Abstract
It would be medically and economically desirable to prevent the millions of annual extraintestinal infections and the thousands of associated deaths due to Escherichia coli. Outer membrane proteins are potential vaccine candidates for the prevention of these infections. This study tested the hypotheses that the siderophore receptor IroN is antigenic and that an IroN-specific antibody response confers protection in vivo. Subcutaneous immunization with denatured IroN resulted in a significant IroN immunoglobulin G (IgG)-specific response in serum (P < 0.0001) but not a systemic or mucosal IroN-specific IgA response. In a mouse model of ascending urinary tract infection, subcutaneous immunization with denatured IroN conferred significant protection against renal (P = 0.0135 and 0.0095 in two independent experiments), but not bladder, infection. These data, together with the previously demonstrated role of IroN in virulence, its expression in human biologic fluids, and its prevalence among extraintestinal pathogenic E. coli strains, support further studies on the role of IroN as a vaccine candidate.
Escherichia coli strains of biological significance to humans consist of (i) commensal strains, (ii) intestinal pathogenic strains, and (iii) extraintestinal pathogenic E. coli (ExPEC) (30). ExPEC bacteria are the causative agents of the majority of extraintestinal infections due to E. coli (29). ExPEC bacteria are members of the normal intestinal flora and do not cause gastroenteritis in humans. However, their entry into extraintestinal sites results in a wide variety of infections. The urinary tract is the most common site for extraintestinal infections caused by ExPEC. Further, the urinary tract is the most common site from which ExPEC bacteremia originates (29). ExPEC is also a leading cause of severe sepsis (3, 6, 19, 34), with sepsis ranked as the 10th overall cause of death in the United States (20). Prevention of infection due to ExPEC is desirable from both medical and economic viewpoints.
This need has become more pressing as antibiotic resistance has developed increasingly among E. coli strains causing urinary tract infections (UTI) in the United States, particularly resistance to trimethoprim-sulfamethoxazole, which until recently was the drug of choice for uncomplicated cystitis in many locales (8, 9, 35). Furthermore, the incidence of serious extraintestinal infection due to E. coli increases with age (1, 19). As the proportion of elderly patients increases, so likely will the number of extraintestinal E. coli infections. The combination of increasing numbers of infections and increasing antimicrobial resistance predictably will make the future management of extraintestinal E. coli infections more challenging and costly than ever.
To date, an efficacious vaccine designed to protect against infections has not been approved for human use. The first antivirulence factor vaccine designed to prevent UTI in humans, which has recently entered clinical trials, is directed against the type 1 fimbrial adhesin FimH, a critical determinant of cystitis (17, 18). The results of these trials are awaited with great anticipation. Nonetheless, even if successful, the use of this vaccine will probably not extend to the many other infections caused by extraintestinal E. coli, since protection afforded by this vaccine appears to be through inhibition of bladder-specific adherence mediated by type 1 fimbriae (18). In animal models, passive or active immunization against capsule, O-specific antigen, and iron-regulated outer membrane proteins has afforded protection against systemic infection (4, 14, 33), and immunization with capsule, O-antigen, and P and type 1 fimbriae is protective against UTI due to ExPEC strains expressing these virulence factors (15-18, 21, 22). However, a vaccine based on capsule and/or O-specific antigens is impractical because of their significant antigenic heterogeneity (there are 80 capsular and >100 O-antigen serotypes). On the basis of limited published studies (4), studies on outer membrane proteins as vaccine candidates to protect against the diverse extraintestinal infections caused by this heterogeneous group of E. coli bacteria warrant consideration.
An ideal vaccine candidate needs to be surface exposed, be broadly prevalent among clinical extraintestinal isolates of E. coli, possess epitopes that are conserved, and elicit a protective immune response. Other desirable characteristics include increased expression at the site of infection and a role in the pathogenesis of disease. The expression of IroN by E. coli in various human body fluids (26) and in an avian air sac infection model (5), its prevalence among clinical extraintestinal isolates of E. coli (2, 11, 12), and a role in urovirulence and avian infection have been previously demonstrated (5, 32). In this study, the following hypotheses were tested: (i) immunization with IroN results in the production of antibodies directed against IroN, and (ii) IroN immunization confers protection against an E. coli challenge in a mouse model of ascending UTI.
Bacterial strains and media.
