Abstract
The structure of the organic cation in the title compound, C15H16N3 +·ClO4 −, contains two essentially planar rings. Mean planes fitted through all non-H atoms of each ring system have an r.m.s. deviation of 0.019 Å for the imidazole-based ring and 0.016 Å for the 2,6-dimethylphenyl ring. The angle between the two planes is 86.76 (2)°. In the crystal structure, N—H⋯O interactions form a one-dimensional chain, which propagates in the b-axis direction. C—H⋯O interactions are also found in the crystal packing.
Related literature
For background information on the Groebke–Blackburn synthesis, see: Bienaymé & Bouzid (1998 ▶); Blackburn et al. (1998 ▶); Groebke et al. (1998 ▶). For details of the chemical synthesis, see: Nichol et al. (2011 ▶); Sharma & Li (2011 ▶). For information on graph-set notation to describe hydrogen-bonding motifs, see: Bernstein et al. (1995 ▶).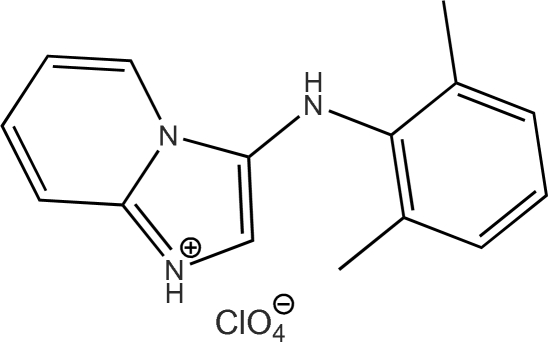
Experimental
Crystal data
C15H16N3 +·ClO4 −
M r = 337.76
Triclinic,

a = 8.6347 (3) Å
b = 8.7663 (3) Å
c = 11.5155 (4) Å
α = 70.668 (2)°
β = 73.131 (2)°
γ = 72.679 (2)°
V = 767.24 (5) Å3
Z = 2
Mo Kα radiation
μ = 0.27 mm−1
T = 100 K
0.26 × 0.16 × 0.16 mm
Data collection
Bruker Kappa APEXII DUO CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.932, T max = 0.957
27864 measured reflections
8640 independent reflections
6871 reflections with I > 2σ(I)
R int = 0.031
Refinement
R[F 2 > 2σ(F 2)] = 0.038
wR(F 2) = 0.111
S = 1.05
8640 reflections
272 parameters
All H-atom parameters refined
Δρmax = 0.61 e Å−3
Δρmin = −0.51 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶) and Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: SHELXTL and publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811014735/kj2175sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811014735/kj2175Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811014735/kj2175Isup3.cdx
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2N⋯O1i | 0.869 (16) | 1.955 (17) | 2.8169 (10) | 170.8 (15) |
| N3—H3N⋯O3 | 0.832 (16) | 2.216 (15) | 2.8899 (10) | 138.3 (14) |
| C2—H2⋯O2ii | 0.911 (14) | 2.547 (14) | 3.3826 (11) | 152.8 (12) |
| C3—H3⋯O4ii | 0.971 (15) | 2.559 (15) | 3.2893 (12) | 132.0 (11) |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
The diffractometer was purchased with funding from NSF grant No. CHE-0741837.
supplementary crystallographic information
Comment
The Groebke–Blackburn reaction is the most popular way to prepare imidazo-azines from 2-aminoazines in a single-step (Groebke et al., 1998; Bienaymé & Bouzid, 1998; Blackburn et al., 1998). We have recently reported developments on this synthetic method (Nichol et al., 2011; Sharma & Li, 2011) and present here the crystal structure of the title compound, determined as part of a larger study.
The asymmetric unit of the title compound is shown in Fig. 1. Molecular dimensions are unexceptional. Both ring systems are essentially planar (a mean plane fitted through atoms N1, N2, N3 C1 > C7 has an r.m.s. deviation of 0.019 Å; a mean plane fitted through atoms N3, C8 > C15 has an r.m.s. deviation of 0.016 Å) and the angle between both planes is 86.76 (2)°.
