Abstract
Human anaplasmosis (formerly human granulocytic ehrlichiosis) and human monocytic ehrlichiosis (HME) are emerging tick-borne infections caused by obligate intracellular bacteria in the family Anaplasmataceae. Clinical findings include fever, headache, myalgia, leukopenia, thrombocytopenia, and hepatic inflammatory injury. Whereas Ehrlichia chaffeensis (HME) often causes meningoencephalitis, this is rare with Anaplasma phagocytophilum infection. The abilities of infected primary host monocytes and neutrophils and of infected HL-60 cells to cross human umbilical vein endothelial cell-derived EA.hy926 cell barriers and human brain microvascular cells (BMEC), a human blood-brain barrier model, were studied. Uninfected monocyte/macrophages crossed endothelial cell barriers six times more efficiently than neutrophils. More E. chaffeensis-infected monocytes transmigrated than uninfected monocytes, whereas A. phagocytophilum suppressed neutrophil transmigration. Differences were not due to barrier dysfunction, as transendothelial cell resistivities were the same for uninfected cell controls. Similar results were obtained for HL-60 cells used as hosts for E. chaffeensis and A. phagocytophilum. Differential transmigration of E. chaffeensis- and A. phagocytophilum-infected leukocytes and HL-60 cells confirmed a role for the pathogen in modifying cell migratory capacity. These results support the hypothesis that Anaplasmataceae intracellular infections lead to unique pathogen-specific host cell functional alterations that are likely important for pathogen survival, pathogenesis, and disease induction.
Human infections caused by obligate intracellular bacteria in the family Anaplasmataceae are on the rise in the United States and around the world (4, 29). The predominant genera that include human pathogens are Anaplasma and Ehrlichia, and a unifying characteristic is that the bacteria infect peripheral blood leukocytes (10). Similarly, a unifying theme of diseases caused by these pathogens is the induction of a nonspecific febrile illness characterized by leukopenia, thrombocytopenia, and mild liver injury (4, 29). Both infections incite inflammatory tissue injury and increase the potential for opportunistic infections (25, 37). While Anaplasma phagocytophilum, the causative agent of human anaplasmosis (formerly human granulocytic ehrlichiosis, or HGE), infects neutrophils, Ehrlichia chaffeensis infects monocytes and macrophages and causes human monocytic ehrlichiosis. Despite the similar clinical manifestations, one key difference between the two diseases is that approximately 20% of E. chaffeensis infections include central nervous system (CNS) infection (meningoencephalitis or meningitis), whereas human CNS infection with A. phagocytophilum has never been convincingly demonstrated (1, 9, 29, 33). The mechanisms for induction of or resistance to meningoencephalitis after infection with these different bacteria are not understood. However, a limiting step in the development of CNS infection is the ability of the pathogen, or in the case of obligate intracellular pathogens the infected leukocyte, to transmigrate across the microvasculature that comprises the blood-brain barrier (15, 19, 32, 36). In the studies that follow, we present data which show that E. chaffeensis-infected monocytic cells transmigrate across in vitro models of systemic and brain microvascular endothelial cell barriers far more efficiently than A. phagocytophilum-infected cells migrate under similar circumstances. Moreover, these data indicate the critical role of the pathogen in manipulating the infected cell toward transmigration of endothelial cell barriers.
MATERIALS AND METHODS
Preparation of A. phagocytophilum- and E. chaffeensis-infected HL-60 cells.
A. phagocytophilum Webster strain was propagated in HL-60 cells in RPMI 1640 medium (GIBCO-BRL) supplemented with 5% fetal bovine serum (FBS; GIBCO-BRL) and 2 mM l-glutamine (GIBCO-BRL) at 37°C in 5% CO2. Although we first sought to compare the transmigrating abilities of infected primary cells, because of the potential for introducing a confounding factor with the use of different cells we initially took advantage of the bifunctional capacity of HL-60 cells to be used as models for the study of neutrophils and monocyte/macrophages and the capacity of these cells to support the growth of both E. chaffeensis and A. phagocytophilum (16). E. chaffeensis Arkansas strain (provided courtesy of Jackie Dawson, Centers for Disease Control and Prevention, Atlanta, Ga.) was initially propagated in the canine DH82 histiocytic cell line in minimal essential medium (MEM) supplemented with 5% FBS and 2 mM l-glutamine. Cell-free E. chaffeensis bacteria were prepared by lysis of heavily infected DH82 cells after serial passage through a 26-gauge syringe needle. Intact DH82 cells were removed by centrifugation at 500 × g. The supernatant was examined for the presence of any residual infected cells and for cell-free bacteria by Romanowsky staining (HEMA 3; Biochemical Science Inc., Swedesboro, N.J.). Cell-free bacteria in the supernatant were then transferred to uninfected HL-60 cells cultivated in the same medium used for culture of A. phagocytophilum-infected cells. The viability of cells was determined using trypan blue exclusion and was always >95%. Infection of cells was monitored by Romanowsky staining.