The model pathogen CP9 is an E. coli blood isolate cultured from a patient with sepsis and has been previously described in detail (13, 28). CP9 possesses many of the characteristics of typical ExPEC strains (10) and is highly virulent in a UTI model (24), an intraperitoneal infection model (31), and a pneumonia model (27). CP82 is an isogenic, TnphoA-generated derivative of CP9 in which iroN has been disrupted by TnphoA, but this transposon insertion does not result in polarity due to the genomic organization 3′ to iroN (26).
Purification of IroN.
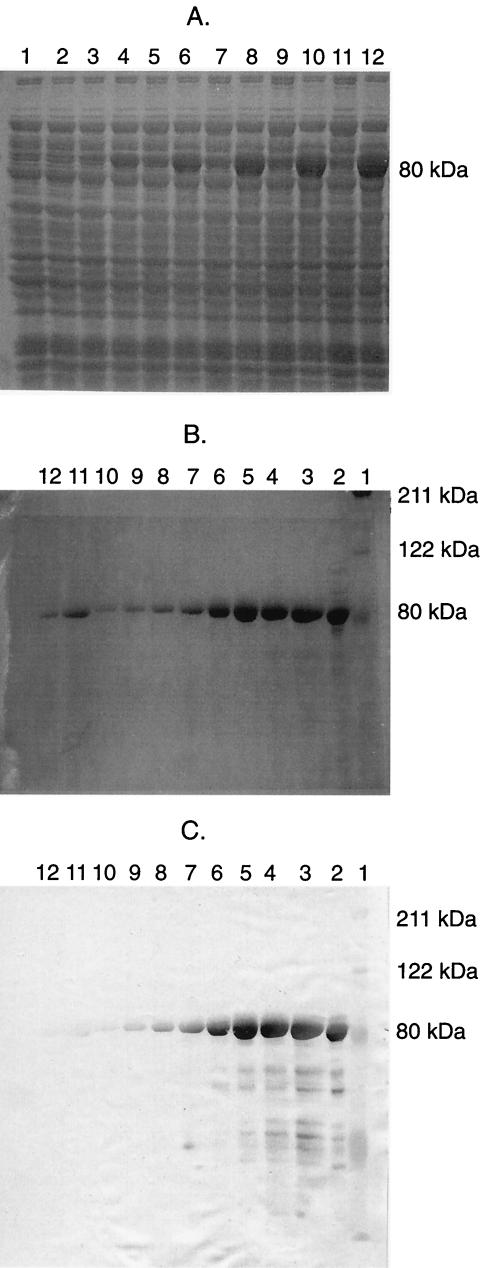
IroN was cloned via PCR-mediated amplification of the entire iroN gene on the basis of the iroN sequence (26), except its signal sequence (2,083 bp). The primers used (forward, 5′-CGCGCGCGGATCCGACGAGACTCTGGTGGTGGA-3′; reverse, 5′-CGCGCGCAAGCTTGAATGATGCGGTAACTCCGG-3′) contained capped BamHI and HindIII sites to facilitate ligation into a pET28a T7/his-tag expression vector (Novagen, Madison, Wis.). The cloned iroN gene was confirmed to be identical to that in strain CP9 by bidirectional DNA sequencing. The pET28a::iroN construct was electroporated into the expression cell line AD494(DE3)pLysS for expression of IroN. AD494(DE3)pLysS/pET28a::iroN was grown overnight in LB medium plus kanamycin. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM to induce the expression of IroN (Fig. 1A). Cells were lysed with BugBuster (Novagen) plus benzoase (25 U/ml). The lysate was pelleted and resuspended in lysis buffer (0.05 M NaH2PO4, 0.01 M Tris, 0.01 M NaCl, 6 M guanidine [final pH 8.0]) for 20 min. The lysate was repelleted, washed in lysis buffer once, and subsequently applied to a TALON cobalt-based immobilized metal affinity chromatography column (Clontech, Palo Alto, Calif.). The affinity-bound IroN was eluted under denaturing conditions (to maintain solubility) with 8 M urea elution buffer at a pH between 5.1 and 5.3 (Fig. 1B). The purified IroN was concentrated in 4 M urea elution buffer with a centrifugal filter device (30,000 molecular weight cutoff; Centricon, Millipore, Bedford, Mass.). A Western blot with the T7-Tag antibody specific to the recombinant protein was done, and it recognized the purified protein (Fig. 1C). IroN was stored at −20°C until further use.
FIG. 1.
