Abstract
We followed 93 subjects with amebic liver abscess (ALA) and 963 close associate controls at 3-month intervals for 36 months to characterize intestinal and humoral antibody responses to the amebic galactose-inhibitable lectin and to determine whether immunity developed to Entamoeba histolytica or Entamoeba dispar infection following cure of ALA. We found that ALA subjects had a higher prevalence and level of intestinal antilectin immunoglobulin A (IgA) and serum anti-LC3 (cysteine-rich recombinant lectin protein) IgA and IgG antibodies, P < 0.01 and P < 0.05, respectively, compared to controls. The intestinal antilectin IgA antibody response was sustained over a longer time period in ALA subjects (71.8% remained positive at 18 months and 52.6% at 36 months, P < 0.001 compared to 17.6% and 10.3% of controls, respectively). ALA subjects were highly immune to E. dispar infection throughout the study (0% infected at 6 and 36 months, compared to 6.5% and 4.9% of control subjects, respectively, P < 0.05). Upon entry into the study, 6.3% of ALA subjects were infected with E. histolytica; the incidence of new E. histolytica infections in controls (as determined by culture) was too low (1.4%) to determine whether ALA subjects exhibited immunity to new infections. We found that stool cultures every 3 months markedly underestimated the occurrence of new E. histolytica infections, as 15.3% of controls seroconverted after 12 months of follow-up. Unfortunately, under the field conditions present in Durban, South Africa, enzyme-linked immunosorbent assay for detection of lectin antigen in stool yielded unreliable results. In summary, subjects cured of ALA exhibited sustained mucosal IgA antibody responses to the amebic galactose-inhibitable lectin and a high level of immunity to E. dispar infection. Determination of immunity to E. histolytica following cure of ALA will require the use of more sensitive and reliable diagnostic methods.
One of the major questions in amebiasis research is whether cure of invasive disease is followed by development of immunity to new intestinal infections and, thus, recurrence of disease. The enteric protozoan Entamoeba histolytica is one of the leading parasitic causes of death worldwide. Disease results from the parasite's ability to invade the colon, causing amebic colitis, or spreading via the portal venous system to the liver, resulting in formation of an amebic liver abscess (ALA). Amebic liver abscesses are more common in adult men and were thought to be fatal if untreated (7). A recent study in Hue, Vietnam, revealed that ALA is even more common than previously realized and may occur frequently in a subclinical manner (10). One large noncontrolled study reported that the rate of recurrence of amebic liver abscesses over 5 years in a high-risk population was less than expected compared to historical controls (14). In a cross-sectional study, the point prevalence of Entamoeba species intestinal infection was lower in subjects who possessed serum antiamebic antibodies (13).
The E. histolytica galactose-inhibitable lectin (12, 22, 26, 27) appears to have a crucial role in colonization of the gut and parasite invasion. The lectin mediates attachment of E. histolytica trophozoites to colonic mucins (11, 12), host epithelial cells and immune effector cells (22, 30). Galactose-inhibitable lectin binding is an absolute requirement for trophozoites to exhibit a lytic effect on host cells (25). The purified lectin in native and recombinant forms is a highly conserved antigen. In over 95% of samples obtained from hundreds of patients cured of amebic colitis or liver abscess studied worldwide, native lectin protein purified from a single cloned E. histolytica isolate is recognized by serum immunoglobulin G (IgG), IgM, and IgA antibodies (1, 3, 5, 6, 21, 32). The same has been found from subjects with noninvasive asymptomatic E. histolytica intestinal infection (28, 31).
Monoclonal antibodies raised to the lectin's carbohydrate-binding domain completely inhibit parasite binding to colonic mucins in vitro (11, 12), suggesting that intestinal antilectin IgA antibodies could prevent parasite colonization of the gut. In a prospective follow-up study of children in Bangladesh, there was a delay in the onset of E. histolytica intestinal infections when intestinal antilectin IgA antibodies were present (17). The lectin in native and recombinant form has been demonstrated to be efficacious as a subunit vaccine in the gerbil model of amebic liver abscess (24, 32).
In Durban, South Africa, E. histolytica and Entamoeba dispar infections are highly endemic (16, 20). E. dispar is a distinct species that is morphologically identical to E. histolytica but is not known to cause disease (15). E. dispar trophozoites possess functional galactose-binding lectin molecules that are 85% homologous with the E. histolytica lectin (25) and have many common epitopes as determined by studies with murine monoclonal antibodies raised to the E. histolytica lectin (23). The purpose of our study was to characterize over time the human mucosal and humoral antilectin antibody responses and to determine whether intestinal immunity to infection exists following cure of invasive amebiasis. These findings provide information that is crucial for the development of an effective lectin-based amebiasis subunit vaccine.
We conducted a prospective cohort study of 93 subjects treated for ALA and 963 controls who were family members or closely associated neighbors. All subjects were enrolled prospectively and followed for at least 36 months. The demographics, risk factors for infection by Entamoeba species, and prevalence of infection with other intestinal parasites will be reported elsewhere.
MATERIALS AND METHODS
Subject recruitment and study enrollment.