Preparation of A. phagocytophilum-infected neutrophils and E. chaffeensis-infected monocytes.
Human peripheral blood neutrophils and monocytes were isolated from EDTA-anticoagulated blood of normal donors by dextran sedimentation followed by Ficoll density gradient centrifugation (Histopaque 1077; Sigma, St. Louis, Mo.). The contaminating residual erythrocytes present in the neutrophil preparations were lysed by exposure to hypotonic solution (0.2% NaCl) for 30 s and then adjusted back to isosmotic conditions with hypertonic (1.8%) NaCl prior to washing in tissue culture medium. The purified cells were suspended in MEM (monocytes) or RPMI 1640 (neutrophils) supplemented with 5% FBS and then incubated at 37°C in 5% CO2 in a humidified environment. The monocyte/macrophage cultures were allowed to become confluent by growth for 3 to 4 days prior to use. Neutrophil cultures were used immediately.
For the infection of neutrophils or monocytes by A. phagocytophilum or E. chaffeensis, cell-free bacteria were prepared by lysis of heavily infected HL-60 cells and DH82 cells after three to five serial passages through a 26-gauge syringe needle. Intact cells were removed by centrifugation as above, and after confirmation of purity by microscopic evaluation the cell-free bacteria were used to infect the purified neutrophil and monocyte/macrophage cultures. After overnight incubation at 37°C in 5% CO2, the viability of cells and the proportion of infected cells were determined as above. Routinely, overnight infection of neutrophils by A. phagocytophilum yielded a preparation with >95% viable neutrophils, of which nearly 100% were infected. Similarly, overnight infection of monocyte/macrophages by E. chaffeensis generally yielded >95% viable cells, of which 50% were infected.
Endothelial cells.
Human brain microvascular endothelial cells (BMEC) transformed by the simian virus 40 large T antigen (35) were propagated in RPMI 1640 medium supplemented with 20% heat-inactivated FBS (Omega Scientific Inc., Tarzana, Calif.), 2 mM l-glutamine, 1 mM MEM sodium pyruvate (GIBCO), 1× MEM nonessential amino acid solution (Sigma), and 1× MEM vitamin solution (Sigma). These cells, which have been shown to exhibit the hallmark characteristics of the endothelium of the blood-brain barrier, have been extensively used to examine how bacteria (Escherichia coli, group B Streptococcus and Streptococcus pneumoniae, and Citrobacter spp.), monocytes, viruses (human immunodeficiency virus), and fungi (Candida albicans) enter the brain (13, 14, 17-19, 21, 28, 32). EA.hy926 cells, which were derived as a fusion of A549 cells with human umbilical vein endothelial cells (HUVEC) and are frequently used as a model of systemic endothelial cells (5, 12), were grown in high-glucose (4.5 g/liter) Dulbecco's modified Eagle medium (GIBCO) supplemented with 10% heat-inactivated FBS and 1× hypoxanthine-thymine supplement (GIBCO). Both endothelial cell cultures were plated and propagated in 25-cm2 flasks (Sarstedt Inc., Newton, N.C.).
Endothelial monolayer transmigration assay.
The confluent cells were removed from the flasks using trypsin-EDTA solution and adjusted to 105/ml. Human BMEC and EA.hy926 cells were seeded (200 μl) on top of collagen-coated semipermeable Transwell polycarbonate tissue culture inserts (6.5-mm diameter [0.33 cm2], with a 3.0-μm pore size; Corning Costar Corp.). This in vitro model allows us separate access to the upper compartment (blood side) and lower compartment (brain or tissue side). The cells were cultured for 5 to 7 days in appropriate medium. Medium in both the top and bottom chambers was changed every other day. One day before the experiments, the culture medium was changed to experimental medium consisting of Ham's F-12 nutrient medium diluted 1:1 with medium M199 supplemented with 20% FBS and 2 mM l-glutamine (experimental medium) and incubated overnight. Electrical resistance measurements using an Endohm chamber with an EVOM voltometer (World Precision Instruments, Sarasota, Fla.) were used to determine monolayer integrity and are expressed as ohms times centimeters squared, as per the manufacturer's recommendation, after adjustment for the resistivity of the membrane itself.
Some endothelial cell cultures were stimulated with 80 ng of recombinant human tumor necrosis factor alpha (TNF-α; R&D Systems, Inc., Minneapolis, Minn.)/ml for 4 h at 37°C in 5% CO2 to induce expression of P-selectin and E-selectin on endothelial cell surfaces. Expression of selectins on these cell lines after exposure to TNF-α was previously confirmed by flow cytometry (data not shown).