Overexpression and purification of IroN. (A) Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of IroN expression over 3 h postinduction in strains AD494(DE3)pLysS/pET28a (control; odd-numbered lanes) and AD494(DE3)pLysS/pET28a::iroN (overexpresses IroN; even-numbered lanes). These strains were grown overnight in Luria-Bertani medium plus kanamycin. IPTG was added to a final concentration of 1 mM to induce the expression of IroN. Lanes (time after addition of IPTG): 1 and 2, 30 min; 3 and 4, 60 min; 5 and 6, 90 min; 7 and 8, 120 min; 9 and 10, 150 min; 11 and 12, 180 min. (B) Affinity purification of IroN. IroN expression was induced with IPTG in AD494(DE3)pLysS/pET28a::iroN, cells were lysed, and the lysate was applied to a TALON cobalt-based immobilized metal affinity chromatography column. The affinity-bound IroN was eluted under denaturing conditions (to maintain solubility) with 8 M urea elution buffer at a pH between 5.1 and 5.3. Lanes: 1, protein size markers; 2, nonpurified sample of the induced culture; 3 to 12, eluted IroN with breakdown products. (C) Western blot of IroN SDS-PAGE in panel B. A T7-Tag antibody (Novagen) to recombinant IroN was used for detection.
Immunization of mice.
To test for an acquired humoral immune response against IroN, BALB/c mice were immunized with purified, denatured IroN. After preimmunization sera were acquired, mice were subcutaneously immunized three times at 2-week intervals with either 35 μg of purified IroN in 4 M urea elution buffer without adjuvant or with 4 M urea elution buffer alone. Two weeks after the third immunization, postimmunization samples were acquired. Comparison of pre- and postimmunization sera, in conjunction with saliva, vaginal wash, and urine samples, was used to evaluate the antibody response that occurred after immunization with IroN. These mice were subsequently used for bacterial challenge experiments with wild-type strain CP9 in the UTI model.
Qualitative determination of antibody response to IroN or whole E. coli CP9.
Because of the large number of samples being assayed, qualitative antibody levels were determined by enzyme-linked immunosorbent assay (ELISA). The wells of ELISA plates (Dynatech Immulon II) were coated overnight at room temperature with either anti-immunoglobulin (Ig; 100 ng/well) or IroN (75 ng/well) in borate-buffered saline (pH 8.2). The next day, plates were washed three times with washing buffer (phosphate-buffered saline, 0.15% Tween 20 [pH 7.2]) and dilutions (in washing buffer) of the samples being tested (sera, 1/1,000 for IgG and 1/10 for IgA; saliva, 1/10; vaginal wash, 1/10; urine, 1/20) were added to the wells (100 μl/well) and incubated overnight at room temperature. On the third day, plates were washed three times with washing buffer. Antibody levels were detected by the addition of a 1/5,000 dilution of the appropriate horseradish peroxidase-conjugated goat anti-mouse Ig (Southern Biotechnology Associates, Inc., Birmingham, Ala.), incubation for 4 h at room temperature, three washes with washing buffer, and addition of o-phenylenediamine dihydrochloride and 30% H2O2 in phosphate-citrate buffer (100 μl/well). The reaction was stopped with 1 M sulfuric acid (100 μl/well) and read on a ELISA plate reader at 490 nm. A standard curve was generated with a mouse immunoglobulin reference serum against goat anti-mouse IgG or IgA. This curve established that the ELISA was linear up to an optical density at 490 nm (OD490) of 1.4. Sample dilutions were chosen so that OD490 measurements would fall within this OD490 range. However, the mean OD490 measurements for IroN-specific IgG from sera of IroN-immunized animals exceeded this value (2.5 and 2.7 in experiments 1 and 2, respectively). Although the measurement of IroN was likely underestimated in these experiments, their purpose, which was to demonstrate an IroN IgG response to immunization, was unequivocally achieved (see below [evaluation of the IroN-specific antibody response after subcutaneous immunization of BALB/c mice with IroN]). The IroN-specific antibody response was determined as the mean IroN IgG- or IgA-specific ELISA OD490 ± the standard error of the mean, and the fold increase was calculated as the ratio of the postimmunization versus the preimmunization OD490 measurement from the IroN ELISA. In experiments 1 and 2, respectively, sera were obtained from 21 nonimmunized control animals and 24 IroN-immunized animals, and measurements were made; saliva samples were obtained from 16 and 10 nonimmunized control animals and from 17 and 12 IroN-immunized animals, and measurements were made; and vaginal wash samples were obtained from 16 and 9 nonimmunized control animals and from 17 and 12 IroN-immunized animals, and measurements were made.
