Abstract
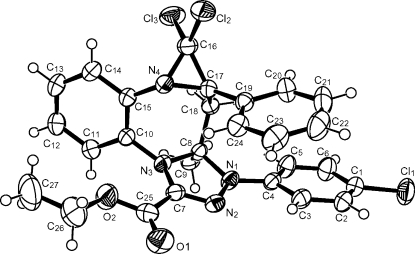
In the title compound, C27H23Cl3N4O2, the seven-membered diazepine ring adopts a boat conformation. The triazole ring makes dihedral angles of 17.24 (8) and 82.86 (8)°, respectively, with the chlorobenzene ring and the benzene ring of the benzodiazepine unit.
Related literature
For background to benzodiazepine derivatives, see: Barltrop et al. (1959 ▶); El Hazazi et al. (2003 ▶); Sharp & Hamilton (1946 ▶). For related structures, see: Chiaroni et al. (1995 ▶); El Hazazi et al. (2000 ▶).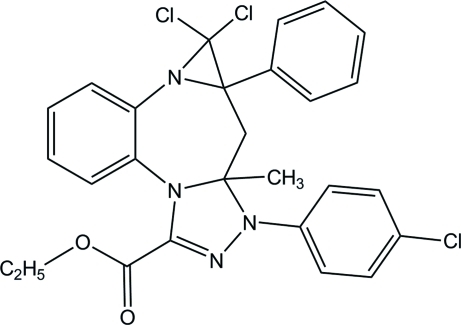
Experimental
Crystal data
C27H23Cl3N4O2
M r = 541.84
Triclinic,

a = 9.679 (3) Å
b = 11.256 (3) Å
c = 12.661 (2) Å
α = 79.09 (2)°
β = 76.46 (2)°
γ = 73.04 (2)°
V = 1271.8 (6) Å3
Z = 2
Mo Kα radiation
μ = 0.39 mm−1
T = 300 K
0.3 × 0.15 × 0.1 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
6860 measured reflections
5536 independent reflections
4616 reflections with I > 2σ(I)
R int = 0.010
2 standard reflections every 60 min intensity decay: 1.0%
Refinement
R[F 2 > 2σ(F 2)] = 0.036
wR(F 2) = 0.101
S = 1.05
5536 reflections
327 parameters
H-atom parameters constrained
Δρmax = 0.24 e Å−3
Δρmin = −0.33 e Å−3
Data collection: CAD-4 EXPRESS (Enraf–Nonius, 1989 ▶); cell refinement: CAD-4 EXPRESS; data reduction: MolEN (Fair, 1990 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811014115/is2697sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811014115/is2697Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Comment
In order to develop work carried out before in our laboratory we were interested in the synthesis of new derivatives benzodiazepinic (El Hazazi et al., 2003). These reactions are either of the reactions of cycloadditions [2 + 1] realising generated carbenes in situ or reactions of transfer of methelyne.
In the present work, we report the synthesis of new benzodiazepine derivatives via addition of dichlorocarbene to [1,2,4]triazolo[4,3-a][1,5]benzodiazepine obtained stereospecifically by the addition of nitrilimines (Sharp et al., 1946) on 1,5-benzodiazepine (Barltrop et al., 1959).
Dichloroazacyclopropanation of [1,2,4]triazolo[4,3-a][1,5]benzodiazepine occurs readily under phase transfer catalysis conditions (liquid-liquid) with chloroform, aqueous sodium hydroxide and benzyltriethylammonium chloride (TBA-Cl) to give the corresponding bichloroadduct 2 (Fig. 1). Thus, the reaction of [1,2,4]triazolo[4,3-a][1,5]benzodiazepine 1 with dichlorocarbene in these conditions produce gem-dichloroaziridino[2,1-d][1,2,4] triazolo[4,3-a][1,5]benzodiazepine 2 in good yield.
The crystallographic study made it possible to determine the stereochemistry of the product 2. The crystalline structure confirms that the condensation of dichlorocarbene is carried out on double bond C=N substituted by the phenyl and shows that the product 2α obtained is of trans relative stereochemistry (Fig. 2). The main geometric features of this group are in good agreement it those observed in similar compound (Chiaroni et al., 1995; El Hazazi et al., 2000).
