Abstract
In the title compound, C17H16O3, the dihedral angle between the aromatic rings is 4.59 (7)° and an intramolecular O—H⋯O hydrogen bond generates an S(6) ring. In the crystal, adjacent molecules are linked by C—H⋯O hydrogen bonds, leading to the formation of [001] supramolecular chains. Weak C—H⋯π interactions consolidate the packing.
Related literature
For a related structure and background references to chalcones, see: Fun et al. (2010 ▶). For related structures, see: Chantrapromma et al. (2009 ▶, 2010 ▶); Fun et al. (2009 ▶); Horkaew et al. (2010 ▶); Lu et al. (2009 ▶); Suwunwong et al. (2009 ▶); Wang et al. (2009 ▶, 2010 ▶); Jasinski et al. (2011 ▶). For hydrogen-bond motifs, see: Bernstein et al. (1995 ▶). For bond-length data, see: Allen et al. (1987 ▶).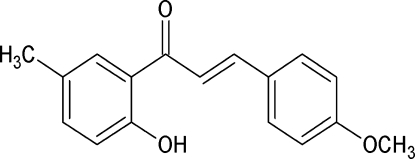
Experimental
Crystal data
C17H16O3
M r = 268.30
Monoclinic,

a = 12.6990 (18) Å
b = 8.8022 (13) Å
c = 13.172 (2) Å
β = 105.565 (2)°
V = 1418.3 (4) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 296 K
0.46 × 0.32 × 0.18 mm
Data collection
Bruker SMART APEXII DUO CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.962, T max = 0.984
11493 measured reflections
4090 independent reflections
2608 reflections with I > 2σ(I)
R int = 0.024
Refinement
R[F 2 > 2σ(F 2)] = 0.047
wR(F 2) = 0.131
S = 1.02
4090 reflections
187 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.16 e Å−3
Δρmin = −0.16 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811015054/hb5847sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811015054/hb5847Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811015054/hb5847Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg1 is the centroid of the C1–C6 ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1O1⋯O2 | 0.95 (2) | 1.65 (2) | 2.5112 (18) | 149.4 (19) |
| C11—H11A⋯O3i | 0.93 | 2.60 | 3.4317 (17) | 149 |
| C16—H16C⋯Cg1ii | 0.96 | 2.81 | 3.5800 (18) | 138 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
The authors thank Universiti Sains Malaysia (USM) for the Research University Grant (No. 1001/PFIZIK/811160). SA thanks the Malaysian Government and USM for the award of a research scholarship. VMK also thanks P. A. College of Engineering for research facilities.
supplementary crystallographic information
Comment
In continuation of our studies on the crystal structures of chalcones (Fun et al., 2010), we now report the synthesis and crystal structure of the title compound, (I). The structures of some related chalcones viz: (Z)-3-(9-anthryl)-1-(4-methoxyphenyl)prop-2-en-1-one (Chantrapromma et al., 2009), (Z)-3-(9-anthryl)- 1-(2-thienyl)prop-2-en-1-one (Fun et al., 2009), (E)-3- (anthracen-9-yl)-1-(4-bromophenyl)prop-2-en-1-one (Suwunwong et al., 2009), (Z)-3-(9-anthryl)-1-(4-bromophenyl)-2-(4-nitro-1H- imidazol-1-yl)prop-2-en-1-one (Lu et al., 2009),(Z)-3- (9-anthryl)-2-(4-nitro-1H-imidazol-1-yl)-1-p-tolylprop- 2-en-1-one (Wang et al., 2009), (E)-3-(9-anthryl)-1- (4-fluorophenyl)-2-(4-nitro-1H-imidazol-1-yl)prop-2-en-1-one (Wang et al., 2010), (E)-3-(anthracen-9-yl)-1-(furan-2-yl) prop-2-en-1-one (Horkaew et al., 2010), and an orthorhombic polymorph of (Z)-3-(9-anthryl)-1-(2-thienyl)prop-2-en-1-one (Chantrapromma et al., 2010) and 2(E)-3-(4-hydroxyphenyl)-1-(4-chlorophenyl) prop-2-en-1-one (Jasinski et al.,2011) have been reported.
