Abstract
In the title compound, C16H19BrO3S, the cyclohexyl ring adopts a chair conformation. In the crystal, molecules are linked through weak C—H⋯O hydrogen bonds and Br⋯O contacts [3.211 (1) Å].
Related literature
For the pharmacological activity of benzofuran compounds, see: Aslam et al. (2009 ▶); Galal et al. (2009 ▶); Khan et al. (2005 ▶). For natural products with benzofuran rings, see: Akgul & Anil (2003 ▶); Soekamto et al. (2003 ▶). For structural studies of related 3-cyclohexylsulfonyl-5-halo-2-methyl-1-benzofuran derivatives, see: Choi et al. (2011a ▶,b
▶). For a review of halogen bonding, see: Politzer et al. (2007 ▶).
Experimental
Crystal data
C16H19BrO3S
M r = 371.28
Triclinic,

a = 6.6992 (2) Å
b = 8.4654 (2) Å
c = 14.1065 (3) Å
α = 101.773 (1)°
β = 99.283 (1)°
γ = 92.067 (1)°
V = 770.94 (3) Å3
Z = 2
Mo Kα radiation
μ = 2.81 mm−1
T = 173 K
0.26 × 0.25 × 0.21 mm
Data collection
Bruker SMART APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.525, T max = 0.591
13840 measured reflections
3542 independent reflections
3247 reflections with I > 2σ(I)
R int = 0.032
Refinement
R[F 2 > 2σ(F 2)] = 0.025
wR(F 2) = 0.066
S = 1.10
3542 reflections
192 parameters
H-atom parameters constrained
Δρmax = 0.27 e Å−3
Δρmin = −0.59 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and DIAMOND (Brandenburg, 1998 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053681101539X/lr2008sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053681101539X/lr2008Isup2.hkl
Supplementary material file. DOI: 10.1107/S160053681101539X/lr2008Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C10—H10B⋯O2i | 0.98 | 2.55 | 3.384 (2) | 143 |
Symmetry code: (i) 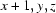 .
.
supplementary crystallographic information
Comment
Many compounds containing a benzofuran skeleton have drawn much attention owing to diverse pharmacological properties such as antibacterial and antifungal, antitumor and antiviral, and antimicrobial activities (Aslam et al., 2009, Galal et al., 2009, Khan et al., 2005). These compounds occur in a wide range of natural products (Akgul & Anil, 2003; Soekamto et al., 2003). As a part of our ongoing study of the substituent effect on the solid state structures of 3-cyclohexylsulfonyl-5-halo-2-methyl-1-benzofuran analogues (Choi et al., 2011a,b), we report herein the crystal structure of the title compound.
In the title molecule (Fig. 1), the benzofuran unit is essentially planar, with a mean deviation of 0.004 (1) Å from the least-squares plane defined by the nine constituent atoms. The cyclohexyl ring is in the chair form. The molecular packing (Fig. 2) is stabilized by weak intermolecular C—H···O hydrogen bonds between a methyl H atom and the O atom of the sulfonyl group (Table 1; C10—H10B···O2ii). The crystal packing (Fig. 2) is further stabilized by a Br···O halogen-bonding between the bromine and the O atom of the sulfonyl group [Br1···O2i = 3.211 (1) Å, C4—Br1···O2i = 168.13 (6)°] (Politzer et al., 2007).
Experimental
77% 3-chloroperoxybenzoic acid (448 mg, 2.0 mmol) was added in small portions to a stirred solution of 5-bromo-3-cyclohexylsulfanyl-2,7-dimethyl-1-benzofuran (305 mg, 0.9 mmol) in dichloromethane (40 mL) at 273 K. After being stirred at room temperature for 5h, the mixture was washed with saturated sodium bicarbonate solution and the organic layer was separated, dried over magnesium sulfate, filtered and concentrated at reduced pressure. The residue was purified by column chromatography (hexane-ethyl acetate, 4:1 v/v) to afford the title compound as a colorless solid [yield 72%, m.p. 440–441 K; Rf = 0.55 (hexane–ethyl acetate, 4:1 v/v)]. Single crystals suitable for X-ray diffraction were prepared by slow evaporation of a solution of the title compound in acetone at room temperature.
