9685 |
| O(2)···C(15)ii | 3.545 (2) | H(8A)···C(12)ii | 2.6574 |
| O(3)···C(5)iii | 3.556 (2) | H(8A)···C(14)ix | 3.4558 |
| O(3)···C(12)iv | 3.407 (2) | H(8A)···H(8C)iv | 3.5954 |
| O(4)···C(12)iv | 3.5306 (19) | H(8A)···H(10)ii | 2.8381 |
| C(2)···C(6)iii | 3.526 (3) | H(8A)···H(12A)ii | 2.2901 |
| C(3)···C(5)iii | 3.538 (3) | H(8A)···H(12B)ii | 2.9188 |
| C(5)···O(3)iv | 3.556 (3) | H(8A)···H(12B)v | 3.3617 |
| C(5)···C(3)iv | 3.538 (3) | H(8A)···H(13B)ix | 3.3436 |
| C(6)···C(2)iv | 3.526 (3) | H(8A)···H(14)ix | 2.6278 |
| C(8)···C(12)ii | 3.578 (3) | H(8A)···H(16B)i | 3.1585 |
| C(8)···C(12)v | 3.509 (7) | H(8B)···O(1)iii | 3.1047 |
| C(12)···O(3)iii | 3.407 (4) | H(8B)···C(12)v | 3.0389 |
| C(12)···O(4)iii | 3.531 (3) | H(8B)···C(16)i | 3.5151 |
| C(12)···C(8)vi | 3.578 (5) | H(8B)···H(10)ii | 3.0967 |
| C(12)···C(8)vii | 3.509 (5) | H(8B)···H(12A)ii | 3.3323 |
| C(13)···O(1)viii | 3.468 (3) | H(8B)···H(12A)v | 2.4836 |
| C(15)···O(2)vi | 3.545 (3) | H(8B)···H(12B)v | 2.8849 |
| C(15)···C(16)iii | 3.556 (4) | H(8B)···H(13A)i | 2.8266 |
| C(16)···C(15)iv | 3.556 (3) | H(8B)···H(14)ii | 3.5027 |
| O(1)···H(6) | 2.5982 | H(8B)···H(15A)i | 3.1979 |
| O(1)···H(8A) | 2.5145 | H(8B)···H(16B)i | 2.7043 |
| O(1)···H(8B) | 2.7752 | H(8C)···O(3)ii | 3.1385 |
| O(2)···H(2) | 2.3550 | H(8C)···O(4)ii | 2.7133 |
| O(3)···H(2) | 2.6517 | H(8C)···C(12)v | 3.1519 |
| O(3)···H(10) | 2.7092 | H(8C)···C(13)ii | 3.3878 |
| O(3)···H(12B) | 3.2512 | H(8C)···C(14)ii | 2.8694 |
| O(4)···H(5) | 2.6652 | H(8C)···C(15)ii | 3.1254 |
| O(4)···H(14) | 2.7276 | H(8C)···H(8A)iii | 3.5954 |
| O(4)···H(15A) | 3.0496 | H(8C)···H(10)ii | 3.4987 |
| C(1)···H(5) | 3.2624 | H(8C)···H(12A)v | 2.9798 |
| C(2)···H(6) | 3.2736 | H(8C)···H(12B)v | 2.5373 |
| C(2)···H(9A) | 2.5735 | H(8C)···H(13B)ix | 3.2663 |
| C(2)···H(9B) | 2.9122 | H(8C)···H(14)ix | 3.2138 |
| C(3)···H(5) | 3.2890 | H(8C)···H(14)ii | 2.4852 |
| C(3)···H(9A) | 2.5109 | H(8C)···H(15A)ii | 3.5154 |
| C(3)···H(9B) | 2.7551 | H(8C)···H(15B)ii | 2.9371 |
| C(4)···H(2) | 3.2784 | H(9A)···C(11)iv | 3.4606 |
| C(4)···H(6) | 3.2707 | H(9A)···C(11)x | 3.3711 |