The molecular structure is shown in Fig. 1. An intramolecular O1—H1O1···O2 hydrogen bond (Table 1) stabilizes the molecular structure and forms an S(6) ring motif (Bernstein et al., 1995). The dihedral angle between the phenyl (C1–C6) ring and the methoxy-substituted phenyl (C10–C15) ring is 4.59 (7)°. Bond lengths (Allen et al., 1987) and angles are within normal ranges and are comparable to the related structures (Fun et al., 2010).
In the crystal packing (Fig. 2), the molecules are linked into infinite one-dimensional chain along the c-axis by intermolecular C11—H11A···O3 hydrogen bonds (Table 1). There are also C—H···π interactions (Table 1) which involves C16 and phenyl ring (Cg1 = C1–C6).
Experimental
2-Hydroxy-5-methylacetophenone (1.50 g, 0.01 mol) was mixed with 4-methoxybenzaldehyde (1.36 g, 0.01 mol) and dissolved in ethanol (40 ml). To this solution, 5 ml of KOH (50%) was added at 278 K. The reaction mixture stirred for 6 h and poured on to crushed ice. The pH of this mixture was adjusted to 3–4 with 2 M HCl aqueous solution. The resulting crude yellow solid was filtered, washed successively with dilute HCl solution and distilled water and finally recrystallized from ethanol (95%) to give the pure chalcone. Orange blocks of (I) were grown by the slow evaporation of the solution of the compound in ethyl alcohol (m. p.: 361 K). Composition: Found (Calculated) for C17H16O3, C: 76.10 (76.16); H: 6.01 (6.05).
Refinement
H1O1 atom attached to the O atom was located from the difference map and refined freely [O–H = 0.94 (2) Å]. The remaining H atoms were positioned geometrically [C–H = 0.93 or 0.96 Å] and refined using a riding model with Uiso(H) = 1.2 or 1.5 Ueq(C). A rotating group model was applied to the methyl groups.
Figures
Fig. 1.
The molecular structure of the title compound, showing 50% probability displacement ellipsoids. The dashed line indicates the intramolecular bond.
Fig. 2.
The crystal packing of the title compound, viewed along the b axis.
Crystal data
| C17H16O3 | F(000) = 568 |
| Mr = 268.30 | Dx = 1.256 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 2769 reflections |
| a = 12.6990 (18) Å | θ = 2.8–28.6° |
| b = 8.8022 (13) Å | µ = 0.09 mm−1 |
| c = 13.172 (2) Å | T = 296 K |
| β = 105.565 (2)° | Block, orange |
| V = 1418.3 (4) Å3 | 0.46 × 0.32 × 0.18 mm |
| Z = 4 |
Data collection
| Bruker SMART APEXII DUO CCD diffractometer | 4090 independent reflections |
| Radiation source: fine-focus sealed tube | 2608 reflections with I > 2σ(I) |