Refinement
All H atoms were positioned geometrically and refined using a riding model, with C—H = 0.95 Å for aryl, 1.00 Å for methine, 0.99 Å for methylene and 0.98 Å for methyl H atoms, respectively. Uiso(H) = 1.2Ueq(C) for aryl, methine and methylene, and 1.5Ueq(C) for methyl H atoms.
Figures
Fig. 1.
The molecular structure of the title compound. Displacement ellipsoids are drawn at the 50% probability level.H atoms are presented as a small spheres of arbitrary radius.
Fig. 2.
A view of the Br···O and C—H···O interactions (dotted lines) in the crystal structure of the title compound. [Symmetry codes: (i) - x, - y + 1, - z + 1; (ii) x + 1, y, z; (iii) x - 1, y, z.]
Crystal data
| C16H19BrO3S | Z = 2 |
| Mr = 371.28 | F(000) = 380 |
| Triclinic, P1 | Dx = 1.599 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.6992 (2) Å | Cell parameters from 7893 reflections |
| b = 8.4654 (2) Å | θ = 2.5–27.5° |
| c = 14.1065 (3) Å | µ = 2.81 mm−1 |
| α = 101.773 (1)° | T = 173 K |
| β = 99.283 (1)° | Block, colourless |
| γ = 92.067 (1)° | 0.26 × 0.25 × 0.21 mm |
| V = 770.94 (3) Å3 |
Data collection
| Bruker SMART APEXII CCD diffractometer | 3542 independent reflections |
| Radiation source: rotating anode | 3247 reflections with I > 2σ(I) |
| graphite multilayer | Rint = 0.032 |
| Detector resolution: 10.0 pixels mm-1 | θmax = 27.5°, θmin = 1.5° |
| φ and ω scans | h = −8→8 |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | k = −10→11 |
| Tmin = 0.525, Tmax = 0.591 | l = −18→18 |
| 13840 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.025 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.066 | H-atom parameters constrained |
| S = 1.10 | w = 1/[σ2(Fo2) + (0.0331P)2 + 0.2732P] where P = (Fo2 + 2Fc2)/3 |
| 3542 reflections | (Δ/σ)max = 0.001 |
| 192 parameters | Δρmax = 0.27 e Å−3 |
| 0 restraints | Δρmin = −0.59 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.23387 (3) | 0.56750 (2) | 0.639762 (13) | 0.02642 (7) | |
| S1 | 0.18306 (6) | 0.68046 (5) | 0.21799 (3) | 0.02179 (10) | |
| O1 | 0.68271 (17) | 0.88970 (14) | 0.39103 (9) | 0.0209 (2) | |
| O2 | 0.00575 (19) | 0.67623 (17) | 0.26350 (10) | 0.0308 (3) | |
| O3 | 0.1772 (2) | 0.76300 (17) | 0.13792 (10) | 0.0319 (3) | |
| C1 | 0.3856 (3) | 0.7638 (2) | 0.31083 (12) | 0.0199 (3) | |
| C2 | 0.4083 (2) | 0.7416 (2) | 0.41070 (12) | 0.0186 (3) | |
| C3 | 0.2943 (2) | 0.6642 (2) | 0.46471 (12) | 0.0205 (3) | |