| C(4)···H(13A) | 2.6440 | H(9A)···C(15)ii | 3.5231 |
| C(4)···H(13B) | 2.6731 | H(9A)···H(11A)iv | 2.6402 |
| C(5)···H(13A) | 2.7429 | H(9A)···H(11A)x | 3.5855 |
| C(5)···H(13B) | 2.8201 | H(9A)···H(11B)x | 2.4769 |
| C(6)···H(2) | 3.2707 | H(9A)···H(12B)iv | 3.4008 |
| C(7)···H(2) | 2.6681 | H(9A)···H(15B)ii | 2.8173 |
| C(7)···H(6) | 2.6334 | H(9A)···H(16A)ix | 3.2426 |
| C(7)···H(8A) | 2.5499 | H(9A)···H(16A)ii | 3.4014 |
| C(7)···H(8B) | 2.6766 | H(9B)···O(3)iii | 3.5250 |
| C(7)···H(8C) | 3.1783 | H(9B)···O(4)iii | 3.4870 |
| C(9)···H(2) | 2.5055 | H(9B)···C(1)iii | 3.5419 |
| C(9)···H(11A) | 2.6830 | H(9B)···C(2)iii | 3.2847 |
| C(9)···H(11B) | 3.3775 | H(9B)···C(3)iii | 2.9578 |
| C(9)···H(12A) | 3.4221 | H(9B)···C(4)iii | 2.9243 |
| C(9)···H(12B) | 2.7392 | H(9B)···C(5)iii | 3.2049 |
| C(11)···H(9A) | 2.9697 | H(9B)···C(6)iii | 3.4973 |
| C(11)···H(9B) | 2.6293 | H(9B)···H(15B)ii | 3.4627 |
| C(12)···H(9A) | 3.3820 | H(9B)···H(16A)ii | 3.1460 |
| C(12)···H(9B) | 2.6912 | H(10)···C(8)vi | 3.3147 |
| C(13)···H(5) | 2.5315 | H(10)···C(11)iv | 2.9874 |
| C(13)···H(15A) | 2.6793 | H(10)···C(11)x | 3.4165 |
| C(13)···H(15B) | 3.3759 | H(10)···C(12)iv | 3.0231 |
| C(13)···H(16A) | 3.3933 | H(10)···C(16)xi | 3.0002 |
| C(13)···H(16B) | 2.7007 | H(10)···H(8A)vi | 2.8381 |
| C(15)···H(13A) | 2.6862 | H(10)···H(8B)vi | 3.0967 |
| C(15)···H(13B) | 3.3697 | H(10)···H(8C)vi | 3.4987 |
| C(16)···H(13A) | 2.6193 | H(10)···H(11A)iv | 2.7913 |
| C(16)···H(13B) | 3.0514 | H(10)···H(11A)x | 3.0070 |
| H(2)···H(9A) | 2.1545 | H(10)···H(11B)iv | 3.2057 |
| H(2)···H(9B) | 2.4315 | H(10)···H(11B)x | 3.1032 |
| H(5)···H(6) | 2.3396 | H(10)···H(12A)iv | 3.2582 |
| H(5)···H(13A) | 2.2709 | H(10)···H(12B)iv | 2.8516 |
| H(5)···H(13B) | 2.3861 | H(10)···H(16A)xi | 3.0514 |
| H(9A)···H(10) | 2.4474 | H(10)···H(16B)xi | 2.3527 |
| H(9A)···H(11A) | 3.0045 | H(11A)···O(3)iii | 3.3990 |
| H(9B)···H(10) | 2.9237 | H(11A)···C(9)iii | 3.2119 |
| H(9B)···H(11A) | 2.4243 | H(11A)···C(10)iii | 3.3530 |
| H(9B)···H(11B) | 3.5652 | H(11A)···C(10)xii | 3.2863 |
| H(9B)···H(12B) | 2.5324 | H(11A)···C(11)xii | 3.0519 |