UTI model.
Mouse UTI experiments were done with an atraumatic mouse model of ascending UTI as previously described (24, 25). Two independent experiments (1 and 2) were performed. Each experiment consisted of 21 control animals and 24 immunized animals. As part of the assessment of the antibody response to immunization with IroN, saliva and vaginal wash samples were obtained from selected animals pre- and postimmunization and urine samples were obtained from some animals postimmunization just prior to a bacterial challenge. In brief, halothane-anesthetized female BALB/c mice (18 to 22 g, 8 to 12 weeks old) were catheterized and a challenge inoculum of strain CP9 (wild type) was delivered with a Harvard pump to avoid inoculation-induced vesicoureteral reflux. CP9 was grown overnight in M9 minimal medium containing 0.5% glucose, treated with Chelex to remove any trace Fe present (M9-glucose-Chelex treated). This medium simulates the Fe- and nutrient-limiting environment within the host and ensures expression of IroN (32). In experiment 1, mice were challenged with 2.0 × 106 CFU of CP9. Two, four, and six days postchallenge, urine, bladder, and kidneys were harvested and bacterial titers were determined. In experiment 2, a larger inoculum (1.25 × 107 CFU) of CP9 was used to determine if the renal protection observed after immunization with IroN in experiment 1 was maintained when a larger challenge dose was administered.
The animal studies described here were reviewed and approved by the Buffalo Veterans Administration Institutional Animal Care Committee.
Statistics.
Regression methodologies were chosen to analyze all of the data from the UTI model together rather than perform separate analyses, that is, basic t tests for each of the time points considered. Analysis of all data points across time, or dosage, results in more power to detect differences present and avoids an unnecessary increase in type I error that occurs with point-by-point analyses. To test for immunization effectiveness in the UTI model, multiple regressions were run for all three endpoints (bladder, kidney, and urine bacterial titers) in both experiments 1 and 2. Regressions included the dichotomous immunization status, a continuous time effect, and a time-and-immunization interaction to allow for possibly different slopes. In the event that the interaction was nonsignificant, it was dropped and the regression was rerun. To meet the regression model assumptions, log transforms were applied to all endpoints after an offset of 1 was added to account for zero values. Standard diagnostic techniques were used to assess the regression model fit, and only regression models in which the overall model P value was significant were considered. It was determined that experiments 1 and 2 could not be pooled for statistical analysis, and thus they were analyzed separately.
Evaluation of the IroN-specific antibody response after subcutaneous immunization of BALB/c mice with IroN.
BALB/c mice, which were subsequently used in protection assays, were subcutaneously immunized with purified but denatured IroN or buffer (nonimmunized controls), and the immune response was measured by ELISA as already described. The mean IroN IgG and IgA titers were measured in serum, saliva, vaginal wash, and urine samples.
Assessment of the systemic-compartment immune response demonstrated that in IroN-immunized mice, the mean serum IroN IgG-specific ELISA OD490 was significantly increased postimmunization (2,580 ± 120 and 2,760 ± 80 in experiments 1 and 2, respectively) compared with preimmunization (9 ± 2.8 and 7 ± 2.1) (P < 0.0001 for each experiment [paired t test]). As expected, in buffer-immunized mice, the mean serum IroN IgG-specific ELISA OD490 was not significantly increased postimmunization (11 ± 2.4 and 5 ± 0.9 in experiments 1 and 2, respectively) compared with the preimmunization value (11 ± 4.5 and 3 ± 0.7) (P > 0.1 for each experiment [paired t test]). These mean serum IroN IgG-specific ELISA OD490 values represented increases of 287- and 394-fold in IroN-immunized mice compared to increases of 1.0- and 1.7-fold in buffer-immunized controls (experiments 1 and 2, respectively). In contrast, in IroN-immunized mice, the mean serum IroN IgA-specific ELISA OD490 was not significantly increased (data not shown).