Prior to the experiments, neutrophils, peripheral blood monocyte-derived macrophages, or HL-60 cells that were uninfected or infected with A. phagocytophilum or E. chaffeensis were labeled with PKH67 green fluorescent dye (a cell tracker dye from Sigma Chemical Co.) according to the manufacturer's instructions. After labeling, the cells were washed and checked for adequate labeling by fluorescence microscopy. The labeled cells were suspended to a concentration of 106 cells/ml in experimental medium, of which 200 μl of the cell suspensions was added to the upper chamber containing the appropriate Transwell inserts in 24-well plates. One milliliter of experimental medium was added into the lower wells. Cultures were incubated overnight at 37°C in 5% CO2. After 4 to 5 h, the Transwell inserts were transferred into new 24-well plates with wells containing 1 ml of fresh experimental medium and incubated overnight. Cells in the bottom chambers were counted after 4 to 5 h and after overnight incubation using a fluorescence microscope. Electrical resistance measurements of monolayer integrity were done before and after the experiments. All experiments were conducted in at least triplicate cultures. Experiments utilizing HL-60 cells were conducted four times; those using primary cells were conducted in duplicate.
Pilot experiments using uninfected undifferentiated HL-60 cells, neutrophils, and monocytes revealed cell transmigration of similar magnitude as observed in previous reports (13, 14, 31, 34), with the exception of neutrophil transmigration of human BMEC, which has not previously been assessed.
Statistical analysis.
All statistical evaluations were conducted using paired or unpaired one-tailed Student's t tests; P values of <0.05 were considered significant.
Human subjects.
Neutrophils and monocytes were obtained from human volunteers with the approval of the Johns Hopkins University School of Medicine Institutional Review Board, and all research conducted with these materials was done so in accordance with the Declaration of Helsinki principles.
RESULTS
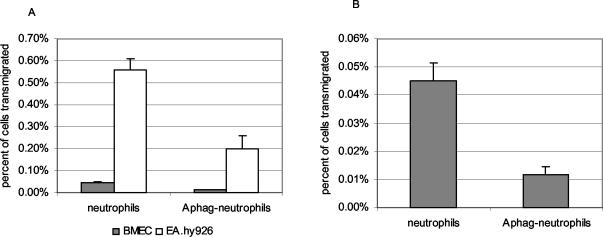
E. chaffeensis-infected monocytes/macrophages cross endothelial cell barriers more efficiently than uninfected or A. phagocytophilum-infected neutrophils.
Resistivity measurements for both cell lines were similar to the observations of other investigators reported in the published literature (6, 14, 21, 28, 34). We first examined the ability of infected and uninfected leukocytes to cross human BMEC and EA.hy926 barriers. True to their blood-brain barrier function, human BMEC consistently formed tighter cell barriers than did EA.hy926 cells; i.e., the resistivities of the BMEC monolayers were an average of 2.3-fold higher than those for EA.hy926, but neither cell line demonstrated significant alterations in resistivity after 4 h of TNF-α stimulation (data not shown).
Thus, it was anticipated that leukocytes would cross EA.hy926 cells more easily than human BMEC. Specifically, normal monocytes/macrophages crossed both endothelial cell barriers approximately six times more efficiently than did neutrophils with or without recombinant TNF-α (rTNFα; P < 0.001) (Fig. 1). While E. chaffeensis-infected monocyte/macrophages crossed rTNF-α-activated endothelial cell barriers as well as or more efficiently than uninfected monocyte/macrophages (BMEC, P = 0.014; EA.hy926, P = 0.075), the ability of A. phagocytophilum-infected neutrophils to cross both BMEC and EA.hy926 cells was usually suppressed compared to the abilities of normal neutrophils (BMEC, P = 0.011; EA.hy926, P = 0.010) (Fig. 2). Similar results were obtained when unstimulated endothelial cell cultures were used, although TNF-α treatment did allow modestly enhanced transmigration of both monocyte/macrophages and neutrophils, whether infected or uninfected (data not shown).
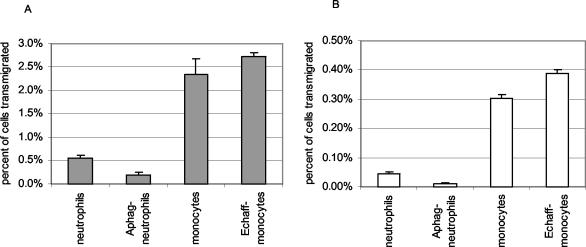
FIG. 1.