| graphite | Rint = 0.024 |
| φ and ω scans | θmax = 29.9°, θmin = 2.8° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −17→17 |
| Tmin = 0.962, Tmax = 0.984 | k = −10→12 |
| 11493 measured reflections | l = −18→18 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.047 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.131 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0547P)2 + 0.1652P] where P = (Fo2 + 2Fc2)/3 |
| 4090 reflections | (Δ/σ)max < 0.001 |
| 187 parameters | Δρmax = 0.16 e Å−3 |
| 0 restraints | Δρmin = −0.16 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.13821 (11) | 0.18686 (15) | 0.32930 (8) | 0.0794 (4) | |
| O2 | 0.26594 (9) | 0.35672 (14) | 0.26545 (7) | 0.0758 (3) | |
| O3 | 0.51456 (8) | 0.87081 (13) | −0.15126 (7) | 0.0666 (3) | |
| C1 | 0.03317 (10) | 0.26022 (14) | 0.04622 (10) | 0.0486 (3) | |
| H1A | 0.0518 | 0.3125 | −0.0078 | 0.058* | |
| C2 | −0.06268 (11) | 0.17724 (16) | 0.02260 (12) | 0.0553 (3) | |
| C3 | −0.08898 (13) | 0.10217 (19) | 0.10552 (15) | 0.0704 (4) | |
| H3A | −0.1538 | 0.0472 | 0.0921 | 0.084* | |
| C4 | −0.02277 (15) | 0.10664 (19) | 0.20568 (15) | 0.0728 (4) | |
| H4A | −0.0427 | 0.0543 | 0.2590 | 0.087* | |
| C5 | 0.07432 (12) | 0.18853 (16) | 0.22906 (11) | 0.0581 (4) | |
| C6 | 0.10376 (10) | 0.26897 (14) | 0.14840 (10) | 0.0468 (3) | |
| C7 | 0.20644 (11) | 0.35632 (16) | 0.17338 (10) | 0.0502 (3) | |
| C8 | 0.23943 (10) | 0.44232 (15) | 0.09183 (10) | 0.0488 (3) | |
| H8A | 0.1929 | 0.4469 | 0.0240 | 0.059* | |
| C9 | 0.33518 (10) | 0.51410 (15) | 0.11356 (10) | 0.0478 (3) | |
| H9A | 0.3790 | 0.5037 | 0.1822 | 0.057* | |
| C10 | 0.38013 (9) | 0.60674 (14) | 0.04375 (9) | 0.0442 (3) | |
| C11 | 0.48166 (10) | 0.67433 (16) | 0.08326 (10) | 0.0501 (3) | |
| H11A | 0.5188 | 0.6589 | 0.1536 | 0.060* | |
| C12 | 0.52938 (10) | 0.76377 (16) | 0.02169 (10) | 0.0504 (3) | |
| H12A | 0.5973 | 0.8081 | 0.0504 | 0.060* | |
| C13 | 0.47519 (10) | 0.78657 (15) | −0.08275 (9) | 0.0477 (3) | |
| C14 | 0.37276 (11) | 0.72165 (17) | −0.12373 (10) | 0.0581 (4) | |
| H14A | 0.3355 | 0.7385 | −0.1939 | 0.070* | |
| C15 | 0.32612 (10) | 0.63348 (16) | −0.06231 (10) | 0.0542 (3) | |
| H15A | 0.2577 | 0.5907 | −0.0912 | 0.065* | |
| C16 | −0.13460 (12) | 0.16543 (19) | −0.08791 (14) | 0.0704 (4) | |
| H16A | −0.1021 | 0.2204 | −0.1347 | 0.106* | |
| H16B | −0.2051 | 0.2077 | −0.0911 | 0.106* | |
| H16C | −0.1427 | 0.0606 | −0.1086 | 0.106* | |
