Abstract
The Staphylococcus aureus MSCRAMM (microbial surface components recognizing adhesive matrix molecules) protein clumping factor A (ClfA) has been shown to be a critical virulence factor in several experimental models of infection. This report describes the generation, characterization, and in vivo evaluation of a murine monoclonal antibody (MAb) against ClfA. Flow cytometric analysis revealed that MAb 12-9 recognized ClfA protein expressed by all of the clinical S. aureus strains obtained from a variety of sources. In assays measuring whole-cell S. aureus binding to human fibrinogen, MAb 12-9 inhibited S. aureus binding by over 90% and displaced up to 35% of the previously adherent S. aureus bacteria. Furthermore, a single infusion of MAb 12-9 was protective against an intravenous challenge with a methicillin-resistant strain of S. aureus in a murine sepsis model (P < 0.0001). These data suggest that anti-ClfA MAb 12-9 should be further investigated as a novel immunotherapy for the treatment and prevention of life-threatening S. aureus infections.
Staphylococcus aureus is an important pathogen that continues to cause a significant number of community-acquired (30) and nosocomial infections (39) worldwide. The sophisticated interplay between the host and bacterium is still not completely understood; however, successful colonization is presumed to be the defining event leading to initiation of an infection. MSCRAMM (microbial surface components recognizing adhesive matrix molecules) proteins are a family of cell surface adhesins that recognize and specifically bind to distinct extracellular components of host tissues or to serum-conditioned implanted biomaterials such as catheters, artificial joints, and vascular grafts (14, 33). Once S. aureus has successfully adhered to and colonized host tissues, expression of specific genes is altered, contributing to a phenotype that is more resistant to eradication by antibiotics (7). Therefore, intervention that impacts early events in the infectious process may lead to a beneficial clinical outcome.
The dramatic increase in methicillin-resistant bacteria, coupled with the recent emergence of vancomycin-resistant isolates (3), has accelerated and broadened the interest in developing novel therapeutics against S. aureus. MSCRAMM proteins provide an excellent target for immunological attack by antibodies. Antibodies against MSCRAMM proteins exhibit at least two biological properties. Initially, the highly specific antibodies prevent microbial adherence (6, 22, 27, 38, 49), as well as recolonization of host tissues or biomaterials. Secondly, the increased level of MSCRAMM protein antibodies bound to the bacterial cell wall facilitates rapid clearance of the organism through opsonophagocytosis (32, 40).
Clumping factor A (ClfA) is an MSCRAMM protein expressed by S. aureus that promotes binding of fibrinogen and fibrin to the bacterial cell surface (23, 25). ClfA is the prototype of a recently identified multigene family of cell surface proteins characterized by a common domain composed of a unique serine-aspartate repeat (17, 31). McDevitt and colleagues (23) originally cloned the gene encoding the fibrinogen-binding protein and showed that the clfA gene encodes a 933-amino-acid polypeptide that contains structural features characteristic of many cell surface-associated proteins from gram-positive bacteria, including a typical cell wall attachment region comprising an LPXTG motif, a hydrophobic transmembrane sequence, and a positively charged C terminus. The fibrinogen-binding domain of ClfA has been localized to a 218-residue segment within region A (22). Initially recognized for its role in fibrinogen binding, ClfA has recently been shown to mediate direct binding to human platelets (4, 44). The biological role of ClfA has been evaluated in experimental animal models of septic arthritis (16) and infective endocarditis (29, 48). In both models, isogenic mutants unable to express ClfA exhibited significantly reduced infectivity compared to complemented strains. These data were further corroborated by studies in which the clfA gene was cloned into a shuttle vector and expressed on the surface of Streptococcus gordonii (48) and Lactococcus lactis (37). The expression of clfA by the carrier strains conferred a significant increase in their ability to cause endocarditis in a rat model. In addition to studies involving genetic manipulation of the clfA gene, passive-immunization studies of mice with anti-ClfA antibodies have shown protection against S. aureus septic arthritis and sepsis-induced death (16). Taken together, these data indicate that ClfA is a valid target for the development of novel immunotherapeutic agents.
This report describes the identification, characterization, and in vivo evaluation of a murine monoclonal antibody (MAb) against ClfA. MAbs were selected on the basis of their ability to inhibit ClfA binding to fibrinogen, their kinetic profile, and their in vivo activity. A panel of more than 2,000 clones against ClfA was initially generated, and on the basis of affinity for ClfA and potent inhibitory activity, one MAb, designated 12-9, was selected for further study. The data presented here demonstrate that MAb 12-9 provides protection against a heterologous S. aureus challenge in a mouse model of sepsis and also possesses the desired biochemical characteristics of a MAb that could lead to a novel therapy for the prevention and treatment of life-threatening S. aureus infections.
MATERIALS AND METHODS
ClfA protein expression.
By PCR, the A domain of clfA (Clf40, representing amino acids 40 to 559) or an N-terminal truncated version (Clf33, representing amino acids 221 to 550) was amplified from S. aureus Newman genomic DNA and subcloned into Escherichia coli expression vector pQE-30 (Qiagen, Valencia, Calif.) for the expression of a recombinant fusion protein containing an N-terminal six-histidine-residue tag as described previously (24). The concentration of purified ClfA protein was analyzed with a bicinchoninic acid assay (Pierce Biochem., Rockford, Ill.). Protein purity was assessed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and endotoxin levels were analyzed by Limulus amebocyte lysate assay (Charles River, Wilmington, Mass.).
Mice and immunizations.
Female BALB/c mice, 4 to 6 weeks old, were purchased from Taconic (Germantown, N.Y.). Mice received a subcutaneous primary injection of 50 μg of rClfA(221-550) or rClfA(40-559) emulsified in complete Freund's adjuvant (Sigma, St. Louis, Mo.). Fourteen days postinjection, mice received an intravenous (i.v.) injection of 10 μg of rClfA(221-550) or rClfA(40-559) in phosphate-buffered saline (PBS). Three days post i.v. injection, mice were sacrificed by CO2 asphyxiation and spleens were removed for cell fusion. All mice were maintained in accordance with National Institutes of Health animal husbandry standards.