Assessment of the mucosal-compartment immune response demonstrated that in IroN-immunized mice, small increases in the mean IroN IgG-specific ELISA OD490 occurred in saliva postimmunization (0.23 ± 0.1 and 0.13 ± 0.07 in experiments 1 and 2, respectively), compared with the preimmunization value (0.007 ± 0.006 and 0.01 ± 0.001) (P = 0.07 and 0.089 [paired t test]), and in vaginal wash postimmunization (6.5 ± 1.9 and 6.5 ± 1.8), compared with the preimmunization value (0.09 ± 0.05 and 0.13 ± 0.07) (P = 0.003 and 0.005 [paired t test]). As expected, no increase occurred in the saliva and vaginal wash samples of buffer-immunized mice (data not shown). These mean IroN ELISA OD490 values represented increases in the saliva samples of IroN-immunized mice of 33- and 13-fold compared to increases of 5.0- and 1.3-fold in those of nonimmunized controls (experiments 1 and 2, respectively) and increases in the vaginal wash samples of IroN-immunized mice of 72- and 50-fold compared to increases of 1.4- and 1.0-fold in those of nonimmunized controls (experiments 1 and 2, respectively). However, because of the large concomitant IroN-specific IgG response in serum, the observed small increase in IroN-specific IgG in the saliva and vaginal wash samples of IroN-immunized mice might have been due to blood contamination from trauma during the collection process. The mean IroN IgA-specific ELISA OD490 was not significantly increased in the saliva or vaginal wash samples of IroN-immunized mice (data not shown).
To assess the immune response within the urinary tract, urine was collected from IroN-immunized and buffer-treated (nonimmunized control) mice. Since preimmunization urine was not available, the antibody responses of buffer-immunized mice were compared to those of IroN-immunized mice. In experiments 1 and 2, respectively, a small increase in the IroN IgG-specific ELISA OD490 occurred in the urine of IroN-immunized mice postimmunization (6.8 ± 1.0 and 12.2 ± 2.0) compared with that of buffer-treated mice (0.016 ± 0.01 and 0.52 ± 0.34) (P < 0.0001 both experiments, nonpaired t test). These mean IroN-ELISA OD490 values represented increases of 425- and 23.5-fold in IroN-immunized mice compared to buffer-immunized mice (experiments 1 and 2, respectively). However, because of the large concomitant IroN-specific IgG response in serum, the observed small increase in IroN-specific IgG in urine may be due to blood contamination from trauma during the collection process. In IroN-immunized mice, the mean IroN IgA-specific ELISA OD490 was not significantly increased in urine (data not shown). Therefore, after subcutaneous immunization of mice with denatured IroN, a significant IroN IgG-specific response, but not a IroN IgA-specific response, occurred.
Evaluation of the protective effects of IroN immunization of BALB/c mice against UTI.
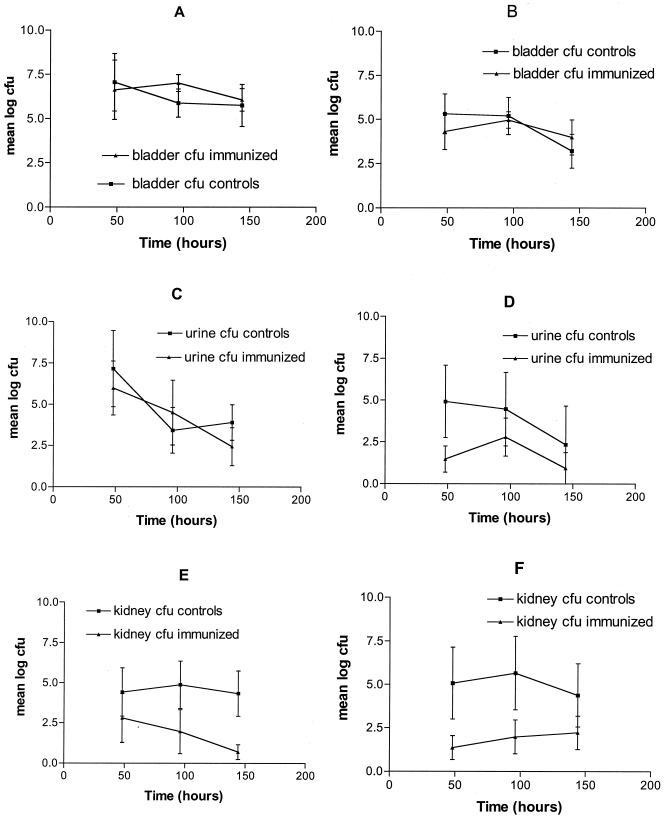
BALB/c mice were immunized with either denatured IroN (immunized) or buffer (nonimmunized controls). Two independent experiments were performed to assess the protective effects of IroN immunization against an intravesicular challenge with wild-type ExPEC strain CP9. Two, four, and six days after a CP9 challenge, the urinary bladder, kidneys, and urine were harvested and the respective titers of CP9 were determined. The overall regression model (see Statistics above) P values in the urinary bladder and urine analyses were not found to be significant (P > 0.12) (Fig. 2 A to D), signifying that neither IroN immunization nor time had a statistically significant effect on the titer of CP9 (data not shown). In contrast, in experiments 1 and 2, the renal titers of CP9 in IroN-immunized mice were significantly (P = 0.0135 and 0.0095, respectively) diminished across time compared to those in controls (Fig. 2E and F). These data strongly support the hypothesis that immunization with IroN confers protection against an E. coli challenge in vivo, in this case against renal infection but not bladder infection.