Experimental
[1,2,4]Triazolo[4,3-a][1,5]benzodiazepine 1 (0.65 mm l) in 2 ml of chloroform were stirred with 2 ml of aqueous 50% NaOH solution and a catalytic amount of triethylbenzylammonium chloride (TBA-Cl). After 4 h the mixture was poured into 5 ml of water and extracted with ether. The organic phase was then dried over anhydrous sodium sulfate and the solvent was removed under reduced pressure. The crude product was chromatographied on a silica gel column (eluent: hexane/ethyl acetate 95/5) and recrystallized from ethanol/chloroform to give a compound 2α
The observation to be noted is that the condensation of dichlorocarbene to [1,2,4]triazolo[4,3-a][1,5]benzodiazepine is streospecific. The structure elucidation of the compound 2 was determinate on spectral data (1H NMR, 13C NMR and mass spectroscopy). The compound revealed in their spectra of mass the molecular peak located at m/z = 541 compatible with their empirical formula. The NMR spectrum of this product shows that the decalage of the chemical shifts of different grouping from monoadduct. In the 13C NMR spectrum of compound, we remarked the absence of the signals attributed to the double bond C5=N6 of cycle diazepinic. The 13C NMR spectrum of product was consistent with the presence of only one diasterioisomer. These spectral analyses do not enable us to determine relative stereochemistry of the aziridino[2,1-d][1,2,4]]triazolo[4,3-a][1,5]benzodiazepine (trans 2α or cis 2β).
Refinement
All H atoms were located in a difference map and then refined using a riding model, with C—H = 0.96 Å and Uiso(H) = 1.2Ueq(C) for CH3, C—H = 0.97 Å and Uiso(H) =1.2Ueq(C) for CH2, and C—H = 0.93 Å and Uiso(H) =1.2Ueq(C) for CH.
Figures
Fig. 1.
The reaction scheme of the title compound
Fig. 2.
The molecular structure of the title compound, with 50% probability ellipsoids.
Crystal data
| C27H23Cl3N4O2 | Z = 2 |
| Mr = 541.84 | F(000) = 560 |
| Triclinic, P1 | Dx = 1.415 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.679 (3) Å | Cell parameters from 25 reflections |
| b = 11.256 (3) Å | θ = 10–15° |
| c = 12.661 (2) Å | µ = 0.39 mm−1 |
| α = 79.09 (2)° | T = 300 K |
| β = 76.46 (2)° | Prism, yellow |
| γ = 73.04 (2)° | 0.3 × 0.15 × 0.1 mm |
| V = 1271.8 (6) Å3 |
Data collection
| Enraf–Nonius CAD-4 diffractometer | Rint = 0.010 |
| Radiation source: fine-focus sealed tube | θmax = 27.0°, θmin = 2.2° |
| graphite | h = −12→2 |
| ω/2θ scans | k = −14→14 |
| 6860 measured reflections | l = −16→16 |
| 5536 independent reflections | 2 standard reflections every 60 min |