Subjects with ALA were recruited at King Edward VIII Hospital and other regional hospitals and clinics in the area around Durban, South Africa. Nurses fluent in Zulu obtained informed consent in English or Zulu. Control subjects included nuclear family members, individuals residing in the same household, and close neighbors living in the same environment. Control subjects were recruited by study nurses through contact with the index case; we sought at least 10 close associate controls for each index case. No criteria for age or gender were applied except age ≥16 years. All subjects provided blood by venipuncture, feces, and throat washings at entry into the study (1 week after commencing treatment of the ALA index case) and at 3-month intervals for a total of at least 36 months of follow-up. Over the duration of the study, only seven of the 100 family groups were lost to follow-up. At the first visit, study nurses filled out a detailed epidemiologic questionnaire based on an oral history obtained from each of the subjects enrolled in the study. The University of Minnesota and University of Natal's institutional review boards for human subjects approved the consent form, questionnaire, and all aspects of the study.
Assays performed included fecal microscopy, stool culture, and zymodeme determination for E. histolytica or E. dispar, enzyme-linked immunosorbent assay (ELISA) for serum anti-LC3 (recombinant cysteine-rich section of the lectin heavy subunit) (32) IgA and IgG antibodies, ELISA for fecal antilectin and anti-LC3 IgA antibodies, and a monoclonal antibody-based antigen capture ELISA to detect E. histolytica- and E. dispar-specific lectin antigen in feces (1, 2, 3, 5, 6).
Stool culture.
Fecal samples were cultured in Robinson's medium (29) for detection of E. histolytica and E. dispar parasites. Primary cultures were performed by adding a small piece of fecal material to a Bijoux bottle containing an agar slope, to which was added 10 mg of starch, 4 drops of 20% erythromycin, and 10 ml of BR medium (Escherichia coli strain B incubated in R medium for 48 h at 37°C). Stock R medium contains 125 g of NaCl, 50 g of citric acid, 12 g of KH2 PO4, 12.4 g of ammonium sulfate, 1.25 g of magnesium sulfate (7H2O), and 100 ml of lactic acid, diluted to 2.5 liters with distilled water. For use, 100 ml of stock was diluted with 7.5 ml of 40% NaOH and 2.5% of bromothymol blue, adjusted to 1 liter with distilled water at pH 7.0, and autoclaved.
After 24 h, the supernatant was removed, leaving the starch-fecal layer. The supernatant was replaced about 2/3 of the way up the slope with BRS medium (equal volume of BR and sheep serum incubated for 24 h at 37°C) diluted 1:4 with phthalate solution (10.2% potassium phthalate, 2% NaOH, pH 6.3).
After 48 h of incubation at 37°C, a drop from the starch layer was mixed with double strength Lugol's iodine and examined microscopically. A second reading was performed after an additional 48 h of incubation. Positive cultures were subcultured every 2 or 3 days with a fresh slope.
Hexokinase isoenzyme electrophoresis.
Entamoeba species were differentiated by electrophoretic migration of hexokinase isoenzymes (16). Briefly, trophozoite lysates were separated in 1% agarose (SeaKem LE, Rockland. Maine) by electrophoresis at 80 V, 22 mA for 1 h at room temperature. The enzyme was stained with phenzin methosulfate (PMS) (10 μg/ml) (Sigma) solution containing NADP (300 μg/ml) (Sigma), glucose (1 mg/ml), glucose-6-phosphate dehydrogenase (1 unit/ml) (Sigma), MgCl3 (7.18 mM) (Fisher Scientific, Itasca, Ill.), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 30 μg/ml) (Sigma), and ATP (1.3 mM) (Sigma) in 0.1 M Tris-HCl, pH 7.4.
Detection of serum anti-LC3 IgG and IgA antibodies by ELISA.
Detection of serum anti-LC3 IgG and IgA antibodies by ELISA was performed as described previously (7, 28, 32). Recombinant 52-kDa LC3 protein was purified as described by Song et al. (32); 96-well microtiter flat-bottomed polystyrene ELISA plates were coated with LC3 protein, and nonreactive sites were blocked with 1% bovine serum albumin. Serum samples were analyzed by ELISA at a 1:1,000 dilution for IgG and at 1:500 for IgA antibodies in phosphate-buffered saline-Tween (1% bovine serum albumin). Following incubation for 2 h at room temperature, alkaline phosphatase-conjugated goat anti-human IgG (Sigma) or IgA antibodies (ICN Biomedicals; Costa Mesa, Calif.) were utilized at 1:5,000 for IgG and 1:2,000 for IgA in phosphate-buffered saline-Tween (1% bovine serum albumin) for 2 h at room temperature. Reading the plates and correcting the results for nonspecific background binding were performed as described (28).
Detection of fecal lectin antigen by ELISA.
The ELISA for detection of 170-kDa lectin antigen was performed as described (3). Briefly, 96-well flat-bottomed microtiter polystyrene ELISA plates (Costar, Corning, N.Y.) were coated with monoclonal antibody 3F4, which recognizes epitopes present in both E. histolytica and E. dispar lectin, or the 8C12 antibody, which is specific for epitopes present only in E. histolytica lectin (23). Feces were mixed in an equal volume of phosphate-buffered saline containing 2 mM phenylmethylsulfonyl fluoride (USB, Cleveland, Ohio). Fecal samples were added at 100 μl per well and incubated for 2 h at room temperature or overnight at 4°C. Alkaline phosphatase-conjugated antilectin monoclonal antibodies 8A3 (recognizing both E. histolytica and E. dispar lectin) and 1G7 (specific for E. histolytica) (23) were added at 1:1,000 dilution and incubated in developing buffer for 2 h at room temperature. Plate reading with correction for nonspecific background was performed as described (28).