In the crystal, N—H···O interactions form a one-dimensional C22(9) chain (Bernstein et al. 1995), which propagates in the b-axis direction (Fig. 2). C—H···O interactions are also found in the crystal packing.
Experimental
The synthesis is described in Sharma & Li (2011).
Refinement
All H atoms were located from a difference Fourier map and are freely refined. N—H distances are 0.869 (16) and 0.832 (16) Å; C—H distances lie in the range 0.911 (4)–1.033 (17) Å.
Figures
Fig. 1.
The asymmetric unit of the title compound with displacement ellipsoids at the 50% probability level.
Fig. 2.
N—H···O interactions (dotted blue lines; dotted red lines indicate continuation) in the title compound.
Crystal data
| C15H16N3+·ClO4− | Z = 2 |
| Mr = 337.76 | F(000) = 352 |
| Triclinic, P1 | Dx = 1.462 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.6347 (3) Å | Cell parameters from 8544 reflections |
| b = 8.7663 (3) Å | θ = 2.5–39.0° |
| c = 11.5155 (4) Å | µ = 0.27 mm−1 |
| α = 70.668 (2)° | T = 100 K |
| β = 73.131 (2)° | Block, colourless |
| γ = 72.679 (2)° | 0.26 × 0.16 × 0.16 mm |
| V = 767.24 (5) Å3 |
Data collection
| Bruker Kappa APEXII DUO CCD diffractometer | 8640 independent reflections |
| Radiation source: fine-focus sealed tube with Miracol optics | 6871 reflections with I > 2σ(I) |
| graphite | Rint = 0.031 |
| φ and ω scans | θmax = 38.6°, θmin = 1.9° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −13→15 |
| Tmin = 0.932, Tmax = 0.957 | k = −15→15 |
| 27864 measured reflections | l = −20→20 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.038 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.111 | All H-atom parameters refined |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.0581P)2 + 0.1118P] where P = (Fo2 + 2Fc2)/3 |
| 8640 reflections | (Δ/σ)max = 0.001 |
| 272 parameters | Δρmax = 0.61 e Å−3 |
| 0 restraints | Δρmin = −0.51 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.98956 (8) | 0.60185 (8) | 0.27803 (6) | 0.01285 (10) | |
| N2 | 0.85450 (9) | 0.82744 (9) | 0.33586 (7) | 0.01631 (12) | |
| H2N | 0.825 (2) | 0.904 (2) | 0.3751 (15) | 0.032 (4)* | |
| N3 | 0.82987 (9) | 0.59792 (9) | 0.13756 (7) | 0.01541 (11) | |
| H3N | 0.782 (2) | 0.5193 (19) | 0.1717 (14) | 0.030 (4)* | |
| C1 | 1.10989 (10) | 0.45913 (10) | 0.27281 (8) | 0.01559 (13) | |