FIG. 2.
Protective effect of subcutaneous immunization with denatured IroN in a model of ascending, unobstructed UTI. BALB/c mice were injected with either IroN (immunized mice) or buffer (nonimmunized controls). Infection was initiated by intravesicular challenge of BALB/c mice with wild-type strain CP9 (2.0 × 106 CFU in experiment 1 and 1.25 × 107 CFU in experiment 2). Two, four, and six days postchallenge, urine, bladder, and kidneys were harvested and bacterial titers were determined. To test for immunization effectiveness, multiple regressions were run for all three endpoints (bladder, kidney, urine bacterial titers) in both experiments 1 and 2. Panels A (experiment 1) and B (experiment 2) are bladder titers, panels C (experiment 1) and D (experiment 2) are urine titers, and panels E (experiment 1) and F (experiment 2) are renal titers. The renal titers of CP9, but not the bladder or urine titers, were significantly (P = 0.0135 and 0.0095, experiments 1 and 2, respectively) diminished across time in IroN-immunized mice compared to those of controls (E and F).
This study was designed to evaluate the potential of the ExPEC siderophore receptor IroN as a vaccine candidate. Subcutaneous immunization with denatured IroN resulted in a significant IroN-specific IgG response in serum (P < 0.0001). Not surprisingly, subcutaneous immunization did not result in a systemic or mucosal IroN-specific IgA response. In a mouse model of ascending UTI, subcutaneous immunization with denatured IroN conferred a significant degree of protection against the development of renal infection (P = 0.0135 and 0.0095 in two independent experiments) but not bladder infection. These data, together with the previously demonstrated role of IroN in virulence (5, 32), its expression in human biologic fluids (26) and in an avian infection model (5), and its prevalence among ExPEC strains (2, 11, 12, 26), support further studies of the role of IroN as a vaccine candidate.
The nature of the measured humoral immune response in the systemic and mucosal compartments was consistent with previous observations when a subcutaneous route of immunization was used (23, 36). A significant systemic-compartment IroN IgG-specific antibody response developed in serum. However, there was no IroN IgA-specific antibody response in either the systemic or the mucosal compartment. A small IroN IgG-specific response was observed in the mucosal compartment, which may in fact represent contamination from serum antibodies because of trauma during the collection process. Interestingly, protection against renal infection, but not bladder infection, was observed with the IroN antibody response after subcutaneous immunization. This finding suggests that perhaps a mucosal-compartment response is needed for protection against cystitis, whereas a systemic-compartment response, at least in part, serves to protect against pyelonephritis.
The findings from the UTI model were encouraging. Although subcutaneous immunization with denatured IroN afforded protection against renal infection and not bladder infection, as discussed above, the humoral immune response that developed is likely far from ideal. Although the relative importance of the mucosal-compartment immune response versus the systemic-compartment immune response in protecting against infection of the various sites within the urinary tract is unclear, it is reasonable to postulate that both responses may be needed for maximal efficacy. Immunization with IroN at a mucosal induction site that results in the generation of a common mucosal immune response (e.g., intranasal immunization) with or without the use of potent mucosal enterotoxin-based adjuvants may significantly enhance the protective effects observed to date (7, 23). Although human trials of the efficacy of vaccination with the type 1 fimbrial adhesin FimH are in progress, preclinical data have demonstrated that the protection afforded by this vaccine appears to be achieved through inhibition of bladder-specific adherence mediated by type 1 fimbriae (18). Therefore, renal protection that results from other vaccine candidates, such as IroN, has the potential to complement vaccines designed to prevent cystitis. In addition, since IroN expression occurs in ascites and blood (26), the development of an appropriate immune response to IroN may also afford protection against infection at non-urinary tract sites. Studies to evaluate this possibility are in progress.
Acknowledgments
This work was supported by VA Merit Review (T.A.R.), National Institutes of Health grants AI 42059 and HL 69763 (T.A.R.), and the John R. Oishei Foundation (T.A.R.).