Detection of fecal antilectin and anti-LC3 IgA antibodies by ELISA.
Native E. histolytica galactose-inhibitable lectin protein (22) and recombinant LC3 protein (32) were purified as described and used in ELISA for detection of fecal antilectin IgA antibodies (7). Briefly, flat-bottomed microtiter plates were coated with lectin protein (0.125 μg/well) and the nonreactive sites were blocked with 1% bovine serum albumin. Fecal samples were mixed with an equal volume of phosphate-buffered saline-2 mM phenylmethylsulfonyl fluoride and added at 100 μl/well for incubation at room temperature for 2 h or overnight at 4°C. Alkaline phosphatase-conjugated goat antihuman IgA antibodies (Sigma) were added at a 1:5,000 dilution in phosphate-buffered saline-Tween containing 1% bovine serum albumin, for incubation at room temperature for 2 h. Plate reading with correction of results for nonspecific background binding was performed as described (7).
Treatment of data.
Assays to detect humoral (anti-LC3 IgG and IgA) or mucosal (fecal anti-LC3 and antilectin IgA) antibody responses utilized a continuous optical density (OD) scale, with a cutoffs for positivity of two standard deviations above a culture-negative control used as a laboratory standard. A subject was considered antibody positive if he had at least two consecutive positive readings at baseline or on follow-up. The same standard was applied to determine whether a subject was E. histolytica or E. dispar culture positive except, as shedding of the parasite may be intermittent, one negative culture between two positive results was accepted as a duplicate positive criterion. Finally, a person was considered negative for any of these immunologic or infective markers if he had at least two consecutive negative assays. Subjects who did not meet the criteria for being positive or negative were excluded from the analyses for that assay or comparison. The baseline period included months 0 to 6, with the follow-up period being months 9 to 18, 21 to 27, and 30 to 36 months; these time periods were used to track longitudinal changes in antibody prevalence or culture results.
Analytic methods.
For continuous data, differences of distributions between groups were evaluated with Wilcoxon rank-sum tests. Accordingly, tests results are presented with median levels of these variables, along with associated 25%-75% interquartile ranges. Contingency table analysis was used to compare proportions (yes/no) between groups: chi-square or Fisher's exact tests (for data that were spare or nonnormally distributed) were used to evaluate differences. It should be noted that while we enrolled ALA cases and close associate controls, the study design is essentially a prospective cohort, with exposure groups defined by disease status as a proxy for prior infection with E. histolytica at baseline (ALA versus control). Similarly, subjects could be scored by culture positivity at baseline, and followed over time to evaluate antibody responses or the occurrence of new infections. Results for all tests were aggregated at baseline or follow-up for all subjects for comparison between ALA and their control subjects.
RESULTS
Results from 36 months of follow-up are available for all subjects who completed the study, 93 ALA and 963 control subjects. Data are aggregated by baseline and follow-up periods. The average age of the ALA subjects was 41.5 years; the gender distribution was 83% male and 17% female. The control subjects were 25% male and 75% female, with an average age of 36.5 years.
Intestinal and serum antilectin antibody responses.
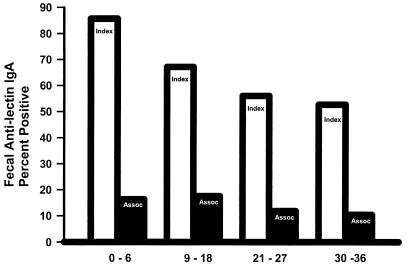
The prevalence in ALA subjects and close associate controls of intestinal antilectin IgA antibodies at baseline (0 to 6 months) and follow-up (9 to 36 months) is illustrated in Fig. 1. ALA subjects had a higher prevalence of intestinal antilectin IgA antibodies at each time period studied compared to controls (85.7%, 67.2%, 56%, and 52.6% positive ELISAs versus 16.3%, 17.6%, 11.8%, and 10.3% in controls, respectively) (P < 0.001 for each time period; Fig. 1). Use of the purified native galactose-inhibitable E. histolytica lectin protein was more sensitive in ELISA for detection of antigen-specific fecal IgA antibodies than the recombinant LC3 protein (P < 0.01; 85.7% compared to 55.2% positive at baseline). The prevalence of intestinal antilectin IgA antibodies decreased over time in ALA and control subjects, compared to baseline values (P = 0.01 and P > 0.05, respectively). Seventy-two percent of the ALA subjects positive for intestinal antilectin IgA antibodies at baseline remained positive at 18 months, compared to only 37.5% of comparable controls (P = 0.0007). By 30 to 36 months, 25.9% of ALA subjects who were positive on entering the study had persistence of intestinal antilectin IgA antibodies, compared to 10.8% of controls (P < 0.038).
FIG. 1.
Prevalence of a positive ELISA for intestinal antilectin IgA antibodies in subjects cured of ALA (light bars) and controls (dark bars). The prevalence of antilectin IgA antibodies was greater in cases than controls at baseline (0 to 6 months) and during each follow-up period (P < 0.0001, for each). There was a significant decrease in the prevalence of antilectin IgA antibodies between baseline and follow-up intervals in ALA cases (P < 0.01 for each comparison) and in close associate controls (0 to 6 and 9 to 18 months compared to 21 to 36 months, P < 0.05).