Total transmigration of infected and uninfected primary monocyte/macrophage cells and neutrophils through TNF-α-stimulated in vitro models of systemic endothelial cells (EA.hy926) (A) and endothelium of the blood-brain barrier (BMEC) (B) after 18 h. The number of transmigrating cells was counted after 5 to 8 h and again after 18 h. E. chaffeensis-infected (Echaff-monocytes) and uninfected monocytes transmigrated through both endothelial cell lines to a greater degree than either uninfected or A. phagocytophilum-infected neutrophils (Aphag-neutrophils) (P < 0.001). Although E. chaffeensis-infected monocytes migrated through both endothelial cell lines more rapidly than uninfected monocytes, by 18 h the difference was significant only for BMEC (P = 0.014).
FIG. 2.
Compared to uninfected neutrophils, A. phagocytophilum infection of neutrophils (Aphag-neutrophils) suppresses transmigration through both EA.hy926 systemic endothelial cell barrier (A and B) and BMEC blood-brain barrier (B) models (P < 0.012) at 18 h. Panel B shows the neutrophil data from panel A expanded to a different scale. Note the significant difference in migration through EA.hy926 cells compared with that through BMEC cells regardless of infection status of neutrophils.
The kinetics of transmigration varied for E. chaffeensis-infected monocyte/macrophages and uninfected cells. Normal monocyte/macrophages crossed both the human BMEC and EA.hy926 barriers to a higher degree during the first 5 h (P = 0.017 and P = 0.001, respectively); while somewhat delayed during the initial 5 h, infected cells crossed to a higher degree between 5 and 18 h (P = 0.007 and P = 0.030, respectively). In contrast, A. phagocytophilum-infected neutrophils did not cross human BMEC during the first 5 h, a time period during which only 0.005% of uninfected neutrophils crossed (BMEC, P = 0.010; EA.hy926, P = 0.001). The slow transmigration persisted so that by 18 h, 0.04 and 0.56% of uninfected neutrophils had transmigrated BMEC and EA.hy926 cell monolayers, respectively. During this same interval, considerably fewer A. phagocytophilum-infected neutrophils had transmigrated compared to uninfected neutrophils (0.01% for BMEC [P = 0.010] and 0.20% for EA.hy925 [P = 0.007]). Although minor differences in the magnitude of transmigrating cells were observed between experiments, the overall differences between transmigration of uninfected cells and cells infected with either bacterium were not statistically different.
Increased transmigration of E. chaffeensis-infected monocyte/macrophages could not be attributed to loss of endothelial cell barrier function, as human BMEC resistivity continued to increase over the entire 18 h of the experiment, and no differences in EA.hy926 resistivity change were noted regardless of whether HL-60 cells were infected or whether endothelial cells were TNF-α activated (P = 0.239 to 0.333).
E. chaffeensis-infected HL-60 cells transmigrate through endothelial cell barriers more than uninfected or A. phagocytophilum-infected HL-60 cells.
The observation that E. chaffeensis- and A. phagocytophilum-infected leukocytes differentially crossed endothelial cell barriers prompted our investigation into whether the effect was related only to the infected cell or whether the infecting bacterium could influence transmigration. Thus, undifferentiated HL-60 cells infected with either E. chaffeensis or A. phagocytophilum were used in identical endothelial cell transmigration assays. As for the assays conducted using primary monocyte/macrophages and neutrophils, many more HL-60 cells, infected and uninfected, passed through EA.hy926 cells than through human BMEC (Fig. 3A). In repeated experiments, only a very small proportion of total cells migrated through human BMEC during the first 5 h, ranging from 0.003% of E. chaffeensis-infected cells to 0 to 0.003% of A. phagocytophilum-infected and uninfected HL-60 cells, respectively. The majority of human BMEC-transmigrating cells were observed during the interval 5 to 18 h after inoculation, when approximately 5- to 10-fold more (maximum, 0.02%) E. chaffeensis-infected HL-60 cells and many fewer uninfected HL-60 cells (maximum, 0.003%) and A. phagocytophilum-infected HL-60 cells (maximum, 0.005%) transmigrated (Fig. 3B). Prior activation of human BMEC with TNF-α did not enhance overall transmigration of either infected or uninfected cells.
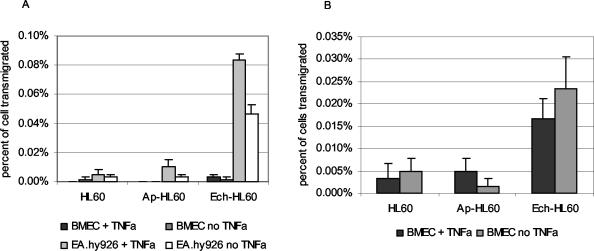
FIG. 3.