MAb production.
Lymphocytes prepared from each spleen were fused to an SP2/0-Ag14 (ATCC 1581) myeloma cell line and subsequently plated in hypoxanthine-aminopterin-thymidine selection medium. Polyethylene glycol-induced cell fusion, subsequent plating, and feeding were all performed in accordance with the production-of-MAbs protocol in Current Protocols in Immunology (51). Resulting hybridomas were screened 14 days following fusion by enzyme-linked immunosorbent assay (ELISA) for antibody recognition of rClfA(40-559) as described below. Five independent fusions were conducted to generate the panel of ClfA clones.
ELISA.
Antibody supernatants that had an optical density at 405 nm that was three or more times the background (medium alone) were considered positive. ELISA-positive clones were kept for further study by expansion into 24-well tissue culture plates and subsequent single-cell cloning.
Measurement of MAb binding by BIAcore.
Surface plasmon resonance (BIAcore 3000; BIAcore, Piscataway, N.J.) was used to test ELISA-positive clones for the ability to bind to rClfA(40-559) and for the ability to inhibit the interaction between rClfA(40-559) and human fibrinogen (Enzyme Research Lab, South Bend, Ind.). Throughout the analysis, the flow rate remained constant at 20 μl/min. Briefly, a rabbit anti-mouse Fcγ antibody was amine coupled to a CM5 chip (BIAcore). Test supernatants were run over the Fcγ chip to allow binding of the test antibody via the Fc region. At time zero, rClfA(40-559) at a concentration of 30 μg/ml was injected over the chip for 3 min, followed by 2 min of dissociation, at which time a 100-μg/ml solution of human fibrinogen in HEPES-buffered saline (BIAcore) was run over the Fcγ complex. The first phase of the analysis measured the relative association and disassociation kinetics of the interaction, while the second phase of the reaction was used to determine the inhibitory activity of the captured MAb.
Antibody scale-up and purification.
Each single-cell cloned hybridoma was grown in 7 liters of Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (HyClone, Logan, Utah), 1 mM sodium pyruvate (Sigma), and 2 mM l-glutamine (Sigma) in a 10-liter spinner flask within a humidified 37°C, 10% CO2 incubator. Hybridoma supernatants were harvested by centrifugation at 4°C and (2,620 × g and kept at −20°C until purification.
To purify the MAbs, supernatants were passed through 0.2-μm-pore-size filters and the immunoglobulin G (IgG) was affinity purified by protein G chromatography. The MAbs were eluted with 0.1 M glycine, pH 2.7, and immediately neutralized with 1/10 volume of 2 M Tris, pH 8.0. Samples containing antibody, as assessed by SDS-polyacrylamide gel electrophoresis, were pooled, and the purified IgG was dialyzed against 10 mM NaH2PO4-0.15 M NaCl-0.001% Tween 80, pH 7.4. The purified antibody was concentrated with Amicon ultrafiltration units and stored at 4°C.
ELISA-based inhibition assays.
Immulon 2-HB high-binding 96-well microtiter plates were coated with 1 μg of rClfA(40-559) per ml in 1× PBS, pH 7.4, and incubated overnight at 4°C. Eighteen hours later, the plates were washed and blocked with a 1% bovine serum albumin (BSA) solution for 1 h. Purified antibodies (anti-ClfA IgG1 MAbs 12-9, 15EC6, and 35-052 and isotype control MAb CRL-1771) were diluted in 1× PBS-0.05% Tween 20-0.1% BSA. Plates were washed, and twofold serial dilutions of purified antibodies were performed across the plate starting from 10 μg/ml. Plates were incubated with purified MAb for 1 h at room temperature. Following incubation with antibody, 20 μg of human fibrinogen per ml was added, the plates were incubated for 1 h at 37°C and washed, and a 1:4,000 dilution of goat anti-fibrinogen-horseradish peroxidase (Abcam Ltd., Cambridge, United Kingdom) in 1× PBS-0.05% Tween 20-0.1% BSA was added. Following incubation for 1 h at room temperature, plates were washed and a 1:1 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS)-H2O2 substrate mixture (KPL, Gaithersburg, Md.) was added. Plates then incubated for 10 min at room temperature, the reaction was stopped by addition of 10% SDS, and absorbance was read at 405 nm with a SpectraMax 190 Plate Reader (Molecular Devices Corp., Sunnyvale, Calif.). All data were analyzed with SOFTmax Pro v.3.1.2. software (Molecular Devices Corp.).
Bacterial strains.
Twenty-six S. aureus strains, representing both community-acquired and hospital-acquired isolates and representing different clonal complexes (10), were received from John Minogue (John Radcliffe Hospital, Oxford, United Kingdom). Strains 560 (SAL1), 203 (SAL2), 451 (SAL4), 206 (SAL5), and 397 (SAL6) (5) were received from Michael Gilmore (University of Oklahoma Health Sciences Center). Clinical isolates 49, 189, 203, and 4046 were received from Brad Allen (Indiana University School of Medicine).Methicillin-resistant S. aureus (MRSA) strain 67-0 was received from Arnold Bayer (Harbor-UCLA), and Newman wild-type (WT) and mutant strains (23) were received from Timothy Foster (Trinity College, Dublin, Ireland).
Flow cytometry.
MAb 12-9 or IgG1 isotype control MAb CRL-1771 was added to appropriate tubes containing the appropriate bacterial solution, vortexed, and incubated on ice for 30 min. Following incubation, the tubes were centrifuged and the supernatant was decanted, resuspended, and then washed twice more by centrifugation. After the final wash, the bacterial pellets were resuspended in a dilution of phycoerythrin-conjugated F(ab′)2 fragment and incubated on ice. The bacteria were washed twice with buffer, transferred to analysis tubes, and then stored on ice until analysis with a Becton Dickinson FACScalibur flow cytometer. The labeled cell suspensions were aspirated through the flow cytometer, and a fluorescence emission measurement (excitation wavelength, 488 nm; emission wavelength, 570 nm) was performed in which at least 10,000 events were collected and analyzed with the Cell Quest software provided with the flow cytometer. Aggregates and debris were omitted from the analysis by gating populations on the basis of the light scatter signal. A marker region was established for each strain to include less than 10% of the gated events as positive for CRL-1771 (serving as an isotype-matched negative control). The established region was used to determine percent positive events for the 12-9 MAb for each strain. In all cases, the background fluorescence recorded with bacteria with F(ab′)2 goat anti-mouse IgG-phycoerythrin alone was less than that obtained for CRL-1771.