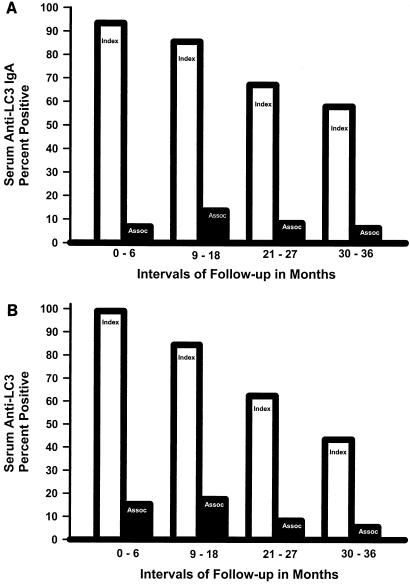
At baseline the prevalence of serum anti-LC3 IgA antibodies was greater in ALA subjects than controls (93.4% compared to 6.9% of controls, P < 0.001, Fig. 2A). Serum anti-LC3 IgA antibodies remained elevated in ALA subjects compared to controls at each interval of follow-up (85.5%, 67.2%, and 57.9% compared to 13.6%, 8.4%, and 6.3%, respectively, P < 0.001 for each, Fig. 2A). There was an increase in serum anti-LC3 IgA antibodies in controls at 18 months (P < 0.05) compared to baseline), indicating new E. histolytica infections. Interestingly, the prevalence of serum anti-LC3 IgG antibodies in ALA subjects declined just as rapidly as that of antilectin IgA antibodies (Fig. 2B, P < 0.001 for each interval compared to baseline). The prevalence of serum anti-LC3 IgG antibodies was higher in ALA subjects than controls at each interval studied (P < 0.001, Fig. 2B).
FIG. 2.
Prevalence of serum anti-LC3 IgA (A) and IgG antibodies (B) for subjects cured of ALA (light bars) and controls (dark bars). The prevalence of a positive test for each antibody studied was greater in ALA subjects than controls at baseline and during each follow-up period (P < 0.001 for each). The prevalence of serum anti-LC3 IgA antibodies increased in controls only at 9 to 18 months (P < 0.001); in ALA cases there was a decrease in the prevalence of anti-LC3 IgG antibodies during each follow-up period (P < 0.001 compared to the previous period.
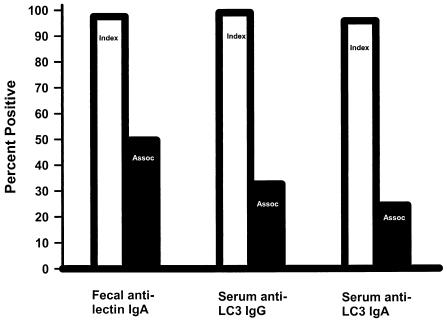
During the entire duration of the study an intestinal anti-lectin IgA antibody response occurred at some point in time in 97.6% of ALA subjects and 49.7% of controls, P < 0.001, Fig. 3. Of interest, in controls fecal antilectin IgA antibodies had a higher commulative prevalence than either serum anti-LC3 IgG or IgA antibodies (49.7% compared to 32.6% and 24.3%, respectively, P < 0.001, Fig. 3). Therefore, an intestinal antilectin IgA antibody response must occur on occasion without seroconversion. In addition, we found that the presence of fecal anti-IgA antibodies in controls was positively associated (P < 0.01) with E. dispar infection. Taken together, this data indicates that E. dispar infection can induce a transient intestinal but not humoral antilectin IgA antibody response.
FIG. 3.
Percentage of ALA (light bars) and controls (dark bars) individuals ever having a positive test over the entire 36 months of the study. ALA subjects had a higher cumulative positive percentage for each antibody studied compared to controls, P < 0.01. Of interest, the cumulative percentage of control subjects with fecal antilectin IgA antibodies was greater than for either serum anti-LC3 IgA or IgG antibodies (P < 0.005).
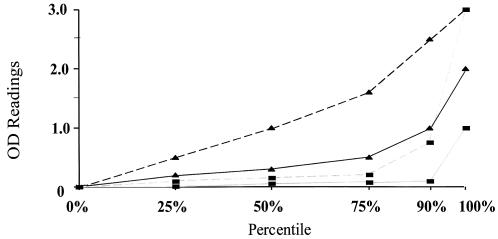
Intestinal and serum ELISA results were analyzed as a continuous variable by comparison of OD values at equal dilutions. At baseline (0 to 6 months), ALA subjects had markedly higher ELISA OD values at the 25th, 50th, 75th, and 90th percentiles for intestinal antilectin IgA and serum anti-LC3 IgA antibodies, compared to ELISA-positive control subjects assayed at identical dilutions of feces or serum (P = 0.0001 for each percentile, Fig. 4). The same was true when ELISA OD levels for serum anti-LC3 IgG antibodies were compared between ALA subjects and ELISA-positive controls (P < 0.001, respectively, for each quartile, data not shown). During the entire 36-month follow-up period, antibody-positive ALA subjects had higher median OD values for all of the antiamebic antibodies studied, compared to ELISA-positive control subjects (P ≤ 0.04 for each, Table 1).
FIG. 4.