| H(10)···H(11A) | 2.9497 | H(11A)···C(16)ii | 3.5854 |
| H(10)···H(11B) | 2.4153 | H(11A)···H(9A)iii | 2.6402 |
| H(10)···H(12A) | 2.4108 | H(11A)···H(9A)xii | 3.5855 |
| H(10)···H(12B) | 2.9459 | H(11A)···H(10)iii | 2.7913 |
| H(11A)···H(12A) | 2.9435 | H(11A)···H(10)xii | 3.0070 |
| H(11A)···H(12B) | 2.4248 | H(11A)···H(11A)x | 3.3981 |
| H(11B)···H(12A) | 2.4247 | H(11A)···H(11A)xii | 3.3981 |
| H(11B)···H(12B) | 2.9436 | H(11A)···H(11B)x | 3.4694 |
| H(13A)···H(14) | 2.9227 | H(11A)···H(11B)xii | 2.5855 |
| H(13A)···H(15A) | 2.5272 | H(11A)···H(16A)ii | 2.8206 |
| H(13A)···H(16A) | 3.5547 | H(11B)···C(9)xii | 3.2146 |
| H(13A)···H(16B) | 2.4089 | H(11B)···C(10)xii | 3.1923 |
| H(13B)···H(14) | 2.4211 | H(11B)···C(11)x | 3.3599 |
| H(13B)···H(16B) | 3.1209 | H(11B)···C(11)xii | 3.3805 |
| H(14)···H(15A) | 2.9345 | H(11B)···C(15)xiii | 3.2233 |
| H(14)···H(15B) | 2.3983 | H(11B)···C(16)xi | 3.4476 |
| H(14)···H(16A) | 2.4103 | H(11B)···C(16)xiii | 3.5454 |
| H(14)···H(16B) | 2.9445 | H(11B)···H(9A)xii | 2.4769 |
| H(15A)···H(16A) | 2.9283 | H(11B)···H(10)iii | 3.2057 |
| H(15A)···H(16B) | 2.4063 | H(11B)···H(10)xii | 3.1032 |
| H(15B)···H(16A) | 2.4063 | H(11B)···H(11A)x | 2.5855 |
| H(15B)···H(16B) | 2.9283 | H(11B)···H(11A)xii | 3.4694 |
| O(1)···H(5)i | 2.8414 | H(11B)···H(11B)x | 3.3990 |
| O(1)···H(8B)iv | 3.1047 | H(11B)···H(11B)xii | 3.3990 |
| O(1)···H(12A)ii | 3.0988 | H(11B)···H(15A)xiii | 3.1240 |
| O(1)···H(13A)i | 2.7280 | H(11B)···H(15B)xiii | 3.0867 |
| O(1)···H(13B)i | 3.3183 | H(11B)···H(16A)xi | 2.9551 |
| O(1)···H(14)ix | 3.5071 | H(11B)···H(16B)xi | 3.1149 |
| O(1)···H(15A)viii | 3.4180 | H(12A)···O(1)vi | 3.0988 |
| O(1)···H(16B)i | 3.5118 | H(12A)···C(8)vi | 3.1867 |
| O(2)···H(14)ix | 2.8256 | H(12A)···C(8)vii | 3.1528 |
| O(2)···H(14)ii | 3.4119 | H(12A)···C(15)xiii | 3.3551 |
| O(2)···H(15B)ii | 2.8026 | H(12A)···H(8A)vi | 2.2901 |
| O(3)···H(5)iii | 3.5191 | H(12A)···H(8B)vi | 3.3323 |
| O(3)···H(8A)vi | 3.2735 | H(12A)···H(8B)vii | 2.4836 |
| O(3)···H(8C)vi | 3.1385 | H(12A)···H(8C)vii | 2.9798 |
| O(3)···H(9B)iv | 3.5250 | H(12A)···H(10)iii | 3.2582 |