Parallel-plate flow chamber and video microscopy system.
The details of the parallel-plate flow chamber, the protein-coating procedure, and the video microscopy system have been previously described (20, 26, 28). Fibrinogen was used to coat the glass slide at room temperature to yield a final concentration of 9.8 ± 0.9 μg/cm−2.
Detachment assay procedure.
To begin the detachment assay, the cell suspension was passed through the flow field at a shear rate of 300 s−1 until approximately five cells attached per field of view. The flow was then stopped, and cells were allowed to settle to the fibrinogen surface for approximately 4 to 5 min, when 30 to 40 cells attached per frame. The percent surface coverage by the attached cells was less than 1% in all cases. PBS buffer was then passed through the system for 3 min at a shear rate of 300 s−1 to remove unattached cells. Finally, the antibody solution (concentrations of 0.006 to 0.047 μmol/liter) was passed through the flow field at the desired shear rates (100, 300, and 1,000 s−1) for 10 min. These shear rates correspond to shear stresses in the range of 0.70 to 16 dynes cm−2. Images were acquired every minute for 10 min. The number of cells attached per frame was determined with NIH Image. Control experiments consisted of passing PBS or an irrelevant isotype-matched mouse antibody (CRL-1771) in place of the MAb solution for the 10 min at the desired shear rate. All detachment assays were run in triplicate, and the values reported represent the mean and standard error of the mean. Analysis of variance was used to determine statistical significance at a confidence level of 95% (α = 0.05).
In vivo sepsis study.
MRSA clinical isolate 67-0 (clfA+ clfB+ fnb+) bacterial cells were taken from a frozen glycerol stock, inoculated onto a single blood agar plate, and grown for 24 h at 37°C. Numerous blood agar plates were inoculated from this plate and incubated overnight. The bacteria were then collected, washed three times with PBS, and resuspended in freezing medium. The bacterial stock was aliquoted, snap-frozen in an ethanol-dry-ice bath, and placed in a −80°C freezer. On the day of injection, aliquots were thawed, combined into one tube, vortexed, and diluted to the appropriate concentration. The final concentration of organisms was calculated by plating on blood agar.
Female BALB/c mice, 5 to 6 weeks of age, were purchased from Taconic. Mice were allowed to acclimate for at least 7 days, randomized, and assigned to treatment groups with stratified body weights. All mice were placed on a 12-h light-dark cycle under the required husbandry standards found in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. In the first experiment, on day −1, mice (30 per group) were treated intraperitoneally with 0.3 mg of purified MAb 12-9 or MAb 35-052. On day 0, the mice were challenged with 2.0 × 107 CFU of MRSA 67-0 cells by a single i.v. injection (0.1 ml) via the tail vein. All animals were followed for 14 to 15 days, at which point all remaining mice were sacrificed. The second animal experiment was conducted as previously described, except that noninhibiting MAb 15EC6 was used as a test agent. In addition, S. aureus strain Newman (clfA+ clfB+ fnb+) was used as the challenge organism.
Statistical analysis.
Statistical evaluation of survival studies was carried out by Kaplan-Meier analysis. Means and standard deviations were calculated (Microsoft Excel) and survival data were analyzed with GraphPad's Prism Version 3 statistical analysis software. Determination of significance was conducted with a two-tailed log rank test (Mantel-Haenszel test). P < 0.05 was considered to be statistically significant.
RESULTS
Characterization of ClfA MAbs.
Hybridomas from the spleen fusions were first screened by ELISA for binding to rClfA(40-559). As a secondary screen, hybridoma clones were analyzed for high-affinity interaction with ClfA by BIAcore. Antibodies that were BIAcore positive were selected and single cell cloned by limiting dilution regardless of their ability to inhibit fibrinogen binding to ClfA. Antibodies from single-cell clones were isotyped with a mouse immunoglobulin isotyping cytometric bead array kit (BD Pharmingen). All of the MAbs described in this study were determined to be of the IgG1 subclass (data not shown). Through the use of this selection strategy, we were able to screen thousands of hybridoma clones and quickly identify hybridomas of interest for scale-up and further study.
BIAcore analysis was subsequently used to assess whether the ClfA MAbs could inhibit ClfA binding to immobilized fibrinogen. BIAcore was also used to determine antibody binding kinetics. Figure 1 shows the binding characteristics of two ClfA MAbs, 12-9 and 15EC6. Both MAbs effectively bound rClfA(40-559), as shown by an increase in the number of resonance units (RU) during the ClfA association phase (points B to C) and demonstrated a slow disassociation phase (points C to D). Binding of fibrinogen to the antibody-ClfA complex was shown by the increase in the number of RU from point D to point E upon the injection of fibrinogen. While fibrinogen clearly bound to the 15EC6-ClfA complex (with a difference of 800 RU), the binding of fibrinogen was significantly inhibited in the case of the 12-9-ClfA complex (with a difference of 200 RU), suggesting that MAb 12-9 recognizes a site on ClfA that is involved in fibrinogen binding. In addition, kinetic analysis for MAb 12-9 interaction with ClfA demonstrated an apparent ka of 1.99 × 106 M−1 s−1 and a kd of 4.18 × 10−4 s−1, while Kd was calculated to be 2.10 × 10−10 M.
FIG. 1.
Characterization of protein interactions by BIAcore. The binding characteristics of anti-ClfA MAbs 12-9 (solid line) and 15EC6 (broken line) compared to those of buffer alone (dotted line) were studied by BIAcore. The progressive real-time analysis begins at time point A, when the MAb of interest was adsorbed to the chip via rabbit anti-mouse Fcγ antibody binding. At time point B, rClfA(40-559) was injected over the chip. Point C represents the termination of the ClfA injection over the chip. Point D represents the initial injection of fibrinogen over the MAb-ClfA complex, while point E represents the termination of the fibrinogen injection over the chip.