Comparison of ELISA OD values at baseline (0 to 6 months) between antibody-positive ALA subjects and controls for intestinal antilectin IgA and serum anti-LC3 IgA antibodies. Fecal antilectin IgA antibody (dashed line) OD values are higher at each percentile (except 100%) for ALA cases (▴) compared to antibody-positive controls (▪) (P = 0.001 for each). The same was true for serum anti-LC3 IgA antibodies (solid line), P < 0.001 comparing ALA cases (▴) to controls (▪).
TABLE 1.
Comparison of OD values between ELISA-positive ALA subjects and close associate controls during 36 months of follow-upa
| ELISA | Median OD
|
|
|---|---|---|
| ELISA-positive ALA subjects | ELISA-positive controls | |
| Intestinal antilectin IgA | 0.657*a | 0.12 |
| Intestinal anti-LC3 IgA | 0.123*a | 0.016 |
| Serum anti-LC3 IgG | 0.641†a | 0.119 |
| Serum anti-LC3 IgA | 0.42†a | 0.068 |
*, P < 0.05 compared to close associates; †, P < 0.01 compared to close associates.
Occurrence of E. histolytica and E. dispar intestinal infections.
By stool culture and zymodeme determination, 6.3% of ALA subjects were infected with E. histolytica during the baseline period (0 to 6 months), compared to only 1.2% of controls (P = 0.001, Table 2). The prevalence of E. histolytica infection over time was at 10.0%, 5.9%, and 3.1% in ALA subjects during follow-up, compared to 2.6%, 0.7%, and 1.0% of control subjects, respectively (P = 0.005, Table 2). Over the duration of the study, by culture and zymodeme determination, 15.6% of ALA subjects and 3.5% of controls were infected with E. histolytica (P = 0.001, Table 2). In ALA subjects there were seven E. histolytica infections detected after 6 months; six of seven were found at 9 to 18 months and only one was found thereafter (Table 2). The same pattern was evident in control subjects; 13 to 16 new infections were detected at 9 to 18 months and only three thereafter (Table 2). In controls, there was no correlation between being positive for intestinal antilectin IgA antibodies or serum antilectin IgA or IgG antibodies and the occurrence or nonoccurrence of new E. histolytica infections (P > 0.1 for each).
TABLE 2.
Prevalence of E. histolytica infection in ALA cases and close associate controlsa
| Group | No. infected/no. in group (% infected) at:
|
||||
|---|---|---|---|---|---|
| 0-6 mo | 9-18 mo | 21-27 mo | 30-36 mo | 0-36 mo | |
| ALA cases | 5/80 (6.3)*a | 8/80 (10)*a | 4/68 (5.9)*a | 2/64 (3.1) | 12/77 (15.6) |
| New infections | 6/8 | 1/4 | 0/2 | ||
| Controls | 9/737 (1.2)†a | 19/40 (2.6)†a | 5/725 (0.7) | 7/665 (1.0) | 25/703 (3.5) |
| New infections | 13/19 | 3/5 | 0/7 | ||
Includes only those subjects who had samples collected at baseline and during follow-up. *, P < 0.05 for prevalence of E. histolytica infection in ALA cases compared to controls during follow-up; †, P < 0.05 for prevalence of E. histolytica infection in controls 9 to 18 months compared to 21 to 27 and 30 to 36 months of follow-up.
Studies of E. dispar intestinal infection revealed that at baseline (0 to 6 months), none of the 85 ALA subjects who submitted adequate fecal samples were infected with E. dispar, compared to 6.5% of controls (P = 0.007, Table 3). During the follow-up period, only 4 of 81 ALA subjects became transiently infected with E. dispar compared to 149 of 713 control subjects (4.9% compared to 20.9%, P < 0.05, Table 3). At the end of the prospective study, 30 to 36 months, none of the ALA subjects were infected with E. dispar, compared to 6.5% of controls (P < 0.05, Table 3). Among the controls, there was no correlation between having a positive serum or fecal antibody test at baseline and immunity against the occurrence of a new E. dispar infection. (P = 0.80).
TABLE 3.
Prevalence of E. dispar infection in ALA cases and close associate controlsa
| Group | No. infected/no. in group (% infected) at:
|
||||
|---|---|---|---|---|---|
| 0-6 mo | 9-18 mo | 21-27 mo | 30-36 mo | 0-36a | |
| ALA cases | 0/85 (0)*a | 2/79 (2.6) | 2/68 (1.52) | 0/63 (0)*a | 4/81b (4.9) |
| New infections | 2/2 | 2/2 | 0/1 | ||
| Controls | 47/719 (6.5) | 74/739 (8.3)*a | 49/719 (5) | 43/658 (4.94)*a | 149/713b (20.9) |
| New infections | 56/74 | 28/49 | 18/43 | ||
Includes only those subjects who had samples collected at baseline and during follow-up.
*, P < 0.05 for lower prevalence of E. dispar infection in ALA cases compared to controls.
We determined that culture of fecal samples at 3-month intervals was not sensitive enough to detect the majority of new E. histolytica infections. By sero-conversion criteria, with serum anti-LC3 IgG antibodies, 15.3% of asymptomatic controls had a new E. histolytica infection during 12 months of follow-up (baseline compared to months 9 to 18), yet culture and zymodeme criteria detected only 1.0% as having a new infection (P > 0.01).