| H3 | 0.1661 | 0.6086 | 0.4362 | 0.025* | |
| C4 | 0.3779 (3) | 0.6731 (2) | 0.56194 (13) | 0.0205 (3) | |
| C5 | 0.5655 (3) | 0.7538 (2) | 0.60597 (12) | 0.0207 (3) | |
| H5 | 0.6147 | 0.7562 | 0.6733 | 0.025* | |
| C6 | 0.6817 (2) | 0.8306 (2) | 0.55345 (12) | 0.0193 (3) | |
| C7 | 0.5946 (2) | 0.8214 (2) | 0.45637 (12) | 0.0187 (3) | |
| C8 | 0.5527 (3) | 0.8528 (2) | 0.30300 (12) | 0.0210 (3) | |
| C9 | 0.8873 (3) | 0.9137 (2) | 0.59697 (14) | 0.0259 (4) | |
| H9A | 0.9904 | 0.8546 | 0.5652 | 0.039* | |
| H9B | 0.9150 | 0.9161 | 0.6676 | 0.039* | |
| H9C | 0.8910 | 1.0246 | 0.5865 | 0.039* | |
| C10 | 0.6222 (3) | 0.9166 (2) | 0.22308 (13) | 0.0278 (4) | |
| H10A | 0.5172 | 0.8903 | 0.1641 | 0.042* | |
| H10B | 0.7471 | 0.8677 | 0.2085 | 0.042* | |
| H10C | 0.6483 | 1.0343 | 0.2435 | 0.042* | |
| C11 | 0.2440 (3) | 0.4760 (2) | 0.17876 (12) | 0.0212 (3) | |
| H11 | 0.2926 | 0.4334 | 0.2389 | 0.025* | |
| C12 | 0.0529 (3) | 0.3725 (2) | 0.12283 (14) | 0.0305 (4) | |
| H12A | −0.0521 | 0.3788 | 0.1652 | 0.037* | |
| H12B | −0.0016 | 0.4137 | 0.0637 | 0.037* | |
| C13 | 0.1038 (3) | 0.1970 (3) | 0.09238 (17) | 0.0399 (5) | |
| H13A | 0.1455 | 0.1532 | 0.1519 | 0.048* | |
| H13B | −0.0187 | 0.1316 | 0.0534 | 0.048* | |
| C14 | 0.2727 (3) | 0.1828 (3) | 0.03208 (16) | 0.0386 (5) | |
| H14A | 0.2253 | 0.2152 | −0.0309 | 0.046* | |
| H14B | 0.3069 | 0.0686 | 0.0169 | 0.046* | |
| C15 | 0.4616 (3) | 0.2888 (2) | 0.08642 (15) | 0.0321 (4) | |
| H15A | 0.5648 | 0.2825 | 0.0431 | 0.039* | |
| H15B | 0.5189 | 0.2477 | 0.1452 | 0.039* | |
| C16 | 0.4139 (3) | 0.4651 (2) | 0.11810 (14) | 0.0277 (4) | |
| H16A | 0.5368 | 0.5293 | 0.1576 | 0.033* | |
| H16B | 0.3723 | 0.5107 | 0.0592 | 0.033* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.02483 (10) | 0.02977 (12) | 0.02630 (10) | −0.00189 (7) | 0.00582 (7) | 0.00932 (8) |
| S1 | 0.0202 (2) | 0.0225 (2) | 0.0199 (2) | 0.00190 (16) | −0.00183 (15) | 0.00234 (17) |
| O1 | 0.0188 (6) | 0.0227 (6) | 0.0203 (6) | −0.0014 (5) | 0.0035 (5) | 0.0031 (5) |
| O2 | 0.0196 (6) | 0.0361 (8) | 0.0324 (7) | 0.0021 (5) | 0.0016 (5) | −0.0002 (6) |
| O3 | 0.0372 (8) | 0.0301 (7) | 0.0265 (7) | 0.0024 (6) | −0.0058 (6) | 0.0103 (6) |
| C1 | 0.0199 (8) | 0.0190 (8) | 0.0187 (8) | 0.0020 (6) | 0.0012 (6) | 0.0007 (6) |
| C2 | 0.0190 (8) | 0.0149 (8) | 0.0196 (8) | 0.0025 (6) | 0.0017 (6) | −0.0005 (6) |