Functional characterization of MAb 12-9.
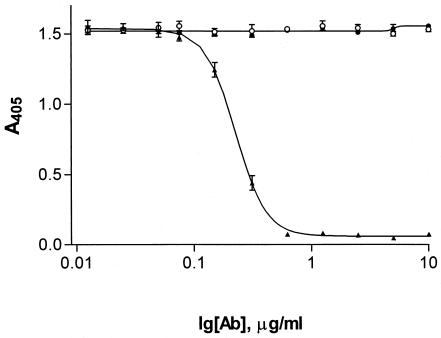
MAb 12-9 was chosen for further characterization on the basis of its ability to inhibit fibrinogen binding to ClfA. Figure 2 demonstrates the ability of MAb 12-9 to inhibit fibrinogen binding to ClfA, compared to 15EC6, a ClfA MAb that previously showed no inhibition by BIAcore analysis. While the IgG1 murine isotype control CRL-1771 (American Type Culture Collection) and 15EC6 showed little inhibition, 12-9 completely inhibited fibrinogen binding to ClfA, yielding a calculated 50% inhibitory concentration of 0.21 μg/ml. In comparison, CRL-1771 and 15EC6 never achieved 50% inhibition within the antibody concentration range tested in this study.
FIG. 2.
Antibody-dependent inhibition of fibrinogen binding to rClfA(40-559). Anti-ClfA MAbs 12-9 (filled triangles) and 15EC6 (filled circles) were tested at various concentrations (0 to 10 μg/ml) for the ability to inhibit human fibrinogen binding to rClfA(40-559) compared to that of isotype control CRL-1771 (open circles). Data are representative of at least three independent experiments.
Surface recognition of ClfA by MAb 12-9 among a panel of S. aureus strains.
The ability of MAb 12-9 to recognize the native ClfA protein was analyzed by flow cytometry. Twenty-one S. aureus isolates, including representatives of 11 different clonal genotype complexes (10), five highly prevalent phylogenetic S. aureus lineages (SALs) (5), and one clinical MRSA isolate were tested for reactivity against MAbs 12-9 and CRL-1771 (an isotype control). S. aureus Newman WT (from which the clfA gene was isolated), an S. aureus Newman ClfA knockout strain (Newman clfA::emr), and an S. aureus Newman protein A knockout strain (Newman spa::kan) served as controls in these studies. Table 1 shows that MAb 12-9 binds the surface of every bacterial strain tested, with the exception of the S. aureus ClfA knockout strain. These data demonstrate that the epitope within ClfA that MAb 12-9 recognizes is highly conserved throughout clinically relevant S. aureus strains. Interestingly, other ClfA MAbs previously identified, but not characterized in depth, recognized only a subset of the strains represented in Table 1 (data not shown).
TABLE 1.
Recognition of native cell surface expression of ClfA by MAb 12-9 among a panel of S. aureus isolates
| Strain | Sequence type | Clonal complex | Positively staining cells (%)
|
|
|---|---|---|---|---|
| MAb CRL-1771 | MAb 12-9 | |||
| 476 | 1 | 1 | 0.17 | 95.01 |
| 451 | 5 | 2 | 0.19 | 81.57 |
| 315 | 8 | 3 | 0.68 | 92.88 |
| 837 | 12 | 4 | 0.27 | 70.84 |
| 207 | 15 | 5 | 4.05 | 92.80 |
| 160 | 34 | 7 | 0.70 | 97.31 |
| 16 | 25 | 8 | 0.66 | 83.58 |
| 959 | 34 | 9 | 8.99 | 94.11 |
| 96 | 47 | 10 | 0.88 | 92.00 |
| 863 | 20 | 11 | 0.79 | 70.84 |
| 150 | 09 | 14 | 1.25 | 48.79 |
| MRSA 67-0 | NDa | ND | 1.25 | 60.97 |
| 560 SAL1 | ND | ND | 6.64 | 58.27 |
| 203 SAL2 | ND | ND | 2.45 | 88.42 |
| 451 SAL4 | ND | ND | 2.48 | 67.07 |
| 206 SAL5 | ND | ND | 2.88 | 74.27 |
| 397 SAL6 | ND | ND | 0.52 | 96.04 |
| Newman WT | ND | ND | 8.82 | 33.21 |
| Newman spa::kan | ND | ND | 5.85 | 71.8 |
| Newman clfA::emr | ND | ND | 1.98 | 1.71 |
ND, not determined.
Antibody-dependent inhibition of adhesion at various shear rates.