| H1 | 1.1040 (18) | 0.3970 (17) | 0.2222 (13) | 0.021 (3)* | |
| C2 | 1.22970 (11) | 0.41819 (11) | 0.33968 (8) | 0.01768 (14) | |
| H2 | 1.3107 (17) | 0.3243 (17) | 0.3371 (13) | 0.021 (3)* | |
| C3 | 1.23050 (11) | 0.52108 (11) | 0.41221 (8) | 0.01805 (14) | |
| H3 | 1.3177 (18) | 0.4918 (18) | 0.4584 (14) | 0.026 (3)* | |
| C4 | 1.11017 (11) | 0.66267 (11) | 0.41743 (8) | 0.01665 (13) | |
| H4 | 1.1091 (17) | 0.7333 (17) | 0.4609 (13) | 0.022 (3)* | |
| C5 | 0.98717 (10) | 0.70098 (9) | 0.34900 (7) | 0.01399 (12) | |
| C6 | 0.77067 (10) | 0.80959 (10) | 0.25805 (8) | 0.01618 (13) | |
| H6 | 0.6720 (18) | 0.8831 (18) | 0.2376 (14) | 0.025 (3)* | |
| C7 | 0.85317 (10) | 0.66985 (9) | 0.22032 (7) | 0.01359 (12) | |
| C8 | 0.77759 (10) | 0.70835 (9) | 0.02641 (7) | 0.01420 (12) | |
| C9 | 0.62087 (11) | 0.72027 (11) | 0.00791 (8) | 0.01716 (13) | |
| C10 | 0.57589 (12) | 0.82519 (12) | −0.10399 (9) | 0.02081 (15) | |
| H10 | 0.4688 (18) | 0.8271 (17) | −0.1195 (13) | 0.023 (3)* | |
| C11 | 0.68217 (13) | 0.91810 (12) | −0.19433 (9) | 0.02220 (16) | |
| H11 | 0.657 (2) | 0.9887 (19) | −0.2776 (15) | 0.033 (4)* | |
| C12 | 0.83699 (12) | 0.90525 (11) | −0.17397 (8) | 0.02083 (15) | |
| H12 | 0.915 (2) | 0.9649 (19) | −0.2370 (15) | 0.032 (4)* | |
| C13 | 0.88820 (11) | 0.79974 (10) | −0.06457 (8) | 0.01662 (13) | |
| C14 | 0.50193 (13) | 0.62394 (14) | 0.10725 (10) | 0.02599 (19) | |
| H14A | 0.549 (2) | 0.498 (2) | 0.1225 (15) | 0.035 (4)* | |
| H14B | 0.483 (2) | 0.655 (2) | 0.1867 (16) | 0.037 (4)* | |
| H14C | 0.394 (2) | 0.652 (2) | 0.0884 (16) | 0.037 (4)* | |
| C15 | 1.05888 (12) | 0.78164 (13) | −0.04629 (9) | 0.02178 (16) | |
| H15A | 1.0548 (18) | 0.8453 (18) | 0.0085 (14) | 0.027 (4)* | |
| H15B | 1.135 (2) | 0.819 (2) | −0.1279 (16) | 0.036 (4)* | |
| H15C | 1.1063 (19) | 0.6657 (19) | −0.0109 (14) | 0.028 (4)* | |
| Cl | 0.65990 (2) | 0.20252 (2) | 0.419315 (17) | 0.01454 (5) | |
| O1 | 0.79691 (9) | 0.07166 (9) | 0.46174 (7) | 0.02290 (13) | |
| O2 | 0.60990 (9) | 0.15985 (9) | 0.32753 (7) | 0.02488 (14) | |
| O3 | 0.71409 (13) | 0.35550 (10) | 0.36274 (8) | 0.03427 (19) | |
| O4 | 0.52640 (10) | 0.21967 (12) | 0.52460 (7) | 0.03483 (19) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0136 (3) | 0.0125 (2) | 0.0123 (2) | −0.0019 (2) | −0.0038 (2) | −0.0031 (2) |