| C3 | 0.0177 (8) | 0.0186 (8) | 0.0228 (8) | −0.0010 (6) | 0.0024 (6) | 0.0004 (7) |
| C4 | 0.0205 (8) | 0.0177 (8) | 0.0235 (8) | 0.0008 (6) | 0.0056 (6) | 0.0038 (7) |
| C5 | 0.0216 (8) | 0.0196 (8) | 0.0193 (8) | 0.0035 (6) | 0.0008 (6) | 0.0019 (7) |
| C6 | 0.0169 (7) | 0.0169 (8) | 0.0216 (8) | 0.0016 (6) | 0.0012 (6) | −0.0003 (6) |
| C7 | 0.0187 (8) | 0.0164 (8) | 0.0209 (8) | 0.0016 (6) | 0.0051 (6) | 0.0024 (6) |
| C8 | 0.0231 (8) | 0.0189 (8) | 0.0191 (8) | 0.0044 (6) | 0.0020 (6) | 0.0003 (7) |
| C9 | 0.0190 (8) | 0.0286 (10) | 0.0270 (9) | −0.0014 (7) | −0.0007 (7) | 0.0027 (8) |
| C10 | 0.0284 (9) | 0.0320 (10) | 0.0245 (9) | 0.0009 (8) | 0.0060 (7) | 0.0085 (8) |
| C11 | 0.0235 (8) | 0.0198 (8) | 0.0176 (8) | −0.0012 (7) | 0.0003 (6) | 0.0010 (7) |
| C12 | 0.0256 (9) | 0.0335 (11) | 0.0267 (9) | −0.0072 (8) | 0.0036 (7) | −0.0043 (8) |
| C13 | 0.0410 (12) | 0.0302 (11) | 0.0400 (12) | −0.0125 (9) | 0.0069 (9) | −0.0095 (9) |
| C14 | 0.0407 (12) | 0.0329 (11) | 0.0334 (11) | 0.0007 (9) | 0.0038 (9) | −0.0107 (9) |
| C15 | 0.0304 (10) | 0.0286 (10) | 0.0339 (10) | 0.0035 (8) | 0.0047 (8) | −0.0008 (8) |
| C16 | 0.0258 (9) | 0.0266 (10) | 0.0300 (9) | 0.0005 (7) | 0.0068 (7) | 0.0032 (8) |
Geometric parameters (Å, °)
| Br1—C4 | 1.9011 (17) | C9—H9C | 0.9800 |
| Br1—O2i | 3.2114 (14) | C10—H10A | 0.9800 |
| S1—O3 | 1.4406 (13) | C10—H10B | 0.9800 |
| S1—O2 | 1.4410 (14) | C10—H10C | 0.9800 |
| S1—C1 | 1.7420 (17) | C11—C16 | 1.525 (3) |
| S1—C11 | 1.7891 (18) | C11—C12 | 1.530 (2) |
| O1—C8 | 1.368 (2) | C11—H11 | 1.0000 |
| O1—C7 | 1.379 (2) | C12—C13 | 1.526 (3) |
| C1—C8 | 1.359 (2) | C12—H12A | 0.9900 |
| C1—C2 | 1.444 (2) | C12—H12B | 0.9900 |
| C2—C7 | 1.390 (2) | C13—C14 | 1.515 (3) |
| C2—C3 | 1.396 (2) | C13—H13A | 0.9900 |
| C3—C4 | 1.381 (2) | C13—H13B | 0.9900 |
| C3—H3 | 0.9500 | C14—C15 | 1.523 (3) |
| C4—C5 | 1.395 (2) | C14—H14A | 0.9900 |
| C5—C6 | 1.387 (2) | C14—H14B | 0.9900 |
| C5—H5 | 0.9500 | C15—C16 | 1.529 (3) |
| C6—C7 | 1.385 (2) | C15—H15A | 0.9900 |
| C6—C9 | 1.500 (2) | C15—H15B | 0.9900 |
| C8—C10 | 1.476 (2) | C16—H16A | 0.9900 |
| C9—H9A | 0.9800 | C16—H16B | 0.9900 |
| C9—H9B | 0.9800 | ||
| C4—Br1—O2i | 168.13 (6) | H10A—C10—H10B | 109.5 |
| O3—S1—O2 | 118.36 (9) | C8—C10—H10C | 109.5 |
| O3—S1—C1 | 109.59 (8) | H10A—C10—H10C | 109.5 |
| O2—S1—C1 | 107.06 (8) | H10B—C10—H10C | 109.5 |
| O3—S1—C11 | 109.54 (8) | C16—C11—C12 | 111.87 (15) |