All fecal samples were subjected to a monoclonal antibody-based ELISA for detection of E. histolytica and E. dispar-specific lectin antigen, a research assay that we applied successfully in a number of studies encompassing hundreds of amebic infections in Cairo, Egypt (3, 6, 7). However, we found that the antigen detection ELISA results for all subjects over the duration of the study did not correlate with stool culture and zymodeme determination (only 6 of 32 positive cultures for E. histolytica and 22 of 91 for E. dispar had true positive ELISAs, Table 4), with clinical group, or with seropositivity (data not shown). Therefore, under the field conditions and analysis as performed in this study, antigen detection ELISA technology was found to be unreliable.
TABLE 4.
Lack of correlation of ELISA results for fecal lectin antigen detection and culture with zymodeme determination for E. histolytica and E. dispara
| Result of stool culture with zymodeme determination for E. histolytica or E. dispar | No. of samples with ELISA result
|
|||
|---|---|---|---|---|
|
E. histolytica antigen
|
E. dispar antigen
|
|||
| Negative | Positive | Negative | Positive | |
| Negative | 1,038 | 147 | 807 | 272 |
| Positive | 26 | 6 | 69 | 22 |
Includes all ALA patients and controls, all observations for baseline and 9 to 18 months of follow-up in which culture and ELISA for lectin antigen were performed, P > 0.5.
DISCUSSION
We now report the first large prospective, controlled cohort study of E. histolytica and E. dispar intestinal infection following cure of ALA. We evaluated 93 subjects starting at 1 week following cure of ALA and 963 controls (immediate family members and/or neighbors). Subjects were evaluated at 3-month intervals thereafter with collection of feces and blood, for a total of 36 months of follow-up.
There was a high prevalence (>85%) of intestinal antilectin IgA antibody responses in ALA subjects, which unexpectedly persisted in over 50% of subjects for 18 months after treatment. The intensity of the intestinal antibody response as measured by ELISA OD readings was greater in ALA subjects than in antibody-positive controls during any time period. To rule out sample coding errors, we utilized a criterion of at least two positive culture results to identify an infected subject. A recently published study genotyped a subset of Entamoeba species isolated from our study subjects and revealed that, except for two rare transient exceptions, the same isolate was found over time in each individual studied, even for up to six positive cultures over 3 years (34). Therefore, in our study use of a duplicate positive criterion should not significantly mask the occurrence of new infections.
Following cure of ALA, subjects were highly immune to intestinal infection by E. dispar. There are no luminal amebicidal agents available for use in South Africa; therefore, we expected a high incidence of E. histolytica and E. dispar intestinal infection among ALA subjects upon entry into the study. Stool cultures revealed a lower than anticipated (16) prevalence of new E. histolytica infections in controls, making it impossible to determine whether ALA subjects, once cleared of their original infection, were immune to new E. histolytica infections by comparison to controls. Use of seroconversion criteria revealed that the rate of new asymptomatic E. histolytica infections in controls was actually sevenfold higher than suggested by the stool culture data. However, seroconversion criteria cannot be applied to ALA subjects, as almost all (>93%) were seropositive upon entry into the study.
Control subjects with intestinal antilectin IgA antibodies had no history of ALA or colitis; mucosal antibody responses were most likely due to a relatively recent asymptomatic E. histolytica or E. dispar intestinal infection. E. histolytica infections are well documented to produce humoral antilectin antibody responses (28, 32). In addition, intestinal antilectin IgA antibodies were more prevalent than humoral antilectin IgA or IgG antibodies and positively associated with new E. dispar infections, suggesting that E. dispar can induce a mucosal but not a humoral immune response. We were able to conclude that the low levels of intestinal antilectin IgA antibodies present in ELISA-positive control subjects are not sufficient to provide immunity to new E. dispar infections.
We suggest that the immunity to E. dispar infection found in our prospective longitudinal study of ALA patients is due to the high levels of intestinal antilectin IgA antibodies present. Haque et al. (17) reported that children previously treated for amebic colitis had a delay in acquisition of new E. histolytica intestinal infections and that this relative immunity correlated with the presence of intestinal antilectin IgA antibodies. Of interest, this immunity was observed only during the first five months of follow-up (17). In a subsequent study, Haque et al. (18) found that intestinal antilectin IgA antibodies were detectable for an average of only 31 days; this is in marked contrast to our findings following either ALA or during follow-up of intestinal IgA in antibody-positive asymptomatic controls.
Given the 85% homology in lectin amino acid sequence and shared epitopes between the E. dispar and E. histolytica lectins (23), we propose that immunity to E. dispar may be a surrogate marker for immunity to E. histolytica. Unfortunately, the insensitivity of stool cultures every 3 months for E. histolytica infection and the lack of specificity of the fecal antigen detection ELISA under the field conditions in Durban, South Africa, prevented our determining directly whether ALA subjects also exhibited immunity to E. histolytica infection. We could not independently evaluate the unique contribution to immunity of high titers of antilectin antibodies to immunity to E. dispar infection. However, given the demonstrated role of IgA antibodies in immunity to intestinal bacterial and parasitic infection (4) and the in vitro adherence-inhibitory activity of murine fecal antilectin IgA antibodies (8), it is likely that intestinal antilectin IgA antibodies are mediating the immunity observed.