| 4616 reflections with I > 2σ(I) | intensity decay: 1.0% |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.036 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.101 | H-atom parameters constrained |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.0518P)2 + 0.3226P] where P = (Fo2 + 2Fc2)/3 |
| 5536 reflections | (Δ/σ)max = 0.001 |
| 327 parameters | Δρmax = 0.24 e Å−3 |
| 0 restraints | Δρmin = −0.33 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 1.31467 (5) | −0.06197 (4) | 0.57617 (5) | 0.06714 (16) | |
| Cl2 | 0.74695 (5) | 0.33836 (4) | 0.03031 (4) | 0.05465 (13) | |
| Cl3 | 0.50575 (5) | 0.37629 (5) | 0.21522 (4) | 0.05877 (14) | |
| O1 | 1.09072 (14) | 0.71899 (13) | 0.20132 (12) | 0.0619 (3) | |
| O2 | 0.85011 (15) | 0.79523 (12) | 0.19906 (12) | 0.0638 (4) | |
| N1 | 0.94972 (12) | 0.42660 (12) | 0.40621 (10) | 0.0369 (3) | |
| N2 | 1.02302 (13) | 0.51079 (12) | 0.34036 (10) | 0.0366 (3) | |
| N3 | 0.78121 (13) | 0.58997 (11) | 0.34267 (10) | 0.0354 (3) | |
| N4 | 0.72403 (13) | 0.50553 (11) | 0.16401 (10) | 0.0361 (3) | |
| C1 | 1.20506 (17) | 0.07966 (15) | 0.52295 (14) | 0.0444 (3) | |
| C2 | 1.26875 (17) | 0.15749 (15) | 0.44036 (15) | 0.0459 (4) | |
| H2 | 1.3689 | 0.1335 | 0.4116 | 0.055* | |
| C3 | 1.18305 (16) | 0.27115 (15) | 0.40070 (13) | 0.0413 (3) | |
| H3 | 1.2258 | 0.3230 | 0.3444 | 0.050* | |
| C4 | 1.03279 (15) | 0.30883 (13) | 0.44432 (11) | 0.0346 (3) | |
| C5 | 0.97014 (17) | 0.22829 (16) | 0.52598 (14) | 0.0470 (4) | |
| H5 | 0.8699 | 0.2514 | 0.5548 | 0.056* | |
| C6 | 1.05614 (19) | 0.11349 (16) | 0.56493 (15) | 0.0507 (4) | |
| H6 | 1.0135 | 0.0596 | 0.6191 | 0.061* | |
| C7 | 0.92343 (15) | 0.60263 (13) | 0.30379 (11) | 0.0345 (3) | |
| C8 | 0.78887 (14) | 0.46551 (13) | 0.40868 (11) | 0.0322 (3) | |
| C9 | 0.69700 (16) | 0.47994 (15) | 0.52411 (12) | 0.0405 (3) | |
| H9A | 0.7524 | 0.5014 | 0.5681 | 0.049* | |
| H9B | 0.6731 | 0.4025 | 0.5568 | 0.049* | |
| H9C | 0.6079 | 0.5450 | 0.5197 | 0.049* | |
| C10 | 0.65238 (15) | 0.65391 (13) | 0.29762 (12) | 0.0355 (3) | |
| C11 | 0.55573 (18) | 0.75943 (15) | 0.34035 (15) | 0.0464 (4) | |
| H11 | 0.5753 | 0.7887 | 0.3978 | 0.056* | |
| C12 | 0.43014 (19) | 0.82118 (16) | 0.29742 (17) | 0.0555 (4) | |
| H12 | 0.3658 | 0.8918 | 0.3259 | 0.067* | |
| C13 | 0.40124 (19) | 0.77727 (16) | 0.21230 (17) | 0.0567 (5) | |
| H13 | 0.3171 | 0.8187 | 0.1837 | 0.068* | |
| C14 | 0.49615 (18) | 0.67216 (16) | 0.16904 (15) | 0.0492 (4) | |
| H14 | 0.4760 | 0.6436 | 0.1115 | 0.059* | |
| C15 | 0.62242 (15) | 0.60913 (13) | 0.21222 (12) | 0.0366 (3) | |