| O2—S1—C11 | 107.55 (8) | C16—C11—S1 | 111.84 (12) |
| C1—S1—C11 | 103.73 (8) | C12—C11—S1 | 109.74 (13) |
| C8—O1—C7 | 107.00 (13) | C16—C11—H11 | 107.7 |
| C8—C1—C2 | 107.69 (15) | C12—C11—H11 | 107.7 |
| C8—C1—S1 | 127.55 (14) | S1—C11—H11 | 107.7 |
| C2—C1—S1 | 124.69 (13) | C13—C12—C11 | 109.64 (16) |
| C7—C2—C3 | 119.34 (15) | C13—C12—H12A | 109.7 |
| C7—C2—C1 | 104.60 (15) | C11—C12—H12A | 109.7 |
| C3—C2—C1 | 136.06 (15) | C13—C12—H12B | 109.7 |
| C4—C3—C2 | 116.33 (15) | C11—C12—H12B | 109.7 |
| C4—C3—H3 | 121.8 | H12A—C12—H12B | 108.2 |
| C2—C3—H3 | 121.8 | C14—C13—C12 | 111.54 (18) |
| C3—C4—C5 | 123.20 (16) | C14—C13—H13A | 109.3 |
| C3—C4—Br1 | 118.84 (12) | C12—C13—H13A | 109.3 |
| C5—C4—Br1 | 117.95 (13) | C14—C13—H13B | 109.3 |
| C6—C5—C4 | 121.37 (15) | C12—C13—H13B | 109.3 |
| C6—C5—H5 | 119.3 | H13A—C13—H13B | 108.0 |
| C4—C5—H5 | 119.3 | C13—C14—C15 | 111.59 (17) |
| C7—C6—C5 | 114.55 (15) | C13—C14—H14A | 109.3 |
| C7—C6—C9 | 122.37 (16) | C15—C14—H14A | 109.3 |
| C5—C6—C9 | 123.07 (16) | C13—C14—H14B | 109.3 |
| O1—C7—C6 | 124.35 (15) | C15—C14—H14B | 109.3 |
| O1—C7—C2 | 110.43 (14) | H14A—C14—H14B | 108.0 |
| C6—C7—C2 | 125.21 (15) | C14—C15—C16 | 111.47 (17) |
| C1—C8—O1 | 110.27 (15) | C14—C15—H15A | 109.3 |
| C1—C8—C10 | 134.88 (16) | C16—C15—H15A | 109.3 |
| O1—C8—C10 | 114.85 (15) | C14—C15—H15B | 109.3 |
| C6—C9—H9A | 109.5 | C16—C15—H15B | 109.3 |
| C6—C9—H9B | 109.5 | H15A—C15—H15B | 108.0 |
| H9A—C9—H9B | 109.5 | C11—C16—C15 | 110.14 (16) |
| C6—C9—H9C | 109.5 | C11—C16—H16A | 109.6 |
| H9A—C9—H9C | 109.5 | C15—C16—H16A | 109.6 |
| H9B—C9—H9C | 109.5 | C11—C16—H16B | 109.6 |
| C8—C10—H10A | 109.5 | C15—C16—H16B | 109.6 |
| C8—C10—H10B | 109.5 | H16A—C16—H16B | 108.1 |
| O3—S1—C1—C8 | −20.06 (19) | C9—C6—C7—C2 | 177.58 (17) |
| O2—S1—C1—C8 | −149.60 (16) | C3—C2—C7—O1 | −179.90 (14) |
| C11—S1—C1—C8 | 96.84 (17) | C1—C2—C7—O1 | 0.31 (18) |
| O3—S1—C1—C2 | 163.32 (14) | C3—C2—C7—C6 | 0.7 (3) |
| O2—S1—C1—C2 | 33.78 (17) | C1—C2—C7—C6 | −179.11 (16) |
| C11—S1—C1—C2 | −79.78 (16) | C2—C1—C8—O1 | 0.13 (19) |
| C8—C1—C2—C7 | −0.26 (19) | S1—C1—C8—O1 | −176.95 (12) |
| S1—C1—C2—C7 | 176.92 (13) | C2—C1—C8—C10 | −179.71 (19) |
| C8—C1—C2—C3 | 179.99 (19) | S1—C1—C8—C10 | 3.2 (3) |
| S1—C1—C2—C3 | −2.8 (3) | C7—O1—C8—C1 | 0.06 (18) |
| C7—C2—C3—C4 | −0.2 (2) | C7—O1—C8—C10 | 179.94 (15) |
| C1—C2—C3—C4 | 179.56 (18) | O3—S1—C11—C16 | 43.02 (15) |