Transmigration of uninfected, A. phagocytophilum-infected (Ap-HL60), and E. chaffeensis-infected HL-60 (Ech-HL60) cells through both EA.hy926 systemic endothelial cell barrier and BMEC blood-brain barrier models mimics the findings with infected primary leukocytes. Note the significantly increased transmigration of E. chaffeensis-infected HL-60 cells through EA.hy926 endothelial cells after 5 h, compared with that of either uninfected or A. phagocytophilum-infected cells (P < 0.006) (A). Similarly, E. chaffeensis-infected HL-60 cells, but not A. phagocytophilum-infected HL-60 cells, transmigrated by 18 h through the BMEC blood-brain barrier model more than uninfected HL-60 cells (P < 0.04), regardless of pretreatment of endothelial cells with TNF-α. A. phagocytophilum-infected HL-60 cells migrated through both EA.hy926 and BMEC endothelial cells to the same degree or less than uninfected cells (B). The results are averages of triplicate cultures and are representative of at least four replicated experiments.
In contrast, significant transmigration through the systemic endothelial cell barrier model EA.hy926 cells occurred throughout the entire 18-h period, including during the first 5 h. TNF-α activation of EA.hy926 cells enhanced early migration of infected and uninfected HL-60 cells, but only E. chaffeensis-infected HL-60 cells transmigrated more through TNF-α-activated EA.hy926 cells overall (P = 0.001), although the difference was small (0.25 versus 0.20%) (Fig. 3A).
E. chaffeensis-infected HL-60 cells transmigrated through rTNF-α-activated (P < 0.001) and unstimulated (P < 0.006) BMEC to a significantly higher degree than uninfected or A. phagocytophilum-infected HL-60 cells after overnight incubation (Fig. 3A), but not by 5 h (data not shown). In contrast, by 5 h E. chaffeensis-infected HL-60 cell transmigration through TNF-α-activated or unstimulated EA.hy926 endothelial cells had occurred to a degree greater than that with either uninfected (P < 0.03) or A. phagocytophilum-infected HL-60 cells (P < 0.04) (Fig. 3B). A. phagocytophilum-infected HL-60 cells migrated similarly to or in smaller numbers than uninfected HL-60 cells at all time points examined, regardless of endothelial cell type and TNF-α stimulation (Fig. 3).
Transendothelial electrical resistivity of EA.hy926 cells and human BMEC.
Transendothelial resistance measurements were conducted in order to estimate overall endothelial cell barrier integrity after conclusion of the experiments. In general, resistivity stayed high throughout all experiments, regardless of manipulations by adding infected or uninfected HL-60 cells, or with TNF-α activation. The average starting resistance reading for the 6.5-mm inserts for human BMEC cultures was 25.9 ± 1.35 Ω · cm2 (range, 21.9 to 28.2), and no significant differences were observed when the cells were TNF-α activated. The overall starting electrical resistance of the EA.hy926 cells was lower, with a mean of 11.4 ± 0.86 Ω · cm2 (range, 9.6 to 12.9), and in only one of four experiments did resistivity become significantly lower with TNF-α pretreatment. The observed differences in electrical resistance clearly highlight the barrier function of BMEC as reflected in the profound reduction in leukocyte migration across BMEC compared to their migration across HUVEC. Inconsistently, E. chaffeensis-infected HL-60 cells stimulated decreased resistivity in either human BMEC or EA.hy926 cultures, but the differences were generally very small and within the range of variation observed at the beginning of experiments, except in one instance for EA.hy926 cells (mean resistivity change, −3.3 Ω · cm2). No differences in resistivity between cultures incubated with uninfected and A. phagocytophilum-infected HL-60 cells were observed. In one experiment, the electrical resistance continued to rise despite incubation with infected and uninfected HL-60 cells, excluding a role for endothelial barrier dysfunction in transmigration.
DISCUSSION
How infectious agents penetrate through the blood-brain barrier as a prerequisite for entry into the CNS, a critical event before meningoencephalitis, is an area of active study by many investigators (13, 17-19, 21, 28). Similarly, the mechanisms by which inflammatory cells respond to CNS and systemic infections and pass through the blood-brain barrier or systemic vasculature into tissues is increasingly the focus of study (5, 14, 15, 32). The entry of obligate intracellular bacteria into the CNS and into tissues poses issues critical to both arenas of study because of the strict requirement of host cell association. Among the most intriguing of these situations is the passage through systemic and brain endothelial cell barriers of leukocytes infected with members of the Anaplasmataceae family, especially including E. chaffeensis and A. phagocytophilum.
From a microbiological perspective, A. phagocytophilum and E. chaffeensis are related but unique organisms. Although there are significant differences between these two organisms, one key aspect that differentiates the two is that the former infects neutrophils and the latter infects mononuclear phagocytes (29). From a clinical perspective, these two pathogens induce similar systemic diseases, with the predominant exception that meningoencephalitis is a frequent component of monocytic ehrlichiosis (E. chaffeensis infection), whereas CNS infection with anaplasmosis or HGE (A. phagocytophilum infection) is exceedingly rare (1, 9, 29). Moreover, monocytic ehrlichiosis has a higher rate of mortality and appears to be a more severe infection, while anaplasmosis (HGE) is associated with moderate inflammatory reactions sometimes accompanied by manifestations of immune suppression and neutrophil dysfunction (1, 25, 29, 30). The results of this study suggest that some of the clinical observations recorded may relate to dramatic differences in how leukocytes penetrate endothelial cell barriers, including the blood-brain barrier, differences that can be modulated in the host leukocyte by the bacterium. This novel observation provides support for the emerging concept that interactions of Anaplasmataceae bacteria with the host cells lead to a dynamic alteration in leukocyte function modulated by the bacterium to a great extent, an event that may substantially contribute to human disease (29, 30).