| N2 | 0.0182 (3) | 0.0136 (3) | 0.0171 (3) | −0.0008 (2) | −0.0050 (2) | −0.0054 (2) |
| N3 | 0.0204 (3) | 0.0135 (3) | 0.0136 (3) | −0.0049 (2) | −0.0072 (2) | −0.0011 (2) |
| C1 | 0.0161 (3) | 0.0143 (3) | 0.0155 (3) | −0.0001 (2) | −0.0046 (2) | −0.0047 (2) |
| C2 | 0.0157 (3) | 0.0180 (3) | 0.0183 (3) | 0.0004 (3) | −0.0061 (3) | −0.0049 (3) |
| C3 | 0.0164 (3) | 0.0210 (3) | 0.0175 (3) | −0.0037 (3) | −0.0065 (3) | −0.0042 (3) |
| C4 | 0.0184 (3) | 0.0180 (3) | 0.0156 (3) | −0.0050 (3) | −0.0055 (3) | −0.0047 (3) |
| C5 | 0.0153 (3) | 0.0135 (3) | 0.0133 (3) | −0.0030 (2) | −0.0035 (2) | −0.0037 (2) |
| C6 | 0.0163 (3) | 0.0144 (3) | 0.0167 (3) | −0.0006 (2) | −0.0054 (2) | −0.0037 (2) |
| C7 | 0.0141 (3) | 0.0132 (3) | 0.0131 (3) | −0.0020 (2) | −0.0047 (2) | −0.0024 (2) |
| C8 | 0.0166 (3) | 0.0133 (3) | 0.0125 (3) | −0.0031 (2) | −0.0051 (2) | −0.0018 (2) |
| C9 | 0.0175 (3) | 0.0185 (3) | 0.0154 (3) | −0.0046 (3) | −0.0059 (3) | −0.0018 (3) |
| C10 | 0.0213 (4) | 0.0218 (4) | 0.0188 (3) | −0.0018 (3) | −0.0104 (3) | −0.0020 (3) |
| C11 | 0.0290 (4) | 0.0189 (3) | 0.0161 (3) | −0.0023 (3) | −0.0094 (3) | 0.0000 (3) |
| C12 | 0.0270 (4) | 0.0178 (3) | 0.0149 (3) | −0.0072 (3) | −0.0039 (3) | 0.0006 (3) |
| C13 | 0.0187 (3) | 0.0157 (3) | 0.0148 (3) | −0.0048 (3) | −0.0028 (2) | −0.0030 (2) |
| C14 | 0.0204 (4) | 0.0336 (5) | 0.0227 (4) | −0.0128 (4) | −0.0065 (3) | 0.0016 (4) |
| C15 | 0.0186 (4) | 0.0249 (4) | 0.0222 (4) | −0.0087 (3) | −0.0026 (3) | −0.0048 (3) |
| Cl | 0.01653 (8) | 0.01267 (7) | 0.01320 (7) | −0.00157 (5) | −0.00390 (6) | −0.00279 (5) |
| O1 | 0.0223 (3) | 0.0221 (3) | 0.0274 (3) | 0.0056 (2) | −0.0147 (3) | −0.0118 (3) |
| O2 | 0.0258 (3) | 0.0247 (3) | 0.0311 (4) | 0.0006 (3) | −0.0171 (3) | −0.0124 (3) |
| O3 | 0.0591 (6) | 0.0231 (3) | 0.0265 (4) | −0.0231 (4) | −0.0188 (4) | 0.0067 (3) |
| O4 | 0.0263 (4) | 0.0410 (5) | 0.0207 (3) | 0.0058 (3) | 0.0040 (3) | −0.0066 (3) |
Geometric parameters (Å, °)
| N1—C1 | 1.3741 (10) | C8—C13 | 1.4039 (11) |
| N1—C5 | 1.3687 (10) | C9—C10 | 1.3966 (12) |
| N1—C7 | 1.3992 (10) | C9—C14 | 1.5071 (13) |
| N2—H2N | 0.869 (16) | C10—H10 | 0.985 (14) |
| N2—C5 | 1.3408 (11) | C10—C11 | 1.3849 (14) |
| N2—C6 | 1.3740 (11) | C11—H11 | 0.999 (16) |
| N3—H3N | 0.832 (16) | C11—C12 | 1.3891 (14) |
| N3—C7 | 1.3896 (10) | C12—H12 | 0.966 (16) |
