Abstract
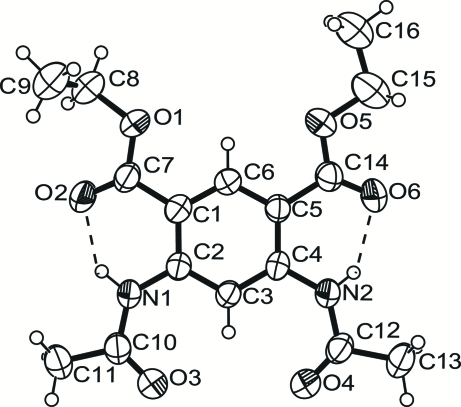
In the title compound, C16H20N2O6, two intramolecular N—H⋯O hydrogen bonds occur, in which the carbonyl O atoms of the ethyl acetate groups serve as the acceptor atoms; both motifs generate S(6) rings. In the crystal, molecules are linked by weak C—H⋯O links (with the acceptor O atoms part of the amide groups), generating [001] chains.
Related literature
For background to intramolecular hydrogen bonds this class of compound, see: Zhu et al. (2000 ▶); Yuan et al. (2004 ▶); Feng et al. (2009 ▶); Yan et al. (2010 ▶); Zhang et al. (2008 ▶). For a related structure, see: Zhang et al. (2006 ▶).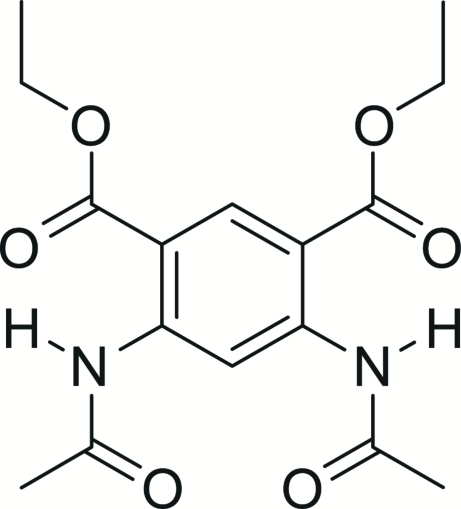
Experimental
Crystal data
C16H20N2O6
M r = 336.34
Triclinic,

a = 7.951 (3) Å
b = 10.249 (3) Å
c = 11.109 (4) Å
α = 76.70 (3)°
β = 77.42 (3)°
γ = 76.50 (2)°
V = 843.7 (5) Å3
Z = 2
Mo Kα radiation
μ = 0.10 mm−1
T = 292 K
0.50 × 0.46 × 0.40 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
3149 measured reflections
3094 independent reflections
1826 reflections with I > 2σ(I)
R int = 0.004
3 standard reflections every 150 reflections intensity decay: 4.5%
Refinement
R[F 2 > 2σ(F 2)] = 0.079
wR(F 2) = 0.268
S = 1.09
3094 reflections
223 parameters
4 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.59 e Å−3
Δρmin = −0.65 e Å−3
Data collection: DIFRAC (Gabe et al., 1993 ▶); cell refinement: DIFRAC; data reduction: NRCVAX (Gabe et al., 1989 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811013845/hb5828sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811013845/hb5828Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1N⋯O2 | 0.88 (4) | 1.92 (3) | 2.676 (4) | 144 (3) |
| N2—H2N⋯O6 | 0.81 (4) | 1.96 (4) | 2.656 (4) | 143 (3) |
| C9—H9B⋯O3i | 0.96 | 2.54 | 3.445 (6) | 157 |
| C16—H16A⋯O4ii | 0.96 | 2.57 | 3.485 (7) | 160 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
The authors acknowledge the National Natural Science Foundation of China (20774059) for funding this work, and the Analytical & Testing Center of Sichuan University for the X-ray analysis.
supplementary crystallographic information
Comment
Hydrogen bonds play a vital role in assisting formation of aromatic oligoamide foldmers and macrocycles (Zhu et al., 2000; Yuan et al., 2004; Feng et al., 2009). It is important to examine the structural feature of the backbones that is responsible for the formation of foldmers and macrocycles. Research has focused on the role of intramolecular hydrogen bonds in regulating conformations and interactions both in solution and the solid state (Yan et al., 2010; Zhang et al. 2008). Herein, we report on the crystal structure of the aromatic monomer containing intramolecular hydrogen bonds, which could be applied as important building blocks to construct macrocycles.