We thank Bruce Davidson for assistance with flow cytometry and Tim Murphy for critically reviewing the manuscript.
Editor: J. T. Barbieri
REFERENCES
- 1.Angus, D., W. Linde-Zwirble, J. Lidlicker, G. Clermont, J. Carcillo, and M. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303-1310. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, R., L. Zhang, B. Foxman, A. Siitonen, M. Jantunen, H. Saxen, and C. Marrs. 2002. Molecular epidemiology of 3 putative virulence genes for Escherichia coli urinary tract infection—usp, iha, and iroNE. coli. J. Infect. Dis. 185:1521-1524. [DOI] [PubMed] [Google Scholar]
- 3.Bernard, G., J. Vincent, P. Laterre, S. LaRosa, J. Dhainaut, A. Lopez-Rodriguez, J. Steingrub, G. Garber, J. Helterbrand, E. Ely, and C. J. Fisher. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699-709. [DOI] [PubMed] [Google Scholar]
- 4.Bolin, C. A., and A. E. Jensen. 1987. Passive immunization with antibodies against iron-regulated outer membrane proteins protects turkeys from Escherichia coli septicemia. Infect. Immun. 55:1239-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dozois, C., F. Daigle, and R. I. Curtiss. 2003. Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc. Natl. Acad. Sci. USA 100:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fluit, A., F. Schmitz, and J. Verhoef. 2001. Frequency of isolation of pathogens from bloodstream, nosocomial pneumonia, skin and soft tissue, and urinary tract infections occurring in European patients. Eur. J. Clin. Microbiol. Infect. Dis. 20:188-191. [DOI] [PubMed] [Google Scholar]
- 7.Fujihashi, K., T. Koga, F. van Ginkel, Y. Hagiwara, and J. McGhee. 2002. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine 20:2431-2438. [DOI] [PubMed] [Google Scholar]
- 8.Gupta, K., T. Hooton, and W. Stamm. 2001. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann. Intern. Med. 135:41-50. [DOI] [PubMed] [Google Scholar]
- 9.Gupta, K., D. Scholes, and W. Stamm. 1999. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA 281:736-738. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, J., P. Delavari, M. Kuskowski, and A. Stell. 2001. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J. Infect. Dis. 183: 78-88. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, J., M. Kuskowski, T. O'Bryan, and J. Maslow. 2002. Epidemiological correlates of virulence genotype and phylogenetic background among Escherichia coli blood isolates from adults with diverse-source bacteremia. J. Infect. Dis. 185: 1439-1447. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, J. R., T. A. Russo, P. I. Tarr, U. Carlino, S. S. Bilge, J. C. Vary, Jr., and A. L. Stell. 2000. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroNE. coli, among Escherichia coli isolates from patients with urosepsis. Infect. Immun. 68:3040-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, J. R., A. E. Stapleton, T. A. Russo, F. Scheutz, J. J. Brown, and J. N. Maslow. 1997. Characteristics and prevalence within serogroup O4 of a J96-like clonal group of uropathogenic Escherichia coli O4:H5 containing the class I and class III alleles of papG. Infect. Immun. 65:2153-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaijser, B., and S. Ahlstedt. 1977. Protective capacity of antibodies against Escherichia coli O and K antigens. Infect. Immun. 17:286-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaijser, B., P. Larsson, W. Nimmich, and T. Soderstrom. 1983. Antibodies to Escherichia coli K and O antigens in protection against acute pyelonephritis. Prog. Allergy 33:275-288. [DOI] [PubMed] [Google Scholar]
- 16.Kaijser, B., P. Larsson, and S. Olling. 1978. Protection against ascending Escherichia coli pyelonephritis in rats and significance of local immunity. Infect. Immun. 20: 78-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langermann, S., R. Mollby, J. Burlein, S. Palaszynski, C. Auguste, A. De Fusco, et al. 2000. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 181: 774-778. [DOI] [PubMed] [Google Scholar]
- 18.Langermann, S., S. Palaszynski, M. Barnhart, G. Auguste, J. Pinkner, J. Burlein, et al. 1997. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 276:607-611. [DOI] [PubMed] [Google Scholar]
- 19.McBean, M., and S. Rajamani. 2001. Increasing rates of hospitalization due to septicemia in the US elderly population, 1986-1997. J. Infect. Dis. 183: 596-603. [DOI] [PubMed] [Google Scholar]