| O(3)···H(11A)iv | 3.3990 | H(12A)···H(15A)xiii | 2.6428 |
| O(3)···H(12B)iv | 2.5506 | H(12A)···H(16B)xi | 2.7473 |
| O(3)···H(13B)iii | 2.9009 | H(12A)···H(16B)xiii | 3.3943 |
| O(4)···H(8C)vi | 2.7133 | H(12B)···O(3)iii | 2.5506 |
| O(4)···H(9B)iv | 3.4870 | H(12B)···O(4)iii | 2.6136 |
| O(4)···H(12B)iv | 2.6136 | H(12B)···C(3)iii | 2.9854 |
| O(4)···H(13B)iii | 3.0664 | H(12B)···C(4)iii | 3.0090 |
| C(1)···H(5)i | 3.1796 | H(12B)···C(8)vii | 3.0774 |
| C(1)···H(6)i | 3.4340 | H(12B)···C(9)iii | 3.3140 |
| C(1)···H(9B)iv | 3.5419 | H(12B)···C(10)iii | 3.4040 |
| C(2)···H(6)iii | 3.4221 | H(12B)···C(13)iii | 3.5333 |
| C(2)···H(9B)iv | 3.2847 | H(12B)···H(8A)vi | 2.9188 |
| C(2)···H(15B)ii | 3.4133 | H(12B)···H(8A)vii | 3.3617 |
| C(3)···H(5)iii | 3.3346 | H(12B)···H(8B)vii | 2.8849 |
| C(3)···H(9B)iv | 2.9578 | H(12B)···H(8C)vii | 2.5373 |
| C(3)···H(12B)iv | 2.9854 | H(12B)···H(9A)iii | 3.4008 |
| C(3)···H(13B)iii | 3.3947 | H(12B)···H(10)iii | 2.8516 |
| C(4)···H(6)i | 3.4694 | H(12B)···H(13B)iii | 3.5216 |
| C(4)···H(9B)iv | 2.9243 | H(12B)···H(14)iii | 3.4659 |
| C(4)···H(12B)iv | 3.0090 | H(13A)···O(1)viii | 2.7280 |
| C(4)···H(13B)iii | 3.4883 | H(13A)···C(7)viii | 3.2771 |
| C(5)···H(6)i | 2.9995 | H(13A)···C(8)viii | 3.5579 |
| C(5)···H(9B)iv | 3.2049 | H(13A)···H(8B)viii | 2.8266 |
| C(6)···H(2)iv | 3.3092 | H(13A)···H(15A)iv | 3.5566 |
| C(6)···H(5)i | 3.1287 | H(13B)···O(1)viii | 3.3183 |
| C(6)···H(6)i | 2.9886 | H(13B)···O(3)iv | 2.9009 |
| C(6)···H(9B)iv | 3.4973 | H(13B)···O(4)iv | 3.0664 |
| C(7)···H(5)i | 3.0449 | H(13B)···C(3)iv | 3.3947 |
| C(7)···H(13A)i | 3.2771 | H(13B)···C(4)iv | 3.4883 |
| C(7)···H(14)ix | 3.1360 | H(13B)···H(8A)xiv | 3.3436 |
| C(8)···H(10)ii | 3.3147 | H(13B)···H(8C)xiv | 3.2663 |
| C(8)···H(12A)ii | 3.1867 | H(13B)···H(12B)iv | 3.5216 |
| C(8)···H(12A)v | 3.1528 | H(13B)···H(15A)iv | 3.0552 |
| C(8)···H(12B)v | 3.0774 | H(14)···O(1)xiv | 3.5071 |
| C(8)···H(13A)i | 3.5579 | H(14)···O(2)xiv | 2.8256 |
| C(8)···H(14)ix | 3.0483 | H(14)···O(2)vi | 3.4119 |
| C(8)···H(14)ii | 3.2335 | H(14)···C(7)xiv | 3.1360 |
| C(8)···H(15B)ii | 3.3790 | H(14)···C(8)xiv | 3.0483 |