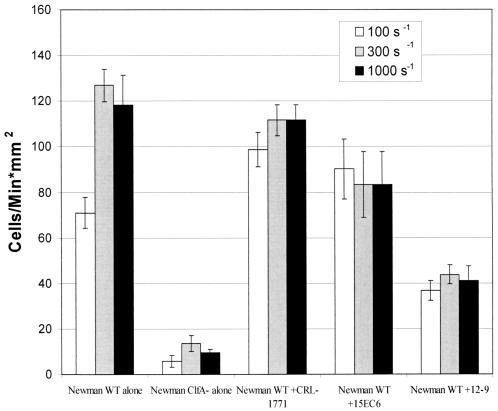
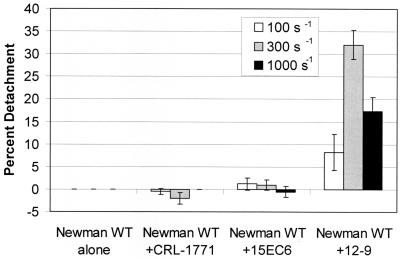
To measure the functional significance of inhibiting fibrinogen binding to ClfA expressed on the surface of S. aureus, we measured the dynamic binding and attachment of whole cells to fibrinogen-coated glass with a parallel-plate flow chamber. S. aureus Newman WT cells adhered to the fibrinogen-coated glass at shear rates of 100 to 1,000 s−1 (Fig. 3). Interestingly, the number of attached cells was uniform between shear rates of 300 and 2,300 s−1 (data not shown). This constant adhesion rate at high rates of shear (300 to 2,300 s−1) indicates that S. aureus binding to fibrinogen is not shear sensitive in the physiologic shear stress range. Inhibition of S. aureus Newman WT cell adherence to fibrinogen was demonstrated by preincubation with MAbs as shown in Fig. 3. It is important to note that the Newman ClfA knockout strain demonstrated a drastically reduced binding rate (<20 cells/min · mm2; Fig. 3; ClfA−). While the binding rate of the Newman WT strain incubated with 12-9 is slightly higher than that of the Newman ClfA knockout strain, studies have shown there was no statistically significant difference between the adhesion rates of these groups when MAb 12-9 was tested at a saturating concentration of 10 μg/ml (data not shown). These data support previous studies demonstrating that while ClfA plays a primary role in enhancing adhesion to fibrinogen-coated surfaces, it also increases the strength of the binding event (11). In this study, MAb 12-9 significantly inhibited the adhesion of S. aureus in the parallel-plate flow chamber assay, while MAb 15EC6, an antibody that binds to ClfA but does not inhibit ClfA binding to fibrinogen (Fig. 1 and 2), resulted in activity similar to that obtained with the isotype control, CRL-1771. The ability of MAb 12-9 to detach adherent S. aureus cells was also determined at various shear rates in the parallel-plate flow chamber assay, as shown in Fig. 4. In these experiments, MAb 12-9 detached 7, 32, and 17% of the bound S. aureus cells at shear rates of 100, 300, and 1,000 s−1, respectively. In contrast, MAbs 15EC6 and CRL-1771 failed to detach significant numbers (<3%) of adherent S. aureus cells (Fig. 4). Interestingly, less bacterial detachment was seen at the 1,000-s−1 flow rate, perhaps because the time of contact between the destabilizing antibody, MAb 12-9, and the target adhesin was insufficient in the flow field at the elevated shear rate.
FIG. 3.
Inhibition of adhesion by preincubation of the bacterial suspension with a MAb at a concentration of 5 μg/ml and shear rates of 100, 300, and 1,000 s−1. Data are representative of at least three independent experiments.
FIG. 4.
Newman destabilization experiments at an antibody concentration of 5 μg/ml and shear rates of 100, 300, and 1,000 s−1. S. aureus Newman WT alone was below the limit of detection. Data are representative of at least three independent experiments.
Efficacy of MAb 12-9 in a mouse model of sepsis.
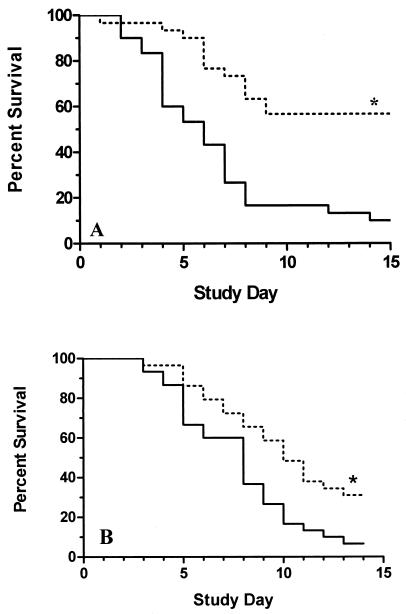
To evaluate whether anti-ClfA antibodies could protect mice against MRSA-induced death, two separate experiments were conducted. To investigate the nonspecific biological activity of MAbs bearing IgG1 Fc domains, MAb 35-052 was compared with MAb 12-9. MAb 35-052 binds recombinant ClfA protein, but it does not recognize surface-expressed ClfA from S. aureus 67-0, as determined by flow cytometric analysis (data not shown). Mice were pretreated by intraperitoneal injection of MAb 12-9 or 35-052. Figure 5A demonstrates significant differences between the relative survival times of the treatment groups. Fifty-seven percent of the mice that received MAb 12-9 survived the bacterial challenge to day 15 (P < 0.0001; Fig. 5A). In contrast, only 10% of the mice treated with the control MAb survived the study period.
FIG. 5.
Prophylaxis in a murine model of S. aureus-induced sepsis. (A) The biological activity of MAbs 12-9 and 35-052 was studied in a mouse septicemia model with MRSA strain 67-0. Percent survival of mice (n = 30) after treatment with anti-ClfA MAb 12-9 (dotted line) or 35-052 (solid line) is shown. *, P < 0.0001. (B) The biological impact of inhibiting fibrinogen binding to S. aureus was evaluated with MAbs 12-9 (n = 29) and 15EC6 (n = 30). Mice were challenged i.v. with S. aureus strain Newman. Percent survival of mice after treatment with anti-ClfA MAbs 12-9 (dotted line) and 15EC6 (solid line) is shown. *, P < 0.006.
The second study was designed to begin to assess the biological impact of inhibiting S. aureus binding to fibrinogen in an in vivo model of S. aureus-induced death. In addition, the efficacy of MAb 12-9 against a different strain of S. aureus (Newman) was evaluated. With these goals in mind, MAb 12-9 (IgG1) was compared directly with MAb 15EC6 (IgG1). MAb 15EC6, which recognizes the native version of ClfA expressed by S. aureus strain Newman (data not shown), did not inhibit ClfA binding to fibrinogen and did not detach adherent S. aureus in the dynamic-flow system (Fig. 3 and 4). Although the overall survival rate of 12-9-treated mice is somewhat lower than in the previous experiment, the MAb with inhibitory activity provided the best protection (P = 0.006) (Fig. 5B). This trend in the data was reproducible in at least three different experiments. The results suggest that inhibiting fibrinogen binding to S. aureus contributes to the overall protective efficacy of MAb 12-9. To our knowledge, this is the first report of a MAb against a cell surface protein from S. aureus that has demonstrated significant in vivo protection.