The title compound comprises two similar six-member hydrogen bonds. There are two possible comformations: (a) one with the ethoxy O atoms involving in the intramolecular NH···OC2H5 six-member hydrogen bonds (conformation a), and (b) the other with carbonyl O atoms that form intramolecualr H-bonds (conformation b) (Fig. 1). However, the crystal structure revealed the existence of two six-member hydrogen bonds that involve carbonyl O atoms, indicating that conformation b is more stable and is sustained by two intromolecular hydrogen bond N1H1···O2 (Table1, Fig. 2). It is expected that this type of hydrogen bonds may be exploited to construct macrocyles and supamolecular architecture with folded conformations. In the crystal, the molecules are linked by C—H···O interactions.
Experimental
For synthesis of the title compound, Pd/C (50 mg) was added to the solution of diethyl 4,6-dinitroisophthalate (500 mg, 1.60 mmol) in CH2Cl2 and the mixture was stirred at room temperature under H2 atmosphere for 6 h. After removal of Pd/C and solvent, the white solid was obtained and then dissolved in CH2Cl2 (50 ml). Et3N (335 mg, 3.31 mmol) was added to the above solution followed by dropping acetyl chloride (350 mg, 4.46 mmol). After stirring 2 h at room temperature, the mixture was washed with distilled water, dried over anhydrous Na2SO4. Removal of solvent under reduced pressure gave the crude product, which was recrystallized from methanol to yield the white product (430 mg, yield 79.8%). 1H NMR (400 MHz, CDCl3): δ 10.95 (s, 2H), 9.98 (s, 1H), 8.74 (s, 1H), 4.35 (m, 4H), 2.25 (s, 6H), 1.20 (t, J = 6.4 Hz, 6H).
The crystal of the title compound was grown from the THF solution. This compound was dissolved in THF and filtered, then the filtrate was allowed to stand undisturbed to give the single-crystal.
Refinement
The H atoms bonded to atom N1 and N2 of the title compound were located from difference Fourier maps and refined isotropically [N1—H = 1.88 (4), N2—H = 0.81 (4) Å]. H atoms bonded to C atoms were placed in calculated positions and refined using a riding model, with C—H = 0.9300 Å and Uiso(H) = 1.2Ueq(C) for aryl H atoms, C—H = 0.9700 Å and Uiso(H) = 1.2Ueq(C) for methylene H atoms, C—H = 0.9600 Å and Uiso(H) = 1.5Ueq(C) for methyl H atoms. The deepest difference hole of -0.65 e.Å-3 is 0.33 Å from atom C16.
Figures
Fig. 1.
The two possible intramolecular hydrogen bond conformations of the title compound.
Fig. 2.
A view of the title compound with displacement ellipsoids shown at the 50% probability level. Hydrogen bonds are indicated by broken lines.
Crystal data
| C16H20N2O6 | Z = 2 |
| Mr = 336.34 | F(000) = 356 |
| Triclinic, P1 | Dx = 1.324 Mg m−3 |
| a = 7.951 (3) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 10.249 (3) Å | Cell parameters from 20 reflections |