| C(8)···H(16B)i | 3.3736 | H(14)···C(8)vi | 3.2335 |
| C(9)···H(11A)iv | 3.2119 | H(14)···H(8A)xiv | 2.6278 |
| C(9)···H(11B)x | 3.2146 | H(14)···H(8B)vi | 3.5027 |
| C(9)···H(12B)iv | 3.3140 | H(14)···H(8C)xiv | 3.2138 |
| C(9)···H(15B)ii | 3.5885 | H(14)···H(8C)vi | 2.4852 |
| C(10)···H(8A)vi | 2.9685 | H(14)···H(12B)iv | 3.4659 |
| C(10)···H(11A)iv | 3.3530 | H(14)···H(15B)iv | 3.4109 |
| C(10)···H(11A)x | 3.2863 | H(15A)···O(1)i | 3.4180 |
| C(10)···H(11B)x | 3.1923 | H(15A)···C(12)xv | 3.4231 |
| C(10)···H(12B)iv | 3.4040 | H(15A)···C(13)iii | 3.5184 |
| C(10)···H(16B)xi | 3.1033 | H(15A)···C(14)iii | 3.4443 |
| C(11)···H(9A)iii | 3.4606 | H(15A)···C(16)iii | 3.0775 |
| C(11)···H(9A)xii | 3.3711 | H(15A)···H(8B)viii | 3.1979 |
| C(11)···H(10)iii | 2.9874 | H(15A)···H(8C)vi | 3.5154 |
| C(11)···H(10)xii | 3.4165 | H(15A)···H(11B)xv | 3.1240 |
| C(11)···H(11A)x | 3.0519 | H(15A)···H(12A)xv | 2.6428 |
| C(11)···H(11B)x | 3.3805 | H(15A)···H(13A)iii | 3.5566 |
| C(11)···H(11B)xii | 3.3599 | H(15A)···H(13B)iii | 3.0552 |
| C(11)···H(16B)xi | 3.4832 | H(15A)···H(16A)iii | 3.1351 |
| C(12)···H(8A)vi | 2.6574 | H(15A)···H(16B)iii | 2.7897 |
| C(12)···H(8B)vii | 3.0389 | H(15B)···O(2)vi | 2.8026 |
| C(12)···H(8C)vii | 3.1519 | H(15B)···C(2)vi | 3.4133 |
| C(12)···H(10)iii | 3.0231 | H(15B)···C(8)vi | 3.3790 |
| C(12)···H(15A)xiii | 3.4231 | H(15B)···C(9)vi | 3.5885 |
| C(12)···H(16B)xi | 3.2856 | H(15B)···C(14)iii | 3.5496 |
| C(13)···H(8C)vi | 3.3878 | H(15B)···C(16)iii | 3.1951 |
| C(13)···H(12B)iv | 3.5333 | H(15B)···H(2)vi | 2.5642 |
| C(13)···H(15A)iv | 3.5184 | H(15B)···H(8C)vi | 2.9371 |
| C(14)···H(8A)xiv | 3.4558 | H(15B)···H(9A)vi | 2.8173 |
| C(14)···H(8C)vi | 2.8694 | H(15B)···H(9B)vi | 3.4627 |
| C(14)···H(15A)iv | 3.4443 | H(15B)···H(11B)xv | 3.0867 |
| C(14)···H(15B)iv | 3.5496 | H(15B)···H(14)iii | 3.4109 |
| C(15)···H(2)vi | 3.5201 | H(15B)···H(16A)iii | 2.7897 |
| C(15)···H(8C)vi | 3.1254 | H(15B)···H(16B)iii | 3.3623 |
| C(15)···H(9A)vi | 3.5231 | H(16A)···C(15)iv | 3.3656 |
| C(15)···H(11B)xv | 3.2233 | H(16A)···H(2)xiv | 3.3954 |