| C16 | 0.68332 (17) | 0.39468 (14) | 0.15834 (13) | 0.0403 (3) | |
| C17 | 0.78959 (15) | 0.38713 (12) | 0.23111 (11) | 0.0333 (3) | |
| C18 | 0.73573 (15) | 0.37907 (13) | 0.35398 (11) | 0.0336 (3) | |
| H18A | 0.7696 | 0.2931 | 0.3866 | 0.040* | |
| H18B | 0.6290 | 0.4013 | 0.3692 | 0.040* | |
| C19 | 0.94952 (15) | 0.32550 (13) | 0.19097 (11) | 0.0351 (3) | |
| C20 | 0.99906 (19) | 0.19656 (15) | 0.21963 (14) | 0.0483 (4) | |
| H20 | 0.9342 | 0.1518 | 0.2625 | 0.058* | |
| C21 | 1.1452 (2) | 0.13472 (18) | 0.18427 (17) | 0.0628 (5) | |
| H21 | 1.1776 | 0.0486 | 0.2031 | 0.075* | |
| C22 | 1.2422 (2) | 0.2009 (2) | 0.12121 (17) | 0.0644 (5) | |
| H22 | 1.3402 | 0.1597 | 0.0985 | 0.077* | |
| C23 | 1.19335 (19) | 0.3282 (2) | 0.09202 (15) | 0.0570 (4) | |
| H23 | 1.2588 | 0.3726 | 0.0494 | 0.068* | |
| C24 | 1.04672 (17) | 0.39071 (15) | 0.12584 (12) | 0.0427 (3) | |
| H24 | 1.0141 | 0.4763 | 0.1046 | 0.051* | |
| C25 | 0.96658 (18) | 0.71092 (15) | 0.22958 (13) | 0.0422 (3) | |
| C26 | 0.8773 (3) | 0.9023 (2) | 0.1207 (2) | 0.0818 (7) | |
| H26A | 0.9327 | 0.8752 | 0.0512 | 0.098* | |
| H26B | 0.9332 | 0.9448 | 0.1478 | 0.098* | |
| C27 | 0.7316 (4) | 0.9875 (2) | 0.1068 (3) | 0.0985 (9) | |
| H27A | 0.6806 | 1.0179 | 0.1751 | 0.118* | |
| H27B | 0.6750 | 0.9428 | 0.0844 | 0.118* | |
| H27C | 0.7451 | 1.0570 | 0.0520 | 0.118* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0493 (3) | 0.0441 (2) | 0.0967 (4) | 0.00124 (19) | −0.0208 (2) | 0.0058 (2) |
| Cl2 | 0.0623 (3) | 0.0590 (3) | 0.0487 (2) | −0.0108 (2) | −0.01682 (19) | −0.02154 (19) |
| Cl3 | 0.0403 (2) | 0.0739 (3) | 0.0728 (3) | −0.0232 (2) | −0.0127 (2) | −0.0197 (2) |
| O1 | 0.0495 (7) | 0.0679 (8) | 0.0696 (8) | −0.0300 (6) | −0.0053 (6) | 0.0043 (7) |
| O2 | 0.0588 (8) | 0.0488 (7) | 0.0836 (9) | −0.0227 (6) | −0.0257 (7) | 0.0213 (6) |
| N1 | 0.0249 (5) | 0.0414 (6) | 0.0421 (6) | −0.0082 (5) | −0.0074 (5) | 0.0008 (5) |
| N2 | 0.0305 (6) | 0.0423 (6) | 0.0388 (6) | −0.0127 (5) | −0.0072 (5) | −0.0041 (5) |
| N3 | 0.0284 (6) | 0.0341 (6) | 0.0449 (7) | −0.0084 (5) | −0.0116 (5) | −0.0022 (5) |
| N4 | 0.0338 (6) | 0.0364 (6) | 0.0382 (6) | −0.0048 (5) | −0.0115 (5) | −0.0060 (5) |
| C1 | 0.0385 (8) | 0.0380 (8) | 0.0562 (9) | −0.0028 (6) | −0.0162 (7) | −0.0068 (7) |
| C2 | 0.0280 (7) | 0.0452 (8) | 0.0620 (10) | −0.0033 (6) | −0.0075 (7) | −0.0117 (7) |
| C3 | 0.0298 (7) | 0.0439 (8) | 0.0488 (8) | −0.0096 (6) | −0.0050 (6) | −0.0060 (6) |
| C4 | 0.0282 (6) | 0.0395 (7) | 0.0364 (7) | −0.0055 (5) | −0.0094 (5) | −0.0066 (6) |