| N3—C8 | 1.4262 (10) | C12—C13 | 1.3951 (12) |
| C1—H1 | 0.939 (14) | C13—C15 | 1.5034 (13) |
| C1—C2 | 1.3602 (12) | C14—H14A | 1.033 (17) |
| C2—H2 | 0.911 (14) | C14—H14B | 0.994 (17) |
| C2—C3 | 1.4203 (12) | C14—H14C | 0.958 (17) |
| C3—H3 | 0.971 (15) | C15—H15A | 0.958 (15) |
| C3—C4 | 1.3671 (12) | C15—H15B | 0.994 (16) |
| C4—H4 | 0.912 (14) | C15—H15C | 0.971 (15) |
| C4—C5 | 1.3999 (11) | Cl—O1 | 1.4528 (7) |
| C6—H6 | 0.941 (15) | Cl—O2 | 1.4375 (7) |
| C6—C7 | 1.3601 (11) | Cl—O3 | 1.4353 (8) |
| C8—C9 | 1.3985 (11) | Cl—O4 | 1.4239 (8) |
| C1—N1—C5 | 121.77 (7) | C8—C9—C10 | 118.57 (8) |
| C1—N1—C7 | 129.50 (7) | C8—C9—C14 | 120.81 (7) |
| C5—N1—C7 | 108.71 (6) | C10—C9—C14 | 120.61 (8) |
| H2N—N2—C5 | 123.8 (11) | C9—C10—H10 | 118.3 (8) |
| H2N—N2—C6 | 126.6 (11) | C9—C10—C11 | 121.14 (8) |
| C5—N2—C6 | 109.48 (7) | H10—C10—C11 | 120.4 (8) |
| H3N—N3—C7 | 114.8 (11) | C10—C11—H11 | 123.1 (9) |
| H3N—N3—C8 | 114.5 (11) | C10—C11—C12 | 119.44 (8) |
| C7—N3—C8 | 116.63 (7) | H11—C11—C12 | 117.3 (9) |
| N1—C1—H1 | 117.3 (9) | C11—C12—H12 | 121.1 (9) |
| N1—C1—C2 | 118.21 (7) | C11—C12—C13 | 121.33 (8) |
| H1—C1—C2 | 124.5 (9) | H12—C12—C13 | 117.5 (9) |
| C1—C2—H2 | 119.5 (9) | C8—C13—C12 | 118.23 (8) |
| C1—C2—C3 | 120.71 (8) | C8—C13—C15 | 121.02 (7) |
| H2—C2—C3 | 119.8 (9) | C12—C13—C15 | 120.74 (8) |
| C2—C3—H3 | 120.1 (9) | C9—C14—H14A | 111.5 (9) |
| C2—C3—C4 | 120.84 (7) | C9—C14—H14B | 108.0 (10) |
| H3—C3—C4 | 119.1 (9) | C9—C14—H14C | 113.1 (10) |
| C3—C4—H4 | 123.1 (9) | H14A—C14—H14B | 109.3 (13) |
| C3—C4—C5 | 117.33 (8) | H14A—C14—H14C | 109.5 (14) |
| H4—C4—C5 | 119.6 (9) | H14B—C14—H14C | 105.1 (14) |
| N1—C5—N2 | 107.30 (7) | C13—C15—H15A | 110.8 (9) |
| N1—C5—C4 | 121.11 (7) | C13—C15—H15B | 111.2 (10) |
| N2—C5—C4 | 131.58 (8) | C13—C15—H15C | 109.7 (9) |
| N2—C6—H6 | 123.8 (9) | H15A—C15—H15B | 108.4 (13) |
| N2—C6—C7 | 108.34 (7) | H15A—C15—H15C | 109.6 (12) |
| H6—C6—C7 | 127.8 (9) | H15B—C15—H15C | 107.1 (13) |
| N1—C7—N3 | 120.77 (7) | O1—Cl—O2 | 108.58 (4) |
| N1—C7—C6 | 106.16 (7) | O1—Cl—O3 | 109.13 (5) |
| N3—C7—C6 | 132.97 (7) | O1—Cl—O4 | 109.21 (5) |
| N3—C8—C9 | 120.05 (7) | O2—Cl—O3 | 109.54 (5) |
| N3—C8—C13 | 118.66 (7) | O2—Cl—O4 | 110.96 (6) |
| C9—C8—C13 | 121.27 (7) | O3—Cl—O4 | 109.39 (6) |
| C5—N1—C1—C2 | −0.63 (12) | C1—N1—C7—C6 | 178.53 (8) |