E. chaffeensis-infected primary monocyte/macrophages rapidly and more effectively penetrate endothelial cell barriers than do either uninfected monocytes/macrophages, neutrophils, or A. phagocytophilum-infected neutrophils. However, this observation could potentially be explained by differences in the nature of the host cells, their receptors, and other factors unrelated to the bacterium itself. This is no moot point, as it is well established that neutrophil migration occurs at a rate less than, by about 14%, that of monocytes from the same donor (27). By using undifferentiated HL-60 cell cultures that are established as models of both neutrophil and macrophage biology (14, 15, 20, 23, 31), we reasoned that it might be possible to ascertain whether the differences in endothelial cell barrier penetration result from cell lineage factors or from bacterium-induced cell changes. That E. chaffeensis-infected HL-60 cells mimic the increased penetration and rate of transmigration observed with primary cells and not observed with uninfected or A. phagocytophilum-infected HL-60 cells suggests a highly significant role for the pathogen in this process. Of additional interest is the lack of any change or the reduction in migration observed when neutrophils and HL-60 cells are infected with A. phagocytophilum. Because transendothelial resistance measurements were not significantly different among the endothelial cell monolayer cultures with infected and uninfected leukocytes, it is highly unlikely that injury to endothelial cell barrier function accounts for the increased transmigration observed with E. chaffeensis. This is consistent with the observation that tight junctions are maintained after paracellular migration of monocytes (14) and neutrophils (6).
The observations are consistent with other data regarding the effects of these species on their respective host cells. E. chaffeensis is known to enter into early endosomes that are then directed into a salvage-recycling pathway to avoid lysosomal fusion of the infected vacuole (3). However, infection in the presence of antibody allows complexes to bind Fc receptors that initiate signal transduction cascades, NF-κB translocation after degradation of IκB, and significant induction of proinflammatory cytokines (24). Empirically, E. chaffeensis infections are more severely inflammatory than A. phagocytophilum infections, and significant degrees of tissue inflammation and cerebrospinal fluid pleocytosis, which may include infected cells, are often observed (11, 30, 33, 37). In contrast, infection of neutrophils with A. phagocytophilum leads to inhibition of the respiratory burst via repression of rac-2 mRNA (7) and downregulation of gp91phox or proteolysis of p22phox (2, 26), secretion of chemokines but not proinflammatory cytokines (22), inhibition of neutrophil phagocytosis and microbicidal activity (38), and loss of surface selectin expression that mediates interactions with endothelial cells (8). The loss of surface selectin may explain the lack of transmigration through tight endothelial cell barriers, such as that in the in vitro blood-brain barrier used here, in spite of the unimpaired ability of infected cells to migrate through Transwell filters without endothelial cells or through porous endothelial cell barriers such as with the HUVEC-derived EA.hy926 cells. Moreover, these data are consistent with clinical observations that CNS infection by A. phagocytophilum is a rare event.
In this study, we analyzed quantitative transmigration of E. chaffeensis-infected, A. phagocytophilum-infected, and normal cells. The data confirm the significant role of the bacterial pathogens in modifying host cell behavior that potentially contributes directly to human disease. It is not possible to exclude whether the biological behavior observed resulted from direct effects of infected cells, direct effects or bacterial components in the medium, or both. However, the differential effects of the two phylogenetically related organisms suggest that the main effect is mediated at the level of the infected cell.
While increasing information about how A. phagocytophilum affects normal leukocyte and neutrophil function is available, detailed analyses of the mechanisms by which these bacteria control cellular and molecular functions are still lacking, especially for E. chaffeensis. The ability to transmigrate endothelial cells, especially those of the blood-brain barrier, requires specific cellular alterations, including those critical for binding and initiating transient endothelial cell alterations that permit passage. Thus, fruitful areas for continued study might examine how E. chaffeensis effects surface adhesion molecule expression on monocytes, how infected cells or products of infection in monocytes affect endothelial cell actin architecture and integrity of junctional components, and whether bacterial components, host cell components, or both directly influence transmigration. Likewise, beneficial areas for study of A. phagocytophilum infections may allow a greater focus on how cells that are in part functionally paralyzed continue to manage to induce continued inflammatory injury unrelated to the degree of bacteria present. Regardless, such studies will continue to emphasize the crucial differences in the biology among members of the Anaplasmataceae rather than proposing broad similarities in clinical disease by shared pathogenetic mechanisms.