| C17 | 0.61893 (12) | 0.93974 (19) | −0.11419 (12) | 0.0645 (4) | |
| H17A | 0.6362 | 0.9942 | −0.1708 | 0.097* | |
| H17B | 0.6731 | 0.8627 | −0.0886 | 0.097* | |
| H17C | 0.6181 | 1.0090 | −0.0581 | 0.097* | |
| H1O1 | 0.2008 (17) | 0.245 (2) | 0.3290 (16) | 0.107 (7)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.1019 (9) | 0.0879 (9) | 0.0533 (6) | −0.0055 (7) | 0.0295 (6) | 0.0145 (6) |
| O2 | 0.0760 (7) | 0.1013 (9) | 0.0451 (5) | −0.0174 (6) | 0.0078 (5) | 0.0079 (5) |
| O3 | 0.0642 (6) | 0.0840 (8) | 0.0509 (5) | −0.0187 (5) | 0.0142 (5) | 0.0088 (5) |
| C1 | 0.0523 (7) | 0.0438 (7) | 0.0542 (7) | 0.0022 (6) | 0.0222 (6) | −0.0008 (6) |
| C2 | 0.0514 (7) | 0.0456 (8) | 0.0729 (9) | 0.0012 (6) | 0.0239 (6) | −0.0062 (6) |
| C3 | 0.0651 (9) | 0.0595 (10) | 0.0961 (13) | −0.0098 (7) | 0.0380 (9) | −0.0011 (9) |
| C4 | 0.0863 (11) | 0.0626 (10) | 0.0845 (11) | −0.0079 (9) | 0.0488 (10) | 0.0088 (8) |
| C5 | 0.0756 (9) | 0.0509 (8) | 0.0567 (8) | 0.0042 (7) | 0.0331 (7) | 0.0038 (6) |
| C6 | 0.0552 (7) | 0.0413 (7) | 0.0496 (7) | 0.0036 (6) | 0.0241 (6) | −0.0015 (5) |
| C7 | 0.0570 (7) | 0.0503 (8) | 0.0454 (6) | 0.0025 (6) | 0.0172 (6) | −0.0010 (5) |
| C8 | 0.0537 (7) | 0.0499 (7) | 0.0428 (6) | −0.0015 (6) | 0.0130 (5) | −0.0013 (5) |
| C9 | 0.0503 (6) | 0.0489 (7) | 0.0435 (6) | 0.0024 (6) | 0.0114 (5) | −0.0028 (5) |
| C10 | 0.0445 (6) | 0.0453 (7) | 0.0427 (6) | 0.0028 (5) | 0.0113 (5) | −0.0040 (5) |
| C11 | 0.0458 (6) | 0.0617 (9) | 0.0395 (6) | 0.0009 (6) | 0.0058 (5) | 0.0004 (6) |
| C12 | 0.0400 (6) | 0.0621 (9) | 0.0462 (6) | −0.0029 (6) | 0.0065 (5) | −0.0027 (6) |
| C13 | 0.0483 (6) | 0.0521 (8) | 0.0434 (6) | −0.0004 (6) | 0.0135 (5) | −0.0012 (5) |
| C14 | 0.0554 (7) | 0.0723 (10) | 0.0401 (6) | −0.0100 (7) | 0.0016 (6) | 0.0034 (6) |
| C15 | 0.0465 (7) | 0.0632 (9) | 0.0481 (7) | −0.0098 (6) | 0.0042 (5) | −0.0018 (6) |
| C16 | 0.0544 (8) | 0.0657 (10) | 0.0876 (11) | −0.0045 (7) | 0.0131 (8) | −0.0117 (8) |
| C17 | 0.0614 (8) | 0.0662 (10) | 0.0707 (9) | −0.0104 (7) | 0.0257 (7) | −0.0025 (7) |
Geometric parameters (Å, °)
| O1—C5 | 1.3517 (18) | C9—C10 | 1.4554 (17) |
| O1—H1O1 | 0.94 (2) | C9—H9A | 0.9300 |
| O2—C7 | 1.2449 (15) | C10—C11 | 1.3881 (17) |
| O3—C13 | 1.3624 (15) | C10—C15 | 1.4011 (17) |
| O3—C17 | 1.4198 (17) | C11—C12 | 1.3814 (18) |
| C1—C2 | 1.3815 (18) | C11—H11A | 0.9300 |
| C1—C6 | 1.4048 (18) | C12—C13 | 1.3778 (17) |
| C1—H1A | 0.9300 | C12—H12A | 0.9300 |