| C(15)···H(12A)xv | 3.3551 | H(16A)···H(9A)xiv | 3.2426 |
| C(15)···H(16A)iii | 3.3656 | H(16A)···H(9A)vi | 3.4014 |
| C(15)···H(16B)iii | 3.4736 | H(16A)···H(9B)vi | 3.1460 |
| C(16)···H(8B)viii | 3.5151 | H(16A)···H(10)xvi | 3.0514 |
| C(16)···H(10)xvi | 3.0002 | H(16A)···H(11A)vi | 2.8206 |
| C(16)···H(11A)vi | 3.5854 | H(16A)···H(11B)xvi | 2.9551 |
| C(16)···H(11B)xvi | 3.4476 | H(16A)···H(15A)iv | 3.1351 |
| C(16)···H(11B)xv | 3.5454 | H(16A)···H(15B)iv | 2.7897 |
| C(16)···H(15A)iv | 3.0775 | H(16B)···O(1)viii | 3.5118 |
| C(16)···H(15B)iv | 3.1951 | H(16B)···C(8)viii | 3.3736 |
| H(2)···C(6)iii | 3.3092 | H(16B)···C(10)xvi | 3.1033 |
| H(2)···C(15)ii | 3.5201 | H(16B)···C(11)xvi | 3.4832 |
| H(2)···H(6)iii | 3.3350 | H(16B)···C(12)xvi | 3.2856 |
| H(2)···H(15B)ii | 2.5642 | H(16B)···C(15)iv | 3.4736 |
| H(2)···H(16A)ix | 3.3954 | H(16B)···H(8A)viii | 3.1585 |
| H(5)···O(1)viii | 2.8414 | H(16B)···H(8B)viii | 2.7043 |
| H(5)···O(3)iv | 3.5191 | H(16B)···H(10)xvi | 2.3527 |
| H(5)···C(1)viii | 3.1796 | H(16B)···H(11B)xvi | 3.1149 |
| H(5)···C(3)iv | 3.3346 | H(16B)···H(12A)xvi | 2.7473 |
| H(5)···C(6)viii | 3.1287 | H(16B)···H(12A)xv | 3.3943 |
| H(5)···C(7)viii | 3.0449 | H(16B)···H(15A)iv | 2.7897 |
| H(5)···H(6)viii | 2.9456 | H(16B)···H(15B)iv | 3.3623 |
| H(5)···H(6)i | 3.2433 | ||
| C(7)—O(2)—C(8) | 116.68 (12) | H(8A)—C(8)—H(8B) | 109.475 |
| C(3)—O(3)—C(9) | 116.11 (9) | H(8A)—C(8)—H(8C) | 109.468 |
| C(4)—O(4)—C(13) | 117.33 (10) | H(8B)—C(8)—H(8C) | 109.472 |
| C(2)—C(1)—C(6) | 120.17 (12) | O(3)—C(9)—H(9A) | 109.948 |
| C(2)—C(1)—C(7) | 120.42 (11) | O(3)—C(9)—H(9B) | 109.953 |
| C(6)—C(1)—C(7) | 119.40 (11) | C(10)—C(9)—H(9A) | 109.961 |
| C(1)—C(2)—C(3) | 120.30 (12) | C(10)—C(9)—H(9B) | 109.956 |
| O(3)—C(3)—C(2) | 124.69 (11) | H(9A)—C(9)—H(9B) | 108.328 |
| O(3)—C(3)—C(4) | 115.80 (10) | C(9)—C(10)—H(10) | 116.366 |
| C(2)—C(3)—C(4) | 119.50 (11) | C(11)—C(10)—H(10) | 116.367 |
| O(4)—C(4)—C(3) | 115.03 (11) | C(12)—C(10)—H(10) | 116.375 |
| O(4)—C(4)—C(5) | 125.26 (12) | C(10)—C(11)—H(11A) | 117.777 |
| C(3)—C(4)—C(5) | 119.71 (12) | C(10)—C(11)—H(11B) | 117.773 |