Acknowledgments
This work was supported by grant ROI AI44102 to J.S.D. D.J.G. was supported in part by a grant from the Thomas Wilson Sanitarium for Children of Baltimore. J. Park and K.-S. Choi were supported in part by awards from the Korea Science and Engineering Foundation.
We thank Monique Stins and Kwang Sik Kim (Department of Pediatrics, Johns Hopkins School of Medicine) for the transfected human BMEC.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bakken, J. S., and J. S. Dumler. 2000. Human granulocytic ehrlichiosis. Clin. Infect. Dis. 31:554-560. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, R., J. Anguita, D. Roos, and E. Fikrig. 2000. Cutting edge: infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J. Immunol. 164:3946-3949. [DOI] [PubMed] [Google Scholar]
- 3.Barnewall, R. E., Y. Rikihisa, and E. H. Lee. 1997. Ehrlichia chaffeensis inclusions are early endosomes which selectively accumulate transferrin receptor. Infect. Immun. 65:1455-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco, J. R., and J. A. Oteo. 2002. Human granulocytic ehrlichiosis in Europe. Clin. Microbiol. Infect. 8:763-772. [DOI] [PubMed] [Google Scholar]
- 5.Bouis, D., G. A. Hospers, C. Meijer, G. Molema, and N. H. Mulder. 2001. Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis 4:91-102. [DOI] [PubMed] [Google Scholar]
- 6.Burns, A. R., R. A. Bowden, S. D. MacDonell, D. C. Walker, T. O. Odebunmi, E. M. Donnachie, S. I. Simon, M. L. Entman, and C. W. Smith. 2000. Analysis of tight junctions during neutrophil transendothelial migration. J. Cell Sci. 113:45-57. [DOI] [PubMed] [Google Scholar]
- 7.Carlyon, J. A., W. T. Chan, J. Galan, D. Roos, and E. Fikrig. 2002. Repression of rac2 mRNA expression by Anaplasma phagocytophilum is essential to the inhibition of superoxide production and bacterial proliferation. J. Immunol. 169:7009-7018. [DOI] [PubMed] [Google Scholar]
- 8.Choi, K. S., J. Garyu, J. Park, and J. S. Dumler. 2003. Diminished adhesion of Anaplasma phagocytophilum-infected neutrophils to endothelial cells is associated with reduced expression of leukocyte surface selectin. Infect. Immun. 71:4586-4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumler, J. S., and J. S. Bakken. 1998. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu. Rev. Med. 49:201-213. [DOI] [PubMed] [Google Scholar]
- 10.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 11.Dunn, B. E., T. P. Monson, J. S. Dumler, C. C. Morris, A. B. Westbrook, J. L. Duncan, J. E. Dawson, K. G. Sims, and B. E. Anderson. 1992. Identification of Ehrlichia chaffeensis morulae in cerebrospinal fluid mononuclear cells. J. Clin. Microbiol. 30:2207-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edgell, C. J., C. C. McDonald, and J. B. Graham. 1983. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA 80:3734-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiala, M., D. J. Looney, M. Stins, D. D. Way, L. Zhang, X. Gan, F. Chiappelli, E. S. Schweitzer, P. Shapshak, M. Weinand, M. C. Graves, M. Witte, and K. S. Kim. 1997. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol. Med. 3:553-564. [PMC free article] [PubMed] [Google Scholar]
- 14.Giri, R., Y. Shen, M. Stins, S. Du Yan, A. M. Schmidt, D. Stern, K. S. Kim, B. Zlokovic, and V. K. Kalra. 2000. β-Amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am. J. Physiol. Cell Physiol. 279:C1772-C1781. [DOI] [PubMed] [Google Scholar]
- 15.Gloor, S. M., M. Wachtel, M. F. Bolliger, H. Ishihara, R. Landmann, and K. Frei. 2001. Molecular and cellular permeability control at the blood-brain barrier. Brain Res. Rev. 36:258-264. [DOI] [PubMed] [Google Scholar]
- 16.Heimer, R., D. Tisdale, and J. E. Dawson. 1998. A single tissue culture system for the propagation of the agents of the human ehrlichioses. Am. J. Trop. Med. Hyg. 58:812-815. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman, J. A., J. L. Badger, Y. Zhang, S. H. Huang, and K. S. Kim. 2000. Escherichia coli K1 aslA contributes to invasion of brain microvascular endothelial cells in vitro and in vivo. Infect. Immun. 68:5062-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, S. H., C. Wass, Q. Fu, N. V. Prasadarao, M. Stins, and K. S. Kim. 1995. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect. Immun. 63:4470-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, S. H., M. F. Stins, and K. S. Kim. 2000. Bacterial penetration across the blood-brain barrier during the development of neonatal meningitis. Microbes Infect. 2:1237-1244. [DOI] [PubMed] [Google Scholar]