| C5 | 0.0310 (7) | 0.0518 (9) | 0.0487 (9) | −0.0049 (7) | −0.0032 (6) | 0.0020 (7) |
| C6 | 0.0421 (9) | 0.0482 (9) | 0.0530 (9) | −0.0075 (7) | −0.0071 (7) | 0.0056 (7) |
| C7 | 0.0316 (7) | 0.0377 (7) | 0.0377 (7) | −0.0113 (6) | −0.0083 (6) | −0.0078 (6) |
| C8 | 0.0250 (6) | 0.0347 (7) | 0.0359 (7) | −0.0057 (5) | −0.0073 (5) | −0.0038 (5) |
| C9 | 0.0301 (7) | 0.0508 (9) | 0.0400 (8) | −0.0079 (6) | −0.0041 (6) | −0.0116 (6) |
| C10 | 0.0286 (6) | 0.0324 (7) | 0.0460 (8) | −0.0061 (5) | −0.0114 (6) | −0.0038 (6) |
| C11 | 0.0435 (8) | 0.0382 (8) | 0.0587 (10) | −0.0036 (6) | −0.0152 (7) | −0.0137 (7) |
| C12 | 0.0436 (9) | 0.0403 (8) | 0.0784 (12) | 0.0061 (7) | −0.0183 (8) | −0.0160 (8) |
| C13 | 0.0401 (9) | 0.0470 (9) | 0.0814 (13) | 0.0042 (7) | −0.0291 (9) | −0.0072 (9) |
| C14 | 0.0435 (9) | 0.0478 (9) | 0.0594 (10) | −0.0026 (7) | −0.0256 (8) | −0.0093 (7) |
| C15 | 0.0307 (7) | 0.0343 (7) | 0.0444 (8) | −0.0049 (5) | −0.0102 (6) | −0.0056 (6) |
| C16 | 0.0369 (7) | 0.0444 (8) | 0.0428 (8) | −0.0098 (6) | −0.0095 (6) | −0.0123 (6) |
| C17 | 0.0313 (7) | 0.0313 (7) | 0.0374 (7) | −0.0071 (5) | −0.0073 (5) | −0.0053 (5) |
| C18 | 0.0293 (6) | 0.0347 (7) | 0.0371 (7) | −0.0098 (5) | −0.0047 (5) | −0.0046 (5) |
| C19 | 0.0335 (7) | 0.0362 (7) | 0.0340 (7) | −0.0049 (6) | −0.0063 (5) | −0.0077 (5) |
| C20 | 0.0481 (9) | 0.0363 (8) | 0.0540 (9) | −0.0037 (7) | −0.0067 (7) | −0.0052 (7) |
| C21 | 0.0583 (11) | 0.0456 (9) | 0.0701 (12) | 0.0122 (8) | −0.0128 (9) | −0.0124 (9) |
| C22 | 0.0386 (9) | 0.0769 (13) | 0.0635 (12) | 0.0086 (9) | −0.0027 (8) | −0.0211 (10) |
| C23 | 0.0388 (9) | 0.0767 (13) | 0.0500 (10) | −0.0136 (8) | 0.0024 (7) | −0.0103 (9) |
| C24 | 0.0395 (8) | 0.0464 (8) | 0.0393 (8) | −0.0090 (7) | −0.0050 (6) | −0.0052 (6) |
| C25 | 0.0466 (9) | 0.0430 (8) | 0.0424 (8) | −0.0187 (7) | −0.0096 (7) | −0.0062 (6) |
| C26 | 0.0976 (18) | 0.0566 (12) | 0.0926 (17) | −0.0362 (12) | −0.0307 (14) | 0.0282 (11) |
| C27 | 0.129 (2) | 0.0516 (12) | 0.104 (2) | −0.0106 (14) | −0.0397 (18) | 0.0166 (13) |
Geometric parameters (Å, °)
| Cl1—C1 | 1.7500 (17) | C10—C11 | 1.392 (2) |
| Cl2—C16 | 1.7614 (16) | C10—C15 | 1.395 (2) |
| Cl3—C16 | 1.7570 (17) | C11—C12 | 1.389 (2) |
| O1—C25 | 1.196 (2) | C11—H11 | 0.9300 |
| O2—C25 | 1.327 (2) | C12—C13 | 1.381 (3) |
| O2—C26 | 1.455 (2) | C12—H12 | 0.9300 |
| N1—N2 | 1.3830 (17) | C13—C14 | 1.385 (2) |
| N1—C4 | 1.3979 (18) | C13—H13 | 0.9300 |
| N1—C8 | 1.4837 (17) | C14—C15 | 1.399 (2) |
| N2—C7 | 1.2878 (19) | C14—H14 | 0.9300 |
| N3—C7 | 1.3886 (18) | C16—C17 | 1.509 (2) |