| C2—C3—C4—C5 | 0.0 (3) | O2—S1—C11—C16 | 172.87 (12) |
| C2—C3—C4—Br1 | −178.90 (12) | C1—S1—C11—C16 | −73.92 (14) |
| O2i—Br1—C4—C3 | 56.5 (3) | O3—S1—C11—C12 | −81.75 (14) |
| O2i—Br1—C4—C5 | −122.5 (3) | O2—S1—C11—C12 | 48.11 (14) |
| C3—C4—C5—C6 | −0.4 (3) | C1—S1—C11—C12 | 161.32 (13) |
| Br1—C4—C5—C6 | 178.57 (13) | C16—C11—C12—C13 | 57.1 (2) |
| C4—C5—C6—C7 | 0.8 (2) | S1—C11—C12—C13 | −178.14 (14) |
| C4—C5—C6—C9 | −177.76 (17) | C11—C12—C13—C14 | −56.2 (2) |
| C8—O1—C7—C6 | 179.18 (16) | C12—C13—C14—C15 | 55.8 (2) |
| C8—O1—C7—C2 | −0.24 (18) | C13—C14—C15—C16 | −55.0 (3) |
| C5—C6—C7—O1 | 179.69 (15) | C12—C11—C16—C15 | −56.6 (2) |
| C9—C6—C7—O1 | −1.8 (3) | S1—C11—C16—C15 | 179.81 (12) |
| C5—C6—C7—C2 | −1.0 (3) | C14—C15—C16—C11 | 54.9 (2) |
Symmetry codes: (i) −x, −y+1, −z+1.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C10—H10B···O2ii | 0.98 | 2.55 | 3.384 (2) | 143 |
Symmetry codes: (ii) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LR2008).
References
- Akgul, Y. Y. & Anil, H. (2003). Phytochemistry, 63, 939–943. [DOI] [PubMed]
- Aslam, S. N., Stevenson, P. C., Kokubun, T. & Hall, D. R. (2009). Microbiol. Res. 164, 191–195. [DOI] [PubMed]
- Brandenburg, K. (1998). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (2009). APEX2 SADABS and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Choi, H. D., Seo, P. J., Son, B. W. & Lee, U. (2011a). Acta Cryst. E67, o542. [DOI] [PMC free article] [PubMed]
- Choi, H. D., Seo, P. J., Son, B. W. & Lee, U. (2011b). Acta Cryst. E67, o828. [DOI] [PMC free article] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Galal, S. A., Abd El-All, A. S., Abdallah, M. M. & El-Diwani, H. I. (2009). Bioorg. Med. Chem. Lett 19, 2420–2428. [DOI] [PubMed]
- Khan, M. W., Alam, M. J., Rashid, M. A. & Chowdhury, R. (2005). Bioorg. Med. Chem 13, 4796–4805. [DOI] [PubMed]
- Politzer, P., Lane, P., Concha, M. C., Ma, Y. & Murray, J. S. (2007). J. Mol. Model 13, 305–311. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Soekamto, N. H., Achmad, S. A., Ghisalberti, E. L., Hakim, E. H. & Syah, Y. M. (2003). Phytochemistry, 64, 831–834. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053681101539X/lr2008sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053681101539X/lr2008Isup2.hkl
Supplementary material file. DOI: 10.1107/S160053681101539X/lr2008Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