DISCUSSION
The continued emergence of multiple-antibiotic-resistant S. aureus isolates originating from community and nosocomial sources necessitates the development of new approaches to the prevention and treatment of these life-threatening infections. The recent report of a vancomycin-resistant strain of S. aureus from a dialysis patient in Michigan serves to accentuate this public health problem (3). The existence of S. aureus with limited susceptibility to vancomycin represents the potential for infection with a virulent organism for which the therapeutic options are severely limited (42). To date, much of industry's drug development efforts have focused on enhancing the potency, while eliminating the side effects, of currently established classes of antimicrobials (47). The implementation of genomics and high-throughput screening has broached the possibility of developing truly new classes of antimicrobials; however, it may be several years until newly developed compounds can be fully evaluated in a clinical setting. Another viable approach is passive immunization with MAbs or polyclonal antibodies, in combination with antibiotics for the treatment of established infections. Traditionally, these biological approaches to the treatment and prevention of bacterial infections or sepsis have been littered with failures. Because the biological basis of benefit in the previous studies relied on the neutralization of potent immunomodulators that act in concert within a complex series of pathways, the variability of the clinical responses was considerable. In addition, the tremendous heterogeneity in the patient population receiving the early antibody-based products, such as HA-1A MAb (anti-lipid A on lipopolysaccharide) (21, 45) or tumor necrosis factor alpha MAb (1, 8), contributed significantly to the well-documented failures.
In contrast to bacterial sepsis, the use of antibodies to prevent viral infections has had substantial clinical success (41). For example, palivizumab (Synagis), a humanized MAb for the prevention of serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) in pediatric patients, has been shown to reduce RSV hospitalizations (19). Moreover, specific hyperimmune immune globulins against hepatitis B (35) or cytomegalovirus (46) for the prevention of infection in high-risk or exposed patients have been used effectively for a number of years. These data suggest that antibodies could be used successfully in the infectious-disease arena. In fact, a recent review by Keller and Stiehm highlighted the use of passive immunization for the prevention and treatment of infectious diseases (18).
Previously, we reported that SA-IGIV, a donor-selected immune globulin containing elevated levels of polyclonal antibodies against ClfA, was protective in a murine model of MRSA-mediated sepsis (16). To further validate the concept that MSCRAMM proteins are relevant targets for the development of antibody-based therapies, an extensive panel of murine MAbs against ClfA were generated. The ideal characteristics of a MAb for the prevention and treatment of S. aureus infections should include specific high-affinity binding to a conserved, surface-exposed antigen; potent inhibition of bacterial binding to host tissue components; and protective efficacy in animal models. This report describes several assays designed as characterization tools from which one specific clone, designated 12-9, was selected for further study.
BIAcore provided a rapid method by which to analyze antibody-binding kinetics and also to simultaneously determine which antibodies could inhibit recombinant ClfA binding to human fibrinogen. Of the thousands of ClfA MAbs screened, 12-9 exhibited the highest affinity (Kd, 2.10 × 10−10 M) and the slowest off rate (4.18 × 10−4 s−1). Interestingly, MAb 12-9 also possessed the most potent inhibitory activity. Other ClfA MAbs that were analyzed with BIAcore often yielded mixed binding activities, for example, a high affinity and quick off rate or a low affinity and a slow off rate (data not shown). Additionally, these MAbs did not exhibit the same inhibitory activity as MAb 12-9. Taken together, these data suggest that the overall in vitro potency of MAb 12-9 is attributable largely to its binding kinetics. Similarly, in a direct comparison of MAbs recognizing F glycoprotein from RSV, BIAcore analysis revealed that MEDI-493 exhibited a higher affinity, a faster on rate, and a slower off rate than RSHZ19 (15). Interestingly, in subsequent phase III clinical trials with at-risk infants, the more potent MAb, MEDI-493, exhibited superior efficacy (2).
Historically, polyclonal antibodies that have been developed against S. aureus have been limited by their serotype specificity (12, 13), consequently recognizing only 75 to 80% of all S. aureus clinical isolates (43). A more attractive approach is the selection of an antibody that could bind with high affinity to a more significant proportion of S. aureus clinical isolates. With this requirement in mind, a major focus of this study was the selection of a MAb that recognized a conserved epitope expressed by different SALs, particularly virulent and antibiotic-resistant strains. In this study, we analyzed 11 S. aureus isolates representing all 11 clonal variants (10). In addition, other clinical isolates representing methicillin-resistant SAL isolates as described by Booth et al. (5) were studied. Because only the ligand-binding domain of ClfA was used to generate MAb 12-9, it was important to determine that the epitope was present in a native conformation and that the epitope was prevalent among clinically relevant SALs. In a flow cytometry assay, MAb 12-9 effectively recognized every S. aureus isolate analyzed, providing strong evidence that the native ClfA epitope is highly conserved. These data are supportive of previous reports that indicate that the presence of the clfA gene (5, 34) and ClfA-mediated fibrinogen binding (9, 36, 50) is a trait conserved in a vast majority of S. aureus strains. While these data may be semiquantitative in nature, it is important to note that the flow cytometry analysis reveals the percentage of positively staining cells at one point in the time of S. aureus isolate cell growth. It is also important to note that the percentage of positively staining cells was recorded under in vitro growth conditions, while environmental conditions in vivo may contribute to different levels of ClfA surface expression.
Having demonstrated that MAb 12-9 was broadly reactive among S. aureus strains and also inhibited the adherence of whole cells to fibrinogen, we assessed the prophylactic efficacy of this antibody in a murine model of MRSA sepsis. A single infusion of MAb 12-9 prior to a challenge with the heterologous clinical MRSA isolate effectively protected mice against sepsis-associated death. The prolonged protective efficacy of MAb 12-9 is consistent with a projected half-life of approximately 150 to 200 h (data not shown). However, the ability of a single MAb to protect against a significant i.v. challenge was surprising given the fact that this strain also expresses a number of virulence factors.
To summarize, we have shown that MAb 12-9 provides significant protection against lethal infection by S. aureus. We hypothesize that the antibody is effective because of its desirable binding kinetics and its ability to inhibit and destabilize ClfA-fibrinogen interactions. However, in addition to its potent inhibitory activity, one must also take into account the contribution of enhanced phagocytosis of S. aureus to the composite biological activity of the MAb. In fact, flow cytometric assays with a humanized version of MAb 12-9 indicate that the antibody specifically enhances the uptake of ClfA-coated beads by human polymorphonuclear neutrophils (unpublished data). Future studies will focus on delineating the roles that inhibition of fibrinogen binding and opsonophagocytosis play in the overall efficacy of the antibody. Taken together, these studies suggest that MAb therapy may be an efficacious approach to the treatment and prevention of life-threatening S. aureus infections.