- 20.Jacob, G. S., J. K. Welply, P. R. Scudder, C. Kirmaier, S. Z. Abbas, S. C. Howard, J. L. Keene, J. J. Schmuke, K. Broschat, and C. Steininger. 1995. Studies on selectin-carbohydrate interactions. Adv. Exp. Med. Biol. 376:283-290. [DOI] [PubMed] [Google Scholar]
- 21.Jong, A. Y., M. F. Stins, S.-H. Huang, S. H. M. Chen, and K. S. Kim. 2001. Traversal of Candida albicans across human blood-brain barrier in vitro. Infect. Immun. 69:4536-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein, M. B., S. Hu, C. C. Chao, and J. L. Goodman. 2000. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J. Infect. Dis. 182:200-205. [DOI] [PubMed] [Google Scholar]
- 23.Lawson, N. D., and N. Berliner. 1999. Neutrophil maturation and the role of retinoic acid. Exp. Hematol. 27:1355-1367. [DOI] [PubMed] [Google Scholar]
- 24.Lee, E. H., and Y. Rikihisa. 1997. Anti-Ehrlichia chaffeensis antibody complexed with E. chaffeensis induces potent proinflammatory cytokine mRNA expression in human monocytes through sustained reduction of IκB-α and activation of NF-κB. Infect. Immun. 65:2890-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lepidi, H., J. E. Bunnell, M. E. Martin, J. E. Madigan, S. Stuen, and J. S. Dumler. 2000. Comparative pathology, and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am. J. Trop. Med. Hyg. 62:29-37. [DOI] [PubMed] [Google Scholar]
- 26.Mott, J., Y. Rikihisa, and S. Tsunawaki. 2002. Effects of Anaplasma phagocytophilum on NADPH oxidase components in human neutrophils and HL-60 cells. Infect. Immun. 70:1359-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller, W. A., S. A. Weigl, X. Deng, and D. M. Phillips. 1993. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 178:449-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nizet, V., K. S. Kim, M. Stins, M. Jonas, E. Y. Chi, D. Nguyen, and C. E. Rubens. 1997. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 65:5074-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olano, J. P., and D. H. Walker. 2002. Human ehrlichioses. Med. Clin. North Am. 86:375-392. [DOI] [PubMed] [Google Scholar]
- 30.Paddock, C. D., and J. E. Childs. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 16:37-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel, N. A., J. A. Patel, M. F. Stins, K. S. Kim, and S. L. Chang. 2001. Dexamethasone affects cytokine-mediated adhesion of HL-60 human promyelocytic leukemia cells to cultured dermal microvascular endothelial cells. Clin. Immunol. 99:387-394. [DOI] [PubMed] [Google Scholar]
- 32.Persidsky, Y., M. Stins, D. Way, M. H. Witte, M. Weinand, K. S. Kim, P. Bock, H. E. Gendelman, and M. Fiala. 1997. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J. Immunol. 158:3499-3510. [PubMed] [Google Scholar]
- 33.Ratnasamy, N., E. D. Everett, W. E. Roland, G. McDonald, and C. W. Caldwell. 1996. Central nervous system manifestations of human ehrlichiosis. Clin. Infect. Dis. 23:314-319. [DOI] [PubMed] [Google Scholar]
- 34.Sedgwick, J. B., I. Menon, J. E. Gern, and W. W. Busse. 2002. Effects of inflammatory cytokines on the permeability of human lung microvascular endothelial cell monolayers and differential eosinophil transmigration. J. Allergy Clin. Immunol. 110:752-756. [DOI] [PubMed] [Google Scholar]
- 35.Stins, M. F., N. V. Prasadarao, J. Zhou, M. Arditi, and K. S. Kim. 1997. Bovine brain microvascular endothelial cells transfected with SV40-large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease. In Vitro Cell Dev. Biol. Anim. 33:243-247. [DOI] [PubMed] [Google Scholar]
- 36.Stins, M. F., J. Badger, and K. Sik Kim. 2001. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb. Pathog. 30:19-28. [DOI] [PubMed] [Google Scholar]
- 37.Walker, D. H., and J. S. Dumler. 1997. Human monocytic and granulocytic ehrlichioses. Discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch. Pathol. Lab. Med. 121:785-791. [PubMed] [Google Scholar]
- 38.Whist, S. K., A. K. Storset, and H. J. Larsen. 2002. Functions of neutrophils in sheep experimentally infected with Ehrlichia phagocytophila. Vet. Immunol. Immunopathol. 86:183-193. [DOI] [PubMed] [Google Scholar]