A commercially available ELISA (DiaTech Labs) for detection of lectin antigen has been utilized successfully (19). The conditions for performance of the ELISAs in this report are not identical to the commercial assay; in addition, the field conditions in South Africa resulted in a substantial delay (hours) between collection and processing of samples. We found an unacceptably low rate of correlation of lectin antigen positive ELISA results with positive stool cultures for E. histolytica and E. dispar, and therefore we could not apply antigen detection technology in this study. Previously, we successfully performed hundreds of fecal antigen detection ELISAs on samples collected in Cairo, Egypt, with excellent correlation to culture results (3, 4, 6). However, other investigators have found a similar lack of sensitivity and specificity with earlier generations of the DiaTech assay when applied to field conditions in the tropics (E. Tannich and T. F. H. G. Jackson, personal communications). It is important to emphasize that use of the DiaTech assay per the manufacturer's instructions was not performed in this study. Studies utilizing PCR for detection of parasite ribosomal DNA confirm that stool culture with zymodeme determination underestimated the incidence of E. histolytica but not E. dispar infection (9).
In summary, we found that subjects cured of ALA have a high prevalence and level of both intestinal and serum IgA antibodies directed against the amebic galactose-inhibitable adherence lectin. Both E. histolytica and E. dispar contain functional galactose binding lectin molecules with multiple shared epitopes (23, 25). Cure of ALA is followed by a high level of immunity to E. dispar intestinal infection for the entire 36 months of our study. Therefore, our data and those of others (17, 18) indicate that mucosal antilectin IgA antibodies may mediate immunity in adults to new intestinal infections by E. dispar or E. histolytica. Use of a more sensitive and specific diagnostic test (PCR) rather than use of stool culture and zymodeme determination will allow us to more directly address this hypothesis. Numerous strategies have been developed for use of the E. histolytica galactose-inhibitable lectin as a subunit amebiasis vaccine, especially to elicit protective intestinal antilectin IgA antibodies (33). Clearly, if antilectin IgA antibodies have a role in human immunity to E. histolytica intestinal infection, as indicated by this study, such vaccine strategies should continue to be actively pursued.
Acknowledgments
This work was supported by NIH grants PO1-AI36359-01 and UO1-AI35840 from NIAID and from the MRC (South Africa).
We thank Shana Brooks for expert secretarial assistance and Rose Hill for very important data technology services.
Editor: B. B. Finlay
REFERENCES
- 1.Abd-Alla, M., A. El-Hawey, and J. I. Ravdin. 1992. Use of an enzyme linked immunosorbent assay to detect anti-adherence protein antibodies in sera from patients with invasive amebiasis in Cairo, Egypt. Am. Soc. Trop. Med. Hyg. 47:800-804. [DOI] [PubMed] [Google Scholar]
- 2.Abd-Alla, M., T. F. H. G. Jackson, V. Gathiram, El-Hawey, A., and J. I. Ravdin. 1993. Differentiation of pathogenic Entamoeba histolytica infection from nonpathogenic infection by detection of galactose-inhibitable adherence protein antigen in sera and feces. J. Clin. Microbiol. 31:2845-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abd-Alla, M., T. F. H. G. Jackson, and J. I. Ravdin. 1998. Serum IgM antibody response to galactose-inhibitable adherence lectin of Entamoeba histolytica. Am. J. Trop. Med. Hyg. 59:431-434. [DOI] [PubMed] [Google Scholar]
- 4.Abd-Alla, M., and J. I. Ravdin.2003. Mucosal immune response to parasitic infections. In J. Mestecky (ed.), Mucosal immunology, 3rd ed. Academic Press, Philadelphia, Pa.
- 5.Abd-Alla, M. D., A. A. Wahib, and J. I. Ravdin. 2000. Comparison of Antigen-capture ELISA to Stool culture methods for the detection of symptomatic Entamoeba infection in Kafer Daoud, Egypt. Am. Soc. Trop. Med. Hyg. 62:579-582. [DOI] [PubMed] [Google Scholar]
- 6.Abo-El-Maged, I., G. Soong, A. El-Hawey, and J. I. Ravdin. 1996. Humoral and mucosal IgA antibody response to a recombinant 52-kDa cysteine-rich portion of the Entamoeba histolytica galactose-inhibitable lectin correlates with detection of native 170-kDa antigen in serum patients with amebic colitis. J. Infect. Dis. 174:157-162. [DOI] [PubMed] [Google Scholar]
- 7.Adams, E. D., and I. N. MacLeod. 1977. Invasive Amebiasis II. Amebic liver abscess and its complications. Medicine 56:315-325. [DOI] [PubMed] [Google Scholar]
- 8.Beving, D. E., C. G. Soong, and J. I. Ravdin. 1996. Oral immunization with a recombinant cysteine-rich section of galactose-inhibitable lectin elicits an intestinal secretory immunoglobulin A response that has an in vitro adherence-inhibitory activity. Infect. Immun. 64:1473-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blessman, J., H. Buss, P. A. Nu, H. D. Thi, M. Abd-Alla, T. G. F. H. Jackson, J. I. Ravdin, and E. Tannich. 2002. Real time PCR for detection and differentiation of Entamoeba histolytica and Entamoeba dispar in fecal samples. J. Clin. Mircobiol. 40:4413-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blessman, J., L. P. Van, P. A. Nu, H. D. Thi, Muller-Myhsok, B., H. Buss, and E. Tannich. 2002. Epidemiology of amebiasis in a region of high incidence of amebic liver abscess in central Vietnam. Am. Soc. Tro. Med. Hyg. 66:578-583. [DOI] [PubMed] [Google Scholar]
- 11.Chadee, K., W. A. Petri, Jr., D. J. Innes, and J. I. Ravdin. 1987. Rat and human colonic mucins bind to and inhibit the adherence lectin of Entamoeba histolytica. J. Clin. Investig. 80:1245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chadee, K., W. A. Petri, Jr., M. Johnson, M. E. Orozco, and J. I Ravdin. 1988. Binding and characterization of purified rat colonic mucin by Gal/NAc adherence lectin of Entamoeba histolytica. J. Infect. Dis. 158:398-406. [DOI] [PubMed] [Google Scholar]
- 13.Choudhuri, G., V. Prakash, A. Kumar, S. K. Shahi, and M. Sharma. 1991. Protective immunity to Entamoeba histolytica infection in subjects with antiamoebic antibodies residing in a hyperendemic zone. Scand. J. Infect. Dis. 23:771-776. [DOI] [PubMed] [Google Scholar]
- 14.Deleon, A. 1970. Prognostica tardio en el absceso hepatico amibiano. Arch. Ivestig. Med. (Mexico) I(Suppl.):205-209.