| N3—C10 | 1.4326 (18) | C17—C19 | 1.5081 (19) |
| N3—C8 | 1.4804 (18) | C17—C18 | 1.5148 (19) |
| N4—C15 | 1.4209 (19) | C18—H18A | 0.9700 |
| N4—C16 | 1.4322 (19) | C18—H18B | 0.9700 |
| N4—C17 | 1.4936 (18) | C19—C24 | 1.384 (2) |
| C1—C6 | 1.379 (2) | C19—C20 | 1.394 (2) |
| C1—C2 | 1.381 (2) | C20—C21 | 1.390 (3) |
| C2—C3 | 1.382 (2) | C20—H20 | 0.9300 |
| C2—H2 | 0.9300 | C21—C22 | 1.380 (3) |
| C3—C4 | 1.397 (2) | C21—H21 | 0.9300 |
| C3—H3 | 0.9300 | C22—C23 | 1.378 (3) |
| C4—C5 | 1.390 (2) | C22—H22 | 0.9300 |
| C5—C6 | 1.389 (2) | C23—C24 | 1.393 (2) |
| C5—H5 | 0.9300 | C23—H23 | 0.9300 |
| C6—H6 | 0.9300 | C24—H24 | 0.9300 |
| C7—C25 | 1.491 (2) | C26—C27 | 1.484 (4) |
| C8—C9 | 1.533 (2) | C26—H26A | 0.9700 |
| C8—C18 | 1.5506 (19) | C26—H26B | 0.9700 |
| C9—H9A | 0.9600 | C27—H27A | 0.9600 |
| C9—H9B | 0.9600 | C27—H27B | 0.9600 |
| C9—H9C | 0.9600 | C27—H27C | 0.9600 |
| C25—O2—C26 | 117.07 (16) | C13—C14—H14 | 120.1 |
| N2—N1—C4 | 118.46 (11) | C15—C14—H14 | 120.1 |
| N2—N1—C8 | 113.24 (11) | C10—C15—C14 | 119.52 (14) |
| C4—N1—C8 | 127.07 (12) | C10—C15—N4 | 120.50 (12) |
| C7—N2—N1 | 106.12 (12) | C14—C15—N4 | 119.84 (14) |
| C7—N3—C10 | 127.78 (12) | N4—C16—C17 | 60.97 (9) |
| C7—N3—C8 | 108.57 (11) | N4—C16—Cl3 | 121.86 (11) |
| C10—N3—C8 | 119.40 (11) | C17—C16—Cl3 | 120.64 (11) |
| C15—N4—C16 | 122.29 (12) | N4—C16—Cl2 | 114.44 (11) |
| C15—N4—C17 | 122.26 (12) | C17—C16—Cl2 | 120.61 (11) |
| C16—N4—C17 | 62.05 (9) | Cl3—C16—Cl2 | 110.44 (8) |
| C6—C1—C2 | 120.55 (15) | N4—C17—C19 | 116.19 (12) |
| C6—C1—Cl1 | 119.73 (14) | N4—C17—C16 | 56.97 (9) |
| C2—C1—Cl1 | 119.71 (12) | C19—C17—C16 | 117.06 (12) |
| C1—C2—C3 | 119.74 (14) | N4—C17—C18 | 116.74 (11) |
| C1—C2—H2 | 120.1 | C19—C17—C18 | 117.07 (12) |
| C3—C2—H2 | 120.1 | C16—C17—C18 | 119.10 (12) |
| C2—C3—C4 | 120.61 (15) | C17—C18—C8 | 113.50 (11) |
| C2—C3—H3 | 119.7 | C17—C18—H18A | 108.9 |
| C4—C3—H3 | 119.7 | C8—C18—H18A | 108.9 |
| C5—C4—C3 | 118.81 (14) | C17—C18—H18B | 108.9 |
| C5—C4—N1 | 121.63 (13) | C8—C18—H18B | 108.9 |
| C3—C4—N1 | 119.55 (13) | H18A—C18—H18B | 107.7 |
| C6—C5—C4 | 120.48 (14) | C24—C19—C20 | 119.32 (14) |
| C6—C5—H5 | 119.8 | C24—C19—C17 | 122.92 (13) |
| C4—C5—H5 | 119.8 | C20—C19—C17 | 117.74 (14) |
| C1—C6—C5 | 119.76 (16) | C21—C20—C19 | 120.20 (17) |
| C1—C6—H6 | 120.1 | C21—C20—H20 | 119.9 |
| C5—C6—H6 | 120.1 | C19—C20—H20 | 119.9 |
| N2—C7—N3 | 113.87 (13) | C22—C21—C20 | 120.11 (17) |
| N2—C7—C25 | 119.72 (13) | C22—C21—H21 | 119.9 |
| N3—C7—C25 | 126.39 (13) | C20—C21—H21 | 119.9 |