| c = 11.109 (4) Å | θ = 5.4–6.7° |
| α = 76.70 (3)° | µ = 0.10 mm−1 |
| β = 77.42 (3)° | T = 292 K |
| γ = 76.50 (2)° | Block, colourless |
| V = 843.7 (5) Å3 | 0.50 × 0.46 × 0.40 mm |
Data collection
| Enraf–Nonius CAD-4 diffractometer | Rint = 0.004 |
| Radiation source: fine-focus sealed tube | θmax = 25.5°, θmin = 1.9° |
| graphite | h = −9→9 |
| ω/2θ scans | k = −1→12 |
| 3149 measured reflections | l = −12→13 |
| 3094 independent reflections | 3 standard reflections every 150 reflections |
| 1826 reflections with I > 2σ(I) | intensity decay: 4.5% |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.079 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.268 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.09 | w = 1/[σ2(Fo2) + (0.1639P)2 + 0.1078P] where P = (Fo2 + 2Fc2)/3 |
| 3094 reflections | (Δ/σ)max < 0.001 |
| 223 parameters | Δρmax = 0.59 e Å−3 |
| 4 restraints | Δρmin = −0.65 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.9467 (4) | −0.1465 (3) | 0.6464 (2) | 0.0692 (9) | |
| O2 | 1.1334 (4) | −0.2976 (3) | 0.7586 (2) | 0.0706 (9) | |
| O3 | 1.0473 (5) | −0.3487 (3) | 1.2179 (3) | 0.0988 (12) | |
| O4 | 0.7415 (4) | −0.0447 (3) | 1.3243 (3) | 0.0796 (9) | |
| O5 | 0.5572 (4) | 0.2164 (3) | 0.7774 (3) | 0.0692 (8) | |
| O6 | 0.5009 (4) | 0.2628 (3) | 0.9676 (3) | 0.0718 (8) | |
| N1 | 1.0637 (4) | −0.3063 (3) | 1.0068 (3) | 0.0503 (8) | |
| H1N | 1.113 (4) | −0.338 (4) | 0.938 (3) | 0.046 (9)* | |
| N2 | 0.6413 (4) | 0.0776 (3) | 1.1468 (3) | 0.0514 (8) | |
| H2N | 0.581 (5) | 0.150 (4) | 1.120 (3) | 0.044 (10)* | |
| C1 | 0.9150 (4) | −0.1332 (4) | 0.8583 (3) | 0.0477 (8) | |
| C2 | 0.9442 (4) | −0.1847 (3) | 0.9824 (3) | 0.0427 (8) | |
| C3 | 0.8541 (4) | −0.1138 (3) | 1.0777 (3) | 0.0440 (8) | |
| H3 | 0.8745 | −0.1477 | 1.1594 | 0.053* | |
| C4 | 0.7336 (4) | 0.0075 (3) | 1.0518 (3) | 0.0413 (8) | |
| C5 | 0.7021 (4) | 0.0591 (3) | 0.9291 (3) | 0.0449 (8) | |
| C6 | 0.7941 (4) | −0.0127 (3) | 0.8356 (3) | 0.0460 (8) | |
| H6 | 0.7736 | 0.0216 | 0.7539 | 0.055* | |
| C7 | 1.0103 (5) | −0.2022 (4) | 0.7527 (3) | 0.0539 (9) | |
| C8 | 1.0398 (7) | −0.1987 (5) | 0.5319 (4) | 0.0871 (15) | |
| H8A | 1.1655 | −0.2192 | 0.5310 | 0.104* | |
| H8B | 1.0160 | −0.1303 | 0.4581 | 0.104* | |
| C9 | 0.9772 (8) | −0.3269 (6) | 0.5304 (5) | 0.1008 (17) | |
| H9A | 1.0175 | −0.3987 | 0.5961 | 0.151* | |
| H9B | 1.0233 | −0.3543 | 0.4507 | 0.151* | |
| H9C | 0.8512 | −0.3090 | 0.5434 | 0.151* | |
| C10 | 1.1082 (5) | −0.3819 (4) | 1.1169 (4) | 0.0574 (10) | |
| C11 | 1.2385 (6) | −0.5100 (4) | 1.1039 (4) | 0.0680 (11) | |
| H11A | 1.2382 | −0.5696 | 1.1844 | 0.102* | |
| H11B | 1.3533 | −0.4888 | 1.0722 | 0.102* | |
| H11C | 1.2081 | −0.5544 | 1.0466 | 0.102* | |