- 15.Diamond, L., and C. Clark. 1993. A redescription of Entamoeba histolytica Schaudinn, 1903 (amended Walker 1911) separating it from Entamoeba dispar, Brumpt, 1925. J. Eukary. Microbiol. 40:340-344. [DOI] [PubMed] [Google Scholar]
- 16.Gathiram, V., and T. F. H. G. Jackson. 1982. Frequency distribution of Entamoeba histolytica zymodemes in a rural South African population. Lancet 1:719-729. [DOI] [PubMed] [Google Scholar]
- 17.Haque, R., I. M. Ali, R. B. Sack, B. M. Farr, G. Ramakrishnan, and W. A. Petri, Jr. 2001. Amebiasis and mucosal IgA antibody against Entamoeba histolytica adherence lectin in Bangladeshi children. J. Infect. Dis. 183:1787-1793. [DOI] [PubMed] [Google Scholar]
- 18.Haque, R., P. Duggal, I. M. Ali, M. Hossain, D. Mondal, R. B. Sack, B. M. Farr, T. H. Beaty, and W. A. Petri, Jr. 2002. Innate and acquired resistance to amebiasis in Bangladeshi children. J. Infect. Dis. 186:547-552. [DOI] [PubMed] [Google Scholar]
- 19.Haque, R., N. U. Mollah, I. K. Ali, K. Alam, A. Eubanks, D. Lyerly, and W. A. Petri, Jr. 2000. Diagnosis of amebic liver abscess and intestinal infection with the TechLab Entamoeba histolytica II antigen detection and antibody tests. J. Clin. Microbiol. 38:3235-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson, T. F. H. G., S. Reddy, J. Fincham, M. D. Alla, and J. I. Ravdin. 2000. A comparison of cross-sectional and longitudinal seroepidemiologic assessment of Entamoeba-infected populations in South Africa. Achives of Medical Research. 31:S36-37. [DOI] [PubMed] [Google Scholar]
- 21.Kelsall, B. L., T. F. H. G. Jackson, V. Gathiram, R. D. Pearson, and J. I. Ravdin. 1994. Secretory immunoglobulin A antibodies to the galactose-inhibitable adherence protein in the saliva of patients with amebic liver abscess. Am. J. Trop. Med. Hyg. 51:454-459. [PubMed] [Google Scholar]
- 22.Petri, W. A., Jr., R. Smith, P. Schlesinger, and J. I. Ravdin. 1987. Isolation of galactose-binding lectin which mediates the in vitro adherence of Entamoeba histolytica. J. Clin. Investig. 80:1238-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petri, W. A., Jr., T. F. H. G. Jackson, V. Gatheram, K. Kress, and L. D. Saffer. 1990. Pathogenic and nonpathogenic strains of Entamoeba histolytica can be differentiated by monoclonal antibodies to galactose-specfic adherence lectin. Infect. Immun. 28:1802-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petri, W. A., Jr., and J. I. Ravdin. 1991. Protection of gerbils from amebic liver abscess by immunization with the galactose-specific adherence lectin of Entamoeba histolytica. Infect. Immun. 59:97-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilia, D. R., S. Kobayshi, and K. C. Kain. 2001. Entamoeba dispar: molecular characterization of the galactosamine lectin. Exp. Parasitol. 99:226-234. [DOI] [PubMed] [Google Scholar]
- 26.Ravdin, J. I., and R. L. Guerrant. 1981. role of adherence in cytopathogenic mechanism of Entamoeba histolytica. J. Clin. Investig. 68:1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravdin, J. I., J. E. John, L. I. Johnston, D. J. Innes, and R. L. Guerrant. 1985. Adherence of Entamoeba histolytica trophozoites to rat and human colonic mucosal. Infect. Immun. 48:292-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravdin, J., Jackson, T. F. H. G., J. Petri, C. Murphy, B. Ungar, V. Gathiram, J. Skilogianis, and A. Simjee. 1990. Association of serum antibodies to adherence lectin with invasive amebiasis and asymptomatic infection with Entamoeba histolytica