| C2—C3 | 1.392 (2) | C13—C14 | 1.3903 (18) |
| C2—C16 | 1.500 (2) | C14—C15 | 1.3656 (19) |
| C3—C4 | 1.361 (2) | C14—H14A | 0.9300 |
| C3—H3A | 0.9300 | C15—H15A | 0.9300 |
| C4—C5 | 1.390 (2) | C16—H16A | 0.9600 |
| C4—H4A | 0.9300 | C16—H16B | 0.9600 |
| C5—C6 | 1.4082 (18) | C16—H16C | 0.9600 |
| C6—C7 | 1.4729 (18) | C17—H17A | 0.9600 |
| C7—C8 | 1.4641 (18) | C17—H17B | 0.9600 |
| C8—C9 | 1.3315 (17) | C17—H17C | 0.9600 |
| C8—H8A | 0.9300 | ||
| C5—O1—H1O1 | 106.0 (13) | C11—C10—C9 | 119.05 (11) |
| C13—O3—C17 | 118.65 (11) | C15—C10—C9 | 123.64 (11) |
| C2—C1—C6 | 122.82 (12) | C12—C11—C10 | 122.26 (11) |
| C2—C1—H1A | 118.6 | C12—C11—H11A | 118.9 |
| C6—C1—H1A | 118.6 | C10—C11—H11A | 118.9 |
| C1—C2—C3 | 117.18 (14) | C13—C12—C11 | 119.26 (11) |
| C1—C2—C16 | 121.70 (13) | C13—C12—H12A | 120.4 |
| C3—C2—C16 | 121.11 (14) | C11—C12—H12A | 120.4 |
| C4—C3—C2 | 122.06 (15) | O3—C13—C12 | 124.53 (11) |
| C4—C3—H3A | 119.0 | O3—C13—C14 | 115.97 (11) |
| C2—C3—H3A | 119.0 | C12—C13—C14 | 119.50 (12) |
| C3—C4—C5 | 120.69 (14) | C15—C14—C13 | 120.86 (12) |
| C3—C4—H4A | 119.7 | C15—C14—H14A | 119.6 |
| C5—C4—H4A | 119.7 | C13—C14—H14A | 119.6 |
| O1—C5—C4 | 118.37 (13) | C14—C15—C10 | 120.80 (12) |
| O1—C5—C6 | 122.08 (14) | C14—C15—H15A | 119.6 |
| C4—C5—C6 | 119.54 (14) | C10—C15—H15A | 119.6 |
| C1—C6—C5 | 117.70 (12) | C2—C16—H16A | 109.5 |
| C1—C6—C7 | 122.80 (11) | C2—C16—H16B | 109.5 |
| C5—C6—C7 | 119.49 (12) | H16A—C16—H16B | 109.5 |
| O2—C7—C8 | 119.66 (12) | C2—C16—H16C | 109.5 |
| O2—C7—C6 | 119.25 (12) | H16A—C16—H16C | 109.5 |
| C8—C7—C6 | 121.09 (11) | H16B—C16—H16C | 109.5 |
| C9—C8—C7 | 120.81 (12) | O3—C17—H17A | 109.5 |
| C9—C8—H8A | 119.6 | O3—C17—H17B | 109.5 |
| C7—C8—H8A | 119.6 | H17A—C17—H17B | 109.5 |
| C8—C9—C10 | 128.35 (12) | O3—C17—H17C | 109.5 |
| C8—C9—H9A | 115.8 | H17A—C17—H17C | 109.5 |
| C10—C9—H9A | 115.8 | H17B—C17—H17C | 109.5 |
| C11—C10—C15 | 117.30 (12) | ||
| C6—C1—C2—C3 | 1.0 (2) | O2—C7—C8—C9 | 3.8 (2) |
| C6—C1—C2—C16 | −177.83 (13) | C6—C7—C8—C9 | −176.22 (12) |
| C1—C2—C3—C4 | −1.3 (2) | C7—C8—C9—C10 | −178.59 (12) |
| C16—C2—C3—C4 | 177.45 (14) | C8—C9—C10—C11 | 178.78 (13) |
| C2—C3—C4—C5 | 0.5 (3) | C8—C9—C10—C15 | −0.7 (2) |
| C3—C4—C5—O1 | −178.70 (15) | C15—C10—C11—C12 | −0.5 (2) |
| C3—C4—C5—C6 | 0.7 (2) | C9—C10—C11—C12 | −179.97 (12) |
| C2—C1—C6—C5 | 0.22 (19) | C10—C11—C12—C13 | −0.4 (2) |
| C2—C1—C6—C7 | 179.43 (12) | C17—O3—C13—C12 | 0.6 (2) |
| O1—C5—C6—C1 | 178.32 (12) | C17—O3—C13—C14 | −179.83 (13) |