| C12 | 0.6424 (5) | 0.0486 (4) | 1.2735 (3) | 0.0544 (9) | |
| C13 | 0.5091 (6) | 0.1480 (5) | 1.3433 (4) | 0.0723 (12) | |
| H13A | 0.3951 | 0.1530 | 1.3249 | 0.108* | |
| H13B | 0.5399 | 0.2367 | 1.3177 | 0.108* | |
| H13C | 0.5073 | 0.1177 | 1.4320 | 0.108* | |
| C14 | 0.5778 (5) | 0.1888 (4) | 0.8965 (4) | 0.0531 (9) | |
| C15 | 0.4366 (9) | 0.3443 (6) | 0.7425 (5) | 0.1109 (14) | |
| H15A | 0.4848 | 0.4216 | 0.7463 | 0.133* | |
| H15B | 0.3245 | 0.3465 | 0.7987 | 0.133* | |
| C16 | 0.4146 (9) | 0.3488 (6) | 0.6092 (5) | 0.1109 (14) | |
| H16A | 0.3796 | 0.2666 | 0.6051 | 0.166* | |
| H16B | 0.5239 | 0.3565 | 0.5534 | 0.166* | |
| H16C | 0.3262 | 0.4263 | 0.5848 | 0.166* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0823 (19) | 0.0772 (19) | 0.0456 (15) | 0.0082 (15) | −0.0164 (13) | −0.0255 (13) |
| O2 | 0.0655 (17) | 0.0820 (19) | 0.0598 (17) | 0.0178 (15) | −0.0127 (13) | −0.0357 (14) |
| O3 | 0.130 (3) | 0.084 (2) | 0.0577 (19) | 0.040 (2) | −0.0244 (18) | −0.0185 (16) |
| O4 | 0.094 (2) | 0.082 (2) | 0.0529 (16) | 0.0193 (18) | −0.0208 (15) | −0.0222 (14) |
| O5 | 0.0774 (19) | 0.0613 (16) | 0.0625 (17) | 0.0094 (14) | −0.0272 (14) | −0.0076 (13) |
| O6 | 0.083 (2) | 0.0520 (15) | 0.0749 (19) | 0.0166 (14) | −0.0225 (15) | −0.0227 (14) |
| N1 | 0.0532 (17) | 0.0460 (16) | 0.0500 (17) | 0.0046 (13) | −0.0107 (14) | −0.0184 (13) |
| N2 | 0.0526 (18) | 0.0501 (18) | 0.0507 (18) | 0.0036 (15) | −0.0115 (14) | −0.0189 (14) |
| C1 | 0.0476 (19) | 0.0513 (19) | 0.0465 (19) | −0.0098 (16) | −0.0054 (15) | −0.0161 (15) |
| C2 | 0.0392 (17) | 0.0438 (17) | 0.0469 (18) | −0.0060 (14) | −0.0068 (14) | −0.0147 (14) |
| C3 | 0.0455 (18) | 0.0449 (18) | 0.0428 (18) | −0.0052 (15) | −0.0074 (14) | −0.0147 (14) |
| C4 | 0.0353 (16) | 0.0430 (17) | 0.0480 (18) | −0.0066 (14) | −0.0029 (13) | −0.0182 (14) |
| C5 | 0.0436 (18) | 0.0433 (18) | 0.0492 (19) | −0.0035 (15) | −0.0102 (15) | −0.0143 (15) |
| C6 | 0.0485 (19) | 0.0480 (18) | 0.0430 (17) | −0.0067 (15) | −0.0100 (14) | −0.0120 (14) |
| C7 | 0.055 (2) | 0.058 (2) | 0.051 (2) | −0.0040 (18) | −0.0062 (16) | −0.0240 (17) |
| C8 | 0.105 (4) | 0.096 (3) | 0.054 (2) | 0.007 (3) | −0.009 (2) | −0.032 (2) |
| C9 | 0.121 (4) | 0.107 (4) | 0.081 (3) | 0.002 (3) | −0.026 (3) | −0.048 (3) |
| C10 | 0.058 (2) | 0.051 (2) | 0.062 (2) | 0.0021 (17) | −0.0145 (18) | −0.0186 (18) |
| C11 | 0.076 (3) | 0.048 (2) | 0.077 (3) | 0.0043 (19) | −0.021 (2) | −0.0148 (19) |
| C12 | 0.056 (2) | 0.060 (2) | 0.050 (2) | −0.0067 (18) | −0.0058 (17) | −0.0237 (17) |
| C13 | 0.071 (3) | 0.081 (3) | 0.063 (2) | 0.005 (2) | −0.005 (2) | −0.035 (2) |
| C14 | 0.058 (2) | 0.0453 (19) | 0.058 (2) | −0.0045 (17) | −0.0150 (17) | −0.0146 (16) |
| C15 | 0.130 (3) | 0.087 (3) | 0.103 (3) | 0.021 (2) | −0.050 (3) | −0.008 (2) |