Acknowledgments
We thank Brad Allen, Arnold Bayer, Timothy Foster, Michael Gilmore, and John Minogue for providing strains for these studies. We gratefully acknowledge Dawn Bryant, Matt Davis, Cheryl Hooks, and Josh Paxton for technical expertise and assistance in animal studies. Expert technical assistance was also provided by Brad Prater, Jin Wang, and Brenda Ames.
This work was supported in part by National Institutes of Health grant 5R01HL066453-02 (J.R.).
Editor: F. C. Fang
REFERENCES
- 1.Abraham, E., A. Anzueto, G. Gutierrez, S. Tessler, G. San Pedro, R. Wunderink, A. Dal Nogare, S. Nasraway, S. Berman, R. Cooney, H. Levy, R. Baughman, M. Rumbak, R. B. Light, L. Poole, R. Allred, J. Constant, J. Pennington, and S. Porter. 1998. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. Lancet 351:929-933. [PubMed] [Google Scholar]
- 2.Anonymous. 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531-537. [PubMed] [Google Scholar]
- 3.Anonymous. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 4.Bayer, A. S., P. M. Sullam, M. Ramos, C. Li, A. L. Cheung, and M. R. Yeaman. 1995. Staphylococcus aureus induces platelet aggregation via a fibrinogen-dependent mechanism which is independent of principal platelet glycoprotein IIb/IIIa fibrinogen-binding domains. Infect. Immun. 63:3634-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth, M. C., L. M. Pence, P. Mahasreshti, M. C. Callegan, and M. S. Gilmore. 2001. Clonal associations among Staphylococcus aureus isolates from various sites of infection. Infect. Immun. 69:345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan, F. R., T. D. Jones, M. Longstaff, S. Chapman, T. Bellaby, H. Smith, F. Xu, W. D. Hamilton, and J. I. Flock. 1999. Immunogenicity of peptides derived from a fibronectin-binding protein of S. aureus expressed on two different plant viruses. Vaccine 17:1846-1857. [DOI] [PubMed] [Google Scholar]
- 7.Chuard, C., P. Vaudaux, F. A. Waldvogel, and D. P. Lew. 1993. Susceptibility of Staphylococcus aureus growing on fibronectin-coated surfaces to bactericidal antibiotics. Antimicrob. Agents Chemother. 37:625-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen, J., and J. Carlet. 1996. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. Crit. Care Med. 24:1431-1440. [DOI] [PubMed] [Google Scholar]
- 9.Cooke, R. P., and C. T. Jenkins. 1997. Comparison of commercial slide agglutination kits with a tube coagulase test for the rapid identification of Staphylococcus aureus from blood culture. J. Clin. Pathol. 50:164-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day, N. P., C. E. Moore, M. C. Enright, A. R. Berendt, J. M. Smith, M. F. Murphy, S. J. Peacock, B. G. Spratt, and E. J. Feil. 2001. A link between virulence and ecological abundance in natural populations of Staphylococcus aureus. Science 292:114-116. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson, R. B., J. A. Nagel, D. McDevitt, T. J. Foster, R. A. Proctor, and S. L. Cooper. 1995. Quantitative comparison of clumping factor- and coagulase-mediated Staphylococcus aureus adhesion to surface-bound fibrinogen under flow. Infect. Immun. 63:3143-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fattom, A. I., and R. Naso. 1996. Staphylococcal vaccines: a realistic dream. Ann. Med. 28:43-46. [DOI] [PubMed] [Google Scholar]
- 13.Fattom, A. I., J. Sarwar, A. Ortiz, and R. Naso. 1996. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect. Immun. 64:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster, T. J., and M. Hook. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, S., S. D. Griego, D. S. Pfarr, M. L. Doyle, R. Woods, D. Carlin, G. A. Prince, S. Koenig, J. F. Young, and S. B. Dillon. 1999. A direct comparison of the activities of two humanized respiratory syncytial virus monoclonal antibodies: MEDI-493 and RSHZl9. J. Infect. Dis. 180:35-40. [DOI] [PubMed] [Google Scholar]
- 16.Josefsson, E., O. Hartford, L. O'Brien, J. M. Patti, and T. Foster. 2001. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect. Dis. 184:1572-1580. [DOI] [PubMed] [Google Scholar]
- 17.Josefsson, E., K. W. McCrea, D. Ni Eidhin, D. O'Connell, J. Cox, M. Hook, and T. J. Foster. 1998. Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus. Microbiology 144(Pt. 12):3387-3395. [DOI] [PubMed] [Google Scholar]
- 18.Keller, M. A., and E. R. Stiehm. 2000. Passive immunity in prevention and treatment of infectious diseases. Clin. Microbiol. Rev. 13:602-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krilov, L. R. 2002. Palivizumab in the prevention of respiratory syncytial virus disease. Expert Opin. Biol. Ther. 2:763-769. [DOI] [PubMed] [Google Scholar]
- 20.Li, Z. J., N. Mohamed, and J. M. Ross. 2000. Shear stress affects the kinetics of Staphylococcus aureus adhesion to collagen. Biotechnol. Prog. 16:1086-1090. [DOI] [PubMed] [Google Scholar]
- 21.McCloskey, R. V., R. C. Straube, C. Sanders, S. M. Smith, and C. R. Smith. 1994. Treatment of septic shock with human monoclonal antibody HA-1A: a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 121:1-5. [DOI] [PubMed] [Google Scholar]
- 22.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1995. Identification of the ligand-binding domain of the surface-located fibrinogen receptor (clumping factor) of Staphylococcus aureus. Mol. Microbiol. 16:895-907. [DOI] [PubMed] [Google Scholar]
- 23.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 24.McDevitt, D., T. Nanavaty, K. House-Pompeo, E. Bell, N. Turner, L. McIntire, T. Foster, and M. Hook. 1997. Characterization of the interaction between the Staphylococcus aureus clumping factor (ClfA) and fibrinogen. Eur. J. Biochem. 247:416-424. [DOI] [PubMed] [Google Scholar]