- 20.Minino, A., and B. Smith. 2001. Deaths: preliminary data for 2000. Natl. Vital Stat. Rep. 49:1-40. [PubMed] [Google Scholar]
- 21.O'Hanley, P., G. Lalonde, and G. Ji. 1991. Alpha-hemolysin contributes to the pathogenicity of piliated digalactoside-binding Escherichia coli in the kidney: efficacy of an alpha-hemolysin vaccine in preventing renal injury in the BALB/c mouse model of pyelonephritis. Infect. Immun. 59:1153-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pecha, B., D. Low, and P. O'Hanley. 1989. Gal-Gal pili vaccines prevent pyelonephritis by piliated Escherichia coli in a murine model: single component Gal-Gal pili vaccines prevent pyelonephritis by homologous and heterologous piliated E. coli strains. J. Clin. Investig. 83:2102-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell, M., and J. Mestecky. 2002. Humoral immune response to microbial infections in the genital tract. Microbes Infect. 4:667-677. [DOI] [PubMed] [Google Scholar]
- 24.Russo, T. A., J. J. Brown, S. T. Jodush, and J. R. Johnson. 1996. The O4 specific antigen moiety of lipopolysaccharide but not the K54 group 2 capsule is important for urovirulence in an extraintestinal isolate of Escherichia coli. Infect. Immun. 64:2343-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo, T. A., U. B. Carlino, and J. R. Johnson. 2001. Identification of a new iron-regulated virulence gene, ireA, in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 69:6209-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo, T. A., U. B. Carlino, A. Mong, and S. T. Jodush. 1999. Identification of genes in an extraintestinal isolate of Escherichia coli with increased expression after exposure to human urine. Infect. Immun. 67:5306-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo, T. A., B. A. Davidson, R. L. Priore, U. B. Carlino, J. D. Helinski, and P. R. Knight III. 2000. Capsular polysaccharide and O-specific antigen divergently modulate pulmonary neutrophil influx in an Escherichia coli model of gram-negative pneumonitis in rats. Infect. Immun. 68:2854-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo, T., J. Guenther, S. Wenderoth, and M. Frank. 1993. Generation of isogenic K54 capsule-deficient Escherichia coli strains through TnphoA-mediated gene disruption. Mol. Microbiol. 9:357-364. [DOI] [PubMed] [Google Scholar]
- 29.Russo, T., and J. Johnson. 2003. Medical and economic impact of extraintestinal infections due to Escherichia coli: an overlooked epidemic. Microbes Infect. 5:449-456. [DOI] [PubMed] [Google Scholar]
- 30.Russo, T., and J. Johnson. 2000. A proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 181: 1753-1754. [DOI] [PubMed] [Google Scholar]
- 31.Russo, T., Y. Liang, and A. Cross. 1994. The presence of K54 capsular polysaccharide increases the pathogenicity of Escherichia coli in vivo. J. Infect. Dis. 169: 112-118. [DOI] [PubMed] [Google Scholar]
- 32.Russo, T. A., C. D. McFadden, U. B. Carlino-MacDonald, J. M. Beanan, T. J. Barnard, and J. R. Johnson. 2002. IroN functions as a siderophore receptor and is a urovirulence factor in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 70:7156-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salles, M., E. Mandine, R. Zalisz, M. Guenounou, and P. Smets. 1989. Protective effects of murine monoclonal antibodies in experimental septicemia: E. coli antibodies protect against different serotypes of E. coli. J. Infect. Dis. 159: 641-647. [DOI] [PubMed] [Google Scholar]
- 34.Siegman-Igra, Y., B. Fourer, R. Orni-Wasserlauf, Y. Golan, A. Noy, D. Schwartz, and M. Giladi. 2002. Reappraisal of community-acquired bacteremia: a proposal of a new classification for the spectrum of acquisition of bacteremia. Clin. Infect. Dis. 34: 1431-1439. [DOI] [PubMed] [Google Scholar]
- 35.Talan, D., W. Stamm, T. Hooton, G. Moran, T. Burke, A. Iravani, J. Reuning-Scherer, and D. Church. 2000. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women: a randomized trial. JAMA 283:1583-1590. [DOI] [PubMed] [Google Scholar]
- 36.Thapar, M., E. Parr, and M. Parr. 1990. Secretory immune responses in mouse vaginal fluid after pelvic, parenteral or vaginal immunization. Immunology 70:121-125. [PMC free article] [PubMed] [Google Scholar]