| C16 | 0.130 (3) | 0.087 (3) | 0.103 (3) | 0.021 (2) | −0.050 (3) | −0.008 (2) |
Geometric parameters (Å, °)
| O1—C7 | 1.338 (4) | C5—C14 | 1.482 (5) |
| O1—C8 | 1.474 (5) | C6—H6 | 0.9300 |
| O2—C7 | 1.212 (4) | C8—C9 | 1.514 (6) |
| O3—C10 | 1.212 (5) | C8—H8A | 0.9700 |
| O4—C12 | 1.204 (5) | C8—H8B | 0.9700 |
| O5—C14 | 1.326 (4) | C9—H9A | 0.9600 |
| O5—C15 | 1.459 (5) | C9—H9B | 0.9600 |
| O6—C14 | 1.192 (4) | C9—H9C | 0.9600 |
| N1—C10 | 1.359 (5) | C10—C11 | 1.485 (5) |
| N1—C2 | 1.390 (4) | C11—H11A | 0.9600 |
| N1—H1N | 0.88 (4) | C11—H11B | 0.9600 |
| N2—C12 | 1.371 (5) | C11—H11C | 0.9600 |
| N2—C4 | 1.391 (4) | C12—C13 | 1.505 (5) |
| N2—H2N | 0.81 (4) | C13—H13A | 0.9600 |
| C1—C6 | 1.386 (5) | C13—H13B | 0.9600 |
| C1—C2 | 1.408 (5) | C13—H13C | 0.9600 |
| C1—C7 | 1.482 (5) | C15—C16 | 1.517 (7) |
| C2—C3 | 1.393 (4) | C15—H15A | 0.9700 |
| C3—C4 | 1.395 (4) | C15—H15B | 0.9700 |
| C3—H3 | 0.9300 | C16—H16A | 0.9600 |
| C4—C5 | 1.398 (5) | C16—H16B | 0.9600 |
| C5—C6 | 1.388 (4) | C16—H16C | 0.9600 |
| C7—O1—C8 | 117.5 (3) | H9A—C9—H9B | 109.5 |
| C14—O5—C15 | 114.2 (3) | C8—C9—H9C | 109.5 |
| C10—N1—C2 | 130.8 (3) | H9A—C9—H9C | 109.5 |
| C10—N1—H1N | 117 (2) | H9B—C9—H9C | 109.5 |
| C2—N1—H1N | 112 (2) | O3—C10—N1 | 123.3 (3) |
| C12—N2—C4 | 131.2 (3) | O3—C10—C11 | 122.3 (4) |
| C12—N2—H2N | 116 (3) | N1—C10—C11 | 114.4 (3) |
| C4—N2—H2N | 112 (3) | C10—C11—H11A | 109.5 |
| C6—C1—C2 | 118.0 (3) | C10—C11—H11B | 109.5 |
| C6—C1—C7 | 119.7 (3) | H11A—C11—H11B | 109.5 |
| C2—C1—C7 | 122.3 (3) | C10—C11—H11C | 109.5 |
| N1—C2—C3 | 121.5 (3) | H11A—C11—H11C | 109.5 |
| N1—C2—C1 | 118.7 (3) | H11B—C11—H11C | 109.5 |
| C3—C2—C1 | 119.9 (3) | O4—C12—N2 | 124.0 (3) |
| C2—C3—C4 | 120.7 (3) | O4—C12—C13 | 122.9 (4) |
| C2—C3—H3 | 119.6 | N2—C12—C13 | 113.1 (3) |
| C4—C3—H3 | 119.6 | C12—C13—H13A | 109.5 |
| N2—C4—C3 | 121.0 (3) | C12—C13—H13B | 109.5 |
| N2—C4—C5 | 119.0 (3) | H13A—C13—H13B | 109.5 |
| C3—C4—C5 | 120.0 (3) | C12—C13—H13C | 109.5 |
| C6—C5—C4 | 118.3 (3) | H13A—C13—H13C | 109.5 |
| C6—C5—C14 | 119.7 (3) | H13B—C13—H13C | 109.5 |
| C4—C5—C14 | 122.0 (3) | O6—C14—O5 | 121.7 (3) |
| C1—C6—C5 | 123.0 (3) | O6—C14—C5 | 125.2 (3) |
| C1—C6—H6 | 118.5 | O5—C14—C5 | 113.1 (3) |
| C5—C6—H6 | 118.5 | O5—C15—C16 | 106.1 (4) |
| O2—C7—O1 | 122.4 (3) | O5—C15—H15A | 110.5 |
| O2—C7—C1 | 125.2 (3) | C16—C15—H15A | 110.5 |
| O1—C7—C1 | 112.4 (3) | O5—C15—H15B | 110.5 |
| O1—C8—C9 | 108.6 (4) | C16—C15—H15B | 110.5 |
| O1—C8—H8A | 110.0 | H15A—C15—H15B | 108.7 |
| C9—C8—H8A | 110.0 | C15—C16—H16A | 109.5 |
| O1—C8—H8B | 110.0 | C15—C16—H16B | 109.5 |
| C9—C8—H8B | 110.0 | H16A—C16—H16B | 109.5 |
| H8A—C8—H8B | 108.3 | C15—C16—H16C | 109.5 |
| C8—C9—H9A | 109.5 | H16A—C16—H16C | 109.5 |
| C8—C9—H9B | 109.5 | H16B—C16—H16C | 109.5 |