| C(4)—C(5)—C(6) | 120.21 (12) | C(12)—C(11)—H(11A) | 117.769 |
| C(1)—C(6)—C(5) | 120.09 (12) | C(12)—C(11)—H(11B) | 117.772 |
| O(1)—C(7)—O(2) | 123.48 (12) | H(11A)—C(11)—H(11B) | 114.909 |
| O(1)—C(7)—C(1) | 125.10 (12) | C(10)—C(12)—H(12A) | 117.744 |
| O(2)—C(7)—C(1) | 111.42 (11) | C(10)—C(12)—H(12B) | 117.746 |
| O(3)—C(9)—C(10) | 108.69 (9) | C(11)—C(12)—H(12A) | 117.745 |
| C(9)—C(10)—C(11) | 116.52 (11) | C(11)—C(12)—H(12B) | 117.747 |
| C(9)—C(10)—C(12) | 119.44 (11) | H(12A)—C(12)—H(12B) | 114.871 |
| C(11)—C(10)—C(12) | 59.78 (9) | O(4)—C(13)—H(13A) | 110.344 |
| C(10)—C(11)—C(12) | 59.97 (9) | O(4)—C(13)—H(13B) | 110.344 |
| C(10)—C(12)—C(11) | 60.25 (9) | C(14)—C(13)—H(13A) | 110.360 |
| O(4)—C(13)—C(14) | 106.85 (12) | C(14)—C(13)—H(13B) | 110.358 |
| C(13)—C(14)—C(15) | 117.46 (14) | H(13A)—C(13)—H(13B) | 108.585 |
| C(13)—C(14)—C(16) | 117.91 (14) | C(13)—C(14)—H(14) | 116.594 |
| C(15)—C(14)—C(16) | 59.51 (12) | C(15)—C(14)—H(14) | 116.598 |
| C(14)—C(15)—C(16) | 60.61 (12) | C(16)—C(14)—H(14) | 116.603 |
| C(14)—C(16)—C(15) | 59.89 (12) | C(14)—C(15)—H(15A) | 117.706 |
| C(1)—C(2)—H(2) | 119.856 | C(14)—C(15)—H(15B) | 117.711 |
| C(3)—C(2)—H(2) | 119.846 | C(16)—C(15)—H(15A) | 117.702 |
| C(4)—C(5)—H(5) | 119.900 | C(16)—C(15)—H(15B) | 117.720 |
| C(6)—C(5)—H(5) | 119.892 | H(15A)—C(15)—H(15B) | 114.826 |
| C(1)—C(6)—H(6) | 119.947 | C(14)—C(16)—H(16A) | 117.785 |
| C(5)—C(6)—H(6) | 119.960 | C(14)—C(16)—H(16B) | 117.794 |
| O(2)—C(8)—H(8A) | 109.469 | C(15)—C(16)—H(16A) | 117.781 |
| O(2)—C(8)—H(8B) | 109.474 | C(15)—C(16)—H(16B) | 117.788 |
| O(2)—C(8)—H(8C) | 109.470 | H(16A)—C(16)—H(16B) | 114.905 |
| C(8)—O(2)—C(7)—O(1) | −2.4 (2) | C(2)—C(3)—C(4)—O(4) | 179.31 (11) |
| C(8)—O(2)—C(7)—C(1) | 177.99 (12) | C(2)—C(3)—C(4)—C(5) | −1.41 (19) |
| C(3)—O(3)—C(9)—C(10) | 160.99 (10) | O(4)—C(4)—C(5)—C(6) | 179.71 (12) |
| C(9)—O(3)—C(3)—C(2) | 11.35 (17) | C(3)—C(4)—C(5)—C(6) | 0.52 (20) |
| C(9)—O(3)—C(3)—C(4) | −167.98 (11) | C(4)—C(5)—C(6)—C(1) | 0.69 (20) |
| C(4)—O(4)—C(13)—C(14) | 177.72 (12) | O(3)—C(9)—C(10)—C(11) | 153.94 (11) |
| C(13)—O(4)—C(4)—C(3) | 177.39 (11) | O(3)—C(9)—C(10)—C(12) | 85.31 (14) |