| C7—N1—C1—C2 | −179.04 (8) | C5—N1—C7—N3 | 176.88 (7) |
| N1—C1—C2—C3 | −0.38 (13) | C5—N1—C7—C6 | −0.04 (9) |
| C1—C2—C3—C4 | 0.61 (13) | C7—N3—C8—C9 | −114.31 (9) |
| C2—C3—C4—C5 | 0.16 (13) | C7—N3—C8—C13 | 67.62 (10) |
| C6—N2—C5—N1 | 0.59 (9) | N3—C8—C9—C10 | −177.75 (8) |
| C6—N2—C5—C4 | −179.96 (9) | N3—C8—C9—C14 | 3.10 (13) |
| C1—N1—C5—N2 | −179.04 (7) | C13—C8—C9—C10 | 0.27 (13) |
| C1—N1—C5—C4 | 1.44 (12) | C13—C8—C9—C14 | −178.88 (9) |
| C7—N1—C5—N2 | −0.34 (9) | C8—C9—C10—C11 | −1.10 (14) |
| C7—N1—C5—C4 | −179.86 (7) | C14—C9—C10—C11 | 178.05 (10) |
| C3—C4—C5—N1 | −1.16 (12) | C9—C10—C11—C12 | 0.78 (15) |
| C3—C4—C5—N2 | 179.45 (9) | C10—C11—C12—C13 | 0.40 (15) |
| C5—N2—C6—C7 | −0.62 (10) | C11—C12—C13—C8 | −1.19 (14) |
| N2—C6—C7—N1 | 0.39 (9) | C11—C12—C13—C15 | 177.38 (9) |
| N2—C6—C7—N3 | −175.99 (8) | N3—C8—C13—C12 | 178.90 (8) |
| C8—N3—C7—N1 | −138.11 (8) | N3—C8—C13—C15 | 0.33 (12) |
| C8—N3—C7—C6 | 37.84 (13) | C9—C8—C13—C12 | 0.85 (13) |
| C1—N1—C7—N3 | −4.55 (12) | C9—C8—C13—C15 | −177.71 (8) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2N···O1i | 0.869 (16) | 1.955 (17) | 2.8169 (10) | 170.8 (15) |
| N3—H3N···O3 | 0.832 (16) | 2.216 (15) | 2.8899 (10) | 138.3 (14) |
| C2—H2···O2ii | 0.911 (14) | 2.547 (14) | 3.3826 (11) | 152.8 (12) |
| C3—H3···O4ii | 0.971 (15) | 2.559 (15) | 3.2893 (12) | 132.0 (11) |
Symmetry codes: (i) x, y+1, z; (ii) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: KJ2175).
References
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Bienaymé, H. & Bouzid, K. (1998). Angew. Chem. Int. Ed. 37, 2234–2237. [DOI] [PubMed]
- Blackburn, C., Guan, B., Fleming, P., Shiosaki, K. & Tsai, S. (1998). Tetrahedron Lett. 39, 3635–3638.
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Groebke, K., Weber, L. & Mehlin, F. (1998). Synlett, pp. 661–663.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Nichol, G. S., Sharma, A. & Li, H.-Y. (2011). Acta Cryst. E67, o833. [DOI] [PMC free article] [PubMed]
- Sharma, A. & Li, H.-Y. (2011). Synlett. In the press.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811014735/kj2175sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811014735/kj2175Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811014735/kj2175Isup3.cdx
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