| N3—C8—N1 | 97.86 (10) | C23—C22—C21 | 119.85 (17) |
| N3—C8—C9 | 110.22 (12) | C23—C22—H22 | 120.1 |
| N1—C8—C9 | 113.22 (11) | C21—C22—H22 | 120.1 |
| N3—C8—C18 | 111.80 (11) | C22—C23—C24 | 120.47 (18) |
| N1—C8—C18 | 113.43 (11) | C22—C23—H23 | 119.8 |
| C9—C8—C18 | 109.85 (11) | C24—C23—H23 | 119.8 |
| C8—C9—H9A | 109.5 | C19—C24—C23 | 120.03 (16) |
| C8—C9—H9B | 109.5 | C19—C24—H24 | 120.0 |
| H9A—C9—H9B | 109.5 | C23—C24—H24 | 120.0 |
| C8—C9—H9C | 109.5 | O1—C25—O2 | 125.08 (15) |
| H9A—C9—H9C | 109.5 | O1—C25—C7 | 123.70 (16) |
| H9B—C9—H9C | 109.5 | O2—C25—C7 | 111.22 (13) |
| C11—C10—C15 | 119.98 (13) | O2—C26—C27 | 107.1 (2) |
| C11—C10—N3 | 119.99 (14) | O2—C26—H26A | 110.3 |
| C15—C10—N3 | 120.01 (13) | C27—C26—H26A | 110.3 |
| C12—C11—C10 | 120.18 (16) | O2—C26—H26B | 110.3 |
| C12—C11—H11 | 119.9 | C27—C26—H26B | 110.3 |
| C10—C11—H11 | 119.9 | H26A—C26—H26B | 108.6 |
| C13—C12—C11 | 119.76 (16) | C26—C27—H27A | 109.5 |
| C13—C12—H12 | 120.1 | C26—C27—H27B | 109.5 |
| C11—C12—H12 | 120.1 | H27A—C27—H27B | 109.5 |
| C12—C13—C14 | 120.77 (15) | C26—C27—H27C | 109.5 |
| C12—C13—H13 | 119.6 | H27A—C27—H27C | 109.5 |
| C14—C13—H13 | 119.6 | H27B—C27—H27C | 109.5 |
| C13—C14—C15 | 119.79 (16) | ||
| C4—N1—N2—C7 | 170.77 (12) | C16—N4—C15—C10 | 123.80 (15) |
| C8—N1—N2—C7 | 2.53 (16) | C17—N4—C15—C10 | 48.67 (19) |
| C6—C1—C2—C3 | 1.1 (3) | C16—N4—C15—C14 | −60.6 (2) |
| Cl1—C1—C2—C3 | −177.96 (13) | C17—N4—C15—C14 | −135.70 (15) |
| C1—C2—C3—C4 | 0.8 (2) | C15—N4—C16—C17 | −112.30 (14) |
| C2—C3—C4—C5 | −2.0 (2) | C15—N4—C16—Cl3 | −2.34 (19) |
| C2—C3—C4—N1 | 177.17 (14) | C17—N4—C16—Cl3 | 109.96 (14) |
| N2—N1—C4—C5 | 168.12 (14) | C15—N4—C16—Cl2 | 134.90 (12) |
| C8—N1—C4—C5 | −25.5 (2) | C17—N4—C16—Cl2 | −112.80 (12) |
| N2—N1—C4—C3 | −11.0 (2) | C15—N4—C17—C19 | −141.09 (13) |
| C8—N1—C4—C3 | 155.43 (14) | C16—N4—C17—C19 | 106.56 (14) |
| C3—C4—C5—C6 | 1.3 (2) | C15—N4—C17—C16 | 112.35 (15) |
| N1—C4—C5—C6 | −177.85 (15) | C15—N4—C17—C18 | 3.56 (18) |
| C2—C1—C6—C5 | −1.8 (3) | C16—N4—C17—C18 | −108.79 (14) |
| Cl1—C1—C6—C5 | 177.27 (14) | Cl3—C16—C17—N4 | −111.90 (13) |
| C4—C5—C6—C1 | 0.6 (3) | Cl2—C16—C17—N4 | 102.80 (13) |
| N1—N2—C7—N3 | 1.62 (16) | N4—C16—C17—C19 | −105.01 (14) |
| N1—N2—C7—C25 | 179.78 (12) | Cl3—C16—C17—C19 | 143.09 (12) |
| C10—N3—C7—N2 | −161.35 (14) | Cl2—C16—C17—C19 | −2.21 (18) |
| C8—N3—C7—N2 | −5.08 (17) | N4—C16—C17—C18 | 104.62 (14) |
| C10—N3—C7—C25 | 20.6 (2) | Cl3—C16—C17—C18 | −7.28 (18) |
| C8—N3—C7—C25 | 176.90 (13) | Cl2—C16—C17—C18 | −152.58 (11) |