| C(13)—O(4)—C(4)—C(5) | −1.83 (19) | C(9)—C(10)—C(11)—C(12) | −110.19 (13) |
| C(2)—C(1)—C(6)—C(5) | −1.01 (19) | C(9)—C(10)—C(12)—C(11) | 105.34 (13) |
| C(6)—C(1)—C(2)—C(3) | 0.09 (19) | C(11)—C(10)—C(12)—C(11) | 0.0 |
| C(2)—C(1)—C(7)—O(1) | 175.66 (13) | C(12)—C(10)—C(11)—C(12) | 0.0 |
| C(2)—C(1)—C(7)—O(2) | −4.76 (17) | C(10)—C(11)—C(12)—C(10) | 0.0 |
| C(7)—C(1)—C(2)—C(3) | −179.24 (12) | O(4)—C(13)—C(14)—C(15) | −76.77 (16) |
| C(6)—C(1)—C(7)—O(1) | −3.7 (2) | O(4)—C(13)—C(14)—C(16) | −144.94 (14) |
| C(6)—C(1)—C(7)—O(2) | 175.90 (12) | C(13)—C(14)—C(15)—C(16) | −107.83 (16) |
| C(7)—C(1)—C(6)—C(5) | 178.33 (12) | C(13)—C(14)—C(16)—C(15) | 107.08 (17) |
| C(1)—C(2)—C(3)—O(3) | −178.20 (12) | C(15)—C(14)—C(16)—C(15) | 0.0 |
| C(1)—C(2)—C(3)—C(4) | 1.11 (19) | C(16)—C(14)—C(15)—C(16) | 0.0 |
| O(3)—C(3)—C(4)—O(4) | −1.31 (17) | C(14)—C(15)—C(16)—C(14) | 0.0 |
| O(3)—C(3)—C(4)—C(5) | 177.96 (11) |
Symmetry codes: (i) x+1/2, −y+3/2, −z+1; (ii) −x+3/2, −y+1, z+1/2; (iii) x+1, y, z; (iv) x−1, y, z; (v) −x+5/2, −y+1, z+1/2; (vi) −x+3/2, −y+1, z−1/2; (vii) −x+5/2, −y+1, z−1/2; (viii) x−1/2, −y+3/2, −z+1; (ix) −x+1/2, −y+1, z+1/2; (x) x−1/2, −y+1/2, −z+1; (xi) −x+1, y−1/2, −z+1/2; (xii) x+1/2, −y+1/2, −z+1; (xiii) −x+2, y−1/2, −z+1/2; (xiv) −x+1/2, −y+1, z−1/2; (xv) −x+2, y+1/2, −z+1/2; (xvi) −x+1, y+1/2, −z+1/2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C12—H12B···O3iii | 0.99 | 2.55 | 3.4073 (18) | 145 |
Symmetry codes: (iii) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: JH2279).
References
- Bose, P., Sachdeva, Y. P., Rathore, R. S. & Kumar, Y. (2005). WO Patent 2005/026095 A1.
- Hou, J.-J., Cheng, X.-C., Wang, R.-L. & Wang, S.-Q. (2010). Acta Cryst. E66, o2004. [DOI] [PMC free article] [PubMed]
- Jacobson, R. (1998). REQAB. Private communication to the Rigaku Corporation, Tokyo, Japan.
- Rigaku. (2009). CrystalClear-SM Expert and CrystalStructure Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811013158/jh2279sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811013158/jh2279Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