| C4—C5—C6—C1 | −1.1 (2) | C11—C12—C13—O3 | −179.21 (13) |
| O1—C5—C6—C7 | −0.9 (2) | C11—C12—C13—C14 | 1.2 (2) |
| C4—C5—C6—C7 | 179.72 (13) | O3—C13—C14—C15 | 179.21 (13) |
| C1—C6—C7—O2 | −178.73 (13) | C12—C13—C14—C15 | −1.2 (2) |
| C5—C6—C7—O2 | 0.47 (19) | C13—C14—C15—C10 | 0.3 (2) |
| C1—C6—C7—C8 | 1.27 (19) | C11—C10—C15—C14 | 0.5 (2) |
| C5—C6—C7—C8 | −179.54 (12) | C9—C10—C15—C14 | 179.98 (13) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1O1···O2 | 0.95 (2) | 1.65 (2) | 2.5112 (18) | 149.4 (19) |
| C11—H11A···O3i | 0.93 | 2.60 | 3.4317 (17) | 149. |
| C16—H16C···Cg1ii | 0.96 | 2.81 | 3.5800 (18) | 138 |
Symmetry codes: (i) x, −y+3/2, z+1/2; (ii) −x, −y, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HB5847).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Bruker (2009). SADABS, APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Chantrapromma, S., Horkaew, J., Suwunwong, T. & Fun, H.-K. (2009). Acta Cryst. E65, o2673–o2674. [DOI] [PMC free article] [PubMed]
- Chantrapromma, S., Suwunwong, T., Boonnak, N. & Fun, H.-K. (2010). Acta Cryst. E66, o312–o313. [DOI] [PMC free article] [PubMed]
- Fun, H.-K., Hemamalini, M., Samshuddin, S., Narayana, B. & Yathirajan, H. S. (2010). Acta Cryst. E66, o864–o865. [DOI] [PMC free article] [PubMed]
- Fun, H.-K., Suwunwong, T., Boonnak, N. & Chantrapromma, S. (2009). Acta Cryst. E65, o2168–o2169. [DOI] [PMC free article] [PubMed]
- Horkaew, J., Suwunwong, T., Chantrapromma, S., Karalai, C. & Fun, H.-K. (2010). Acta Cryst. E66, o800–o801. [DOI] [PMC free article] [PubMed]
- Jasinski, J. P., Butcher, R. J., Yathirajan, H. S., Sarojini, B. K. & Musthafa Khaleel, V. (2011). Acta Cryst. E67, o756. [DOI] [PMC free article] [PubMed]
- Lu, Y.-H., Wang, G.-Z., Zhou, C.-H. & Zhang, Y.-Y. (2009). Acta Cryst. E65, o1396. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Suwunwong, T., Chantrapromma, S., Karalai, C., Pakdeevanich, P. & Fun, H.-K. (2009). Acta Cryst. E65, o420–o421. [DOI] [PMC free article] [PubMed]
- Wang, G.-Z., Fang, B. & Zhou, C.-H. (2009). Acta Cryst. E65, o2619. [DOI] [PMC free article] [PubMed]
- Wang, X.-L., Wang, G.-Z., Geng, R.-X. & Zhou, C.-H. (2010). Acta Cryst. E66, o320. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811015054/hb5847sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811015054/hb5847Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811015054/hb5847Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