- 25.McDevitt, D., P. Vaudaux, and T. J. Foster. 1992. Genetic evidence that bound coagulase of Staphylococcus aureus is not clumping factor. Infect. Immun. 60:1514-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohamed, N., T. R. Rainier, Jr., and J. M. Ross. 2000. Novel experimental study of receptor-mediated bacterial adhesion under the influence of fluid shear. Biotechnol. Bioeng. 68:628-636. [PubMed] [Google Scholar]
- 27.Mohamed, N., M. A. Teeters, J. M. Patti, M. Hook, and J. M. Ross. 1999. Inhibition of Staphylococcus aureus adherence to collagen under dynamic conditions. Infect. Immun. 67:589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohamed, N., L. Visai, P. Speziale, and J. M. Ross. 2000. Quantification of Staphylococcus aureus cell surface adhesins using flow cytometry. Microb. Pathog. 29:357-361. [DOI] [PubMed] [Google Scholar]
- 29.Moreillon, P., J. M. Entenza, P. Francioli, D. McDevitt, T. J. Foster, P. Francois, and P. Vaudaux. 1995. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect. Immun. 63:4738-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morin, C. A., and J. L. Hadler. 2001. Population-based incidence and characteristics of community-onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998. J. Infect. Dis. 184:1029-1034. [DOI] [PubMed] [Google Scholar]
- 31.Ni Eidhin, D., S. Perkins, P. Francois, P. Vaudaux, M. Hook, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson, I. M., J. M. Patti, T. Bremell, M. Hook, and A. Tarkowski. 1998. Vaccination with a recombinant fragment of collagen adhesin provides protection against Staphylococcus aureus-mediated septic death. J. Clin. Investig. 101:2640-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patti, J. M., B. L. Allen, M. J. Mcgavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 34.Peacock, S. J., C. E. Moore, A. Justice, M. Kantzanou, L. Story, K. Mackie, G. O'Neill, and N. P. Day. 2002. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 70:4987-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrillo, R. P. 2000. Antiviral therapy to prevent and treat hepatitis B virus infection in hepatic allografts. Clin. Transplant. 14(Suppl. 2):25-28. [PubMed] [Google Scholar]
- 36.Personne, P., M. Bes, G. Lina, F. Vandenesch, Y. Brun, and J. Etienne. 1997. Comparative performances of six agglutination kits assessed by using typical and atypical strains of Staphylococcus aureus. J. Clin. Microbiol. 35:1138-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Que, Y. A., J. A. Haefliger, P. Francioli, and P. Moreillon. 2000. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect. Immun. 68:3516-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rennermalm, A., Y. H. Li, L. Bohaufs, C. Jarstrand, A. Brauner, F. R. Brennan, and J. I. Flock. 2001. Antibodies against a truncated Staphylococcus aureus fibronectin-binding protein protect against dissemination of infection in the rat. Vaccine 19:3376-3383. [DOI] [PubMed] [Google Scholar]
- 39.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 1999. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit. Care Med. 27:887-892. [DOI] [PubMed] [Google Scholar]
- 40.Rozalska, B., and T. Wadstrom. 1993. Protective opsonic activity of antibodies against fibronectin-binding proteins (FnBPs) of Staphylococcus aureus. Scand. J. Immunol. 37:575-580. [DOI] [PubMed] [Google Scholar]
- 41.Sawyer, L. A. 2000. Antibodies for the prevention and treatment of viral diseases. Antiviral Res. 47:57-77. [DOI] [PubMed] [Google Scholar]
- 42.Sefton, A. M. 2002. Mechanisms of antimicrobial resistance: their clinical relevance in the new millennium. Drugs 62:557-566. [DOI] [PubMed] [Google Scholar]
- 43.Shinefield, H., S. Black, A. Fattom, G. Horwith, S. Rasgon, J. Ordonez, H. Yeoh, D. Law, J. B. Robbins, R. Schneerson, L. Muenz, S. Fuller, J. Johnson, B. Fireman, H. Alcorn, and R. Naso. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346:491-496. [DOI] [PubMed] [Google Scholar]
- 44.Siboo, I. R., A. L. Cheung, A. S. Bayer, and P. M. Sullam. 2001. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect. Immun. 69:3120-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skurnik, M., P. Mikkola, P. Toivanen, and R. Tertti. 1996. Passive immunization with monoclonal antibodies specific for lipopolysaccharide (LPS) O-side chain protects mice against intravenous Yersinia enterocolitica serotype O:3 infection. APMIS 104:598-602. [DOI] [PubMed] [Google Scholar]
- 46.Snydman, D. R. 2001. Historical overview of the use of cytomegalovirus hyperimmune globulin in organ transplantation. Transplant. Infect. Dis. 3(Suppl. 2):6-13. [DOI] [PubMed] [Google Scholar]
- 47.Strahilevitz, J., and E. Rubinstein. 2002. Novel agents for resistant gram-positive infections—a review. Int. J. Infect. Dis. 6(Suppl. 1):S38-S46. [DOI] [PubMed] [Google Scholar]
- 48.Stutzmann Meier, P., J. M. Entenza, P. Vaudaux, P. Francioli, M. P. Glauser, and P. Moreillon. 2001. Study of Staphylococcus aureus pathogenic genes by transfer and expression in the less virulent organism Streptococcus gordonii. Infect. Immun. 69:657-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Switalski, L. M., J. M. Patti, W. Butcher, A. G. Gristina, P. Speziale, and M. Hook. 1993. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol. Microbiol. 7:99-107. [DOI] [PubMed] [Google Scholar]
- 50.Wilkerson, M., S. McAllister, J. M. Miller, B. J. Heiter, and P. P. Bourbeau. 1997. Comparison of five agglutination tests for identification of Staphylococcus aureus. J. Clin. Microbiol. 35:148-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yokoyama, W. 1995. Production of monoclonal antibodies: induction of immune responses, p. 2.5.4-2.5.8. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology, vol. 1. Wiley and Sons, Hoboken, N.J.