| C7—N3—C8—N1 | 5.69 (13) | N4—C17—C18—C8 | −70.59 (15) |
| C10—N3—C8—N1 | 164.27 (12) | C19—C17—C18—C8 | 73.75 (15) |
| C7—N3—C8—C9 | 124.04 (12) | C16—C17—C18—C8 | −135.88 (13) |
| C10—N3—C8—C9 | −77.37 (15) | N3—C8—C18—C17 | 41.51 (15) |
| C7—N3—C8—C18 | −113.48 (12) | N1—C8—C18—C17 | −67.98 (15) |
| C10—N3—C8—C18 | 45.10 (16) | C9—C8—C18—C17 | 164.20 (12) |
| N2—N1—C8—N3 | −5.08 (14) | N4—C17—C19—C24 | 24.5 (2) |
| C4—N1—C8—N3 | −172.10 (13) | C16—C17—C19—C24 | 88.97 (18) |
| N2—N1—C8—C9 | −121.11 (13) | C18—C17—C19—C24 | −120.05 (15) |
| C4—N1—C8—C9 | 71.87 (18) | N4—C17—C19—C20 | −154.13 (13) |
| N2—N1—C8—C18 | 112.84 (13) | C16—C17—C19—C20 | −89.64 (17) |
| C4—N1—C8—C18 | −54.18 (18) | C18—C17—C19—C20 | 61.34 (18) |
| C7—N3—C10—C11 | −97.33 (19) | C24—C19—C20—C21 | 0.9 (3) |
| C8—N3—C10—C11 | 108.64 (16) | C17—C19—C20—C21 | 179.56 (16) |
| C7—N3—C10—C15 | 83.81 (19) | C19—C20—C21—C22 | 0.5 (3) |
| C8—N3—C10—C15 | −70.22 (18) | C20—C21—C22—C23 | −1.0 (3) |
| C15—C10—C11—C12 | −0.7 (3) | C21—C22—C23—C24 | 0.1 (3) |
| N3—C10—C11—C12 | −179.54 (15) | C20—C19—C24—C23 | −1.7 (2) |
| C10—C11—C12—C13 | 0.2 (3) | C17—C19—C24—C23 | 179.67 (15) |
| C11—C12—C13—C14 | 0.0 (3) | C22—C23—C24—C19 | 1.2 (3) |
| C12—C13—C14—C15 | 0.3 (3) | C26—O2—C25—O1 | 3.4 (3) |
| C11—C10—C15—C14 | 1.0 (2) | C26—O2—C25—C7 | −176.14 (17) |
| N3—C10—C15—C14 | 179.86 (14) | N2—C7—C25—O1 | −0.4 (2) |
| C11—C10—C15—N4 | 176.65 (14) | N3—C7—C25—O1 | 177.46 (15) |
| N3—C10—C15—N4 | −4.5 (2) | N2—C7—C25—O2 | 179.06 (14) |
| C13—C14—C15—C10 | −0.8 (3) | N3—C7—C25—O2 | −3.0 (2) |
| C13—C14—C15—N4 | −176.51 (16) | C25—O2—C26—C27 | −175.3 (2) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IS2697).
References
- Barltrop, J. A., Richards, C. G., Russel, D. M. & Ryback, G. J. (1959). J. Chem. Soc. pp. 1132–1142.
- Chiaroni, A., Riche, C., Baouid, A., Hasnaoui, A., Benharref, A. & Lavergne, J.-P. (1995). Acta Cryst. C51, 1352–1355.
- El Hazazi, S., Baouid, A., Hasnaoui, A. & Pierrot, M. (2000). Acta Cryst. C56, e457–e458. [DOI] [PubMed]
- El Hazazi, S., Baouid, A., Hasnaoui, A. & Compain, P. (2003). Synth. Commun. 33, 19–27.
- Enraf–Nonius (1989). CAD-4 EXPRESS Enraf–Nonius, Deft, The Netherlands.
- Fair, C. K. (1990). MolEN Enraf–Nonius, Delft, The Netherlands.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Sharp, B. & Hamilton, C. S. (1946). J. Am. Chem. Soc. 68, 588–591. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811014115/is2697sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811014115/is2697Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report