| C10—N1—C2—C3 | 4.6 (6) | C14—C5—C6—C1 | 178.7 (3) |
| C10—N1—C2—C1 | −175.5 (3) | C8—O1—C7—O2 | −4.3 (6) |
| C6—C1—C2—N1 | 179.3 (3) | C8—O1—C7—C1 | 175.0 (3) |
| C7—C1—C2—N1 | −1.5 (5) | C6—C1—C7—O2 | 170.3 (4) |
| C6—C1—C2—C3 | −0.8 (5) | C2—C1—C7—O2 | −8.9 (6) |
| C7—C1—C2—C3 | 178.4 (3) | C6—C1—C7—O1 | −9.1 (5) |
| N1—C2—C3—C4 | −179.5 (3) | C2—C1—C7—O1 | 171.8 (3) |
| C1—C2—C3—C4 | 0.6 (5) | C7—O1—C8—C9 | 83.1 (5) |
| C12—N2—C4—C3 | −1.0 (6) | C2—N1—C10—O3 | −2.1 (7) |
| C12—N2—C4—C5 | 178.0 (3) | C2—N1—C10—C11 | 177.7 (3) |
| C2—C3—C4—N2 | 179.0 (3) | C4—N2—C12—O4 | 6.1 (7) |
| C2—C3—C4—C5 | 0.0 (5) | C4—N2—C12—C13 | −175.2 (3) |
| N2—C4—C5—C6 | −179.4 (3) | C15—O5—C14—O6 | 1.0 (6) |
| C3—C4—C5—C6 | −0.4 (5) | C15—O5—C14—C5 | −179.4 (4) |
| N2—C4—C5—C14 | 2.1 (5) | C6—C5—C14—O6 | −174.9 (4) |
| C3—C4—C5—C14 | −178.9 (3) | C4—C5—C14—O6 | 3.6 (6) |
| C2—C1—C6—C5 | 0.4 (5) | C6—C5—C14—O5 | 5.6 (5) |
| C7—C1—C6—C5 | −178.8 (3) | C4—C5—C14—O5 | −176.0 (3) |
| C4—C5—C6—C1 | 0.2 (5) | C14—O5—C15—C16 | −173.5 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···O2 | 0.88 (4) | 1.92 (3) | 2.676 (4) | 144 (3) |
| N2—H2N···O6 | 0.81 (4) | 1.96 (4) | 2.656 (4) | 143 (3) |
| C9—H9B···O3i | 0.96 | 2.54 | 3.445 (6) | 157 |
| C16—H16A···O4ii | 0.96 | 2.57 | 3.485 (7) | 160 |
Symmetry codes: (i) x, y, z−1; (ii) −x+1, −y, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HB5828).
References
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Feng, W., Yamato, K., Yang, L. Q., Ferguson, J. S., Zhong, L. J., Zou, S. L., Yuan, L. H., Zeng, X. C. & Gong, B. (2009). J. Am. Chem. Soc. 131, 2629–2637. [DOI] [PubMed]
- Gabe, E. J., Le Page, Y., Charland, J.-P., Lee, F. L. & White, P. S. (1989). J. Appl. Cryst. 22, 384–387.
- Gabe, E. J., White, P. S. & Enright, G. D. (1993). DIFRAC American Crystallographic Association, Pittsburgh Meeting Abstract, PA 104.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Yan, Y., Qin, B., Ren, C. L., Chen, X. Y., Yip, Y. K., Ye, R. J., Zhang, D. W., Su, H. B. & Zeng, H. Q. (2010). J. Am. Chem. Soc. 132, 5869–5879. [DOI] [PubMed]
- Yuan, L. H., Feng, W., Yamato, K., Sanford, A. R., Xu, D. G., Guo, H. & Gong, B. (2004). J. Am. Chem. Soc. 126, 11120–11121. [DOI] [PubMed]
- Zhang, A. M., Han, Y. H., Yamato, K., Zeng, X. C. & Gong, B. (2006). Org. Lett. 8, 803–806. [DOI] [PubMed]
- Zhang, Y. F., Yamato, K., Zhong, K., Zhu, J., Deng, J. G. & Gong, B. (2008). Org. Lett. 10, 4338–4342. [DOI] [PubMed]
- Zhu, J., Parra, R. D., Zeng, H. Q., Skrzypczak-Jankun, E., Zeng, X. C. & Gong, B. (2000). J. Am. Chem. Soc. 122, 4219–4220.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811013845/hb5828sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811013845/hb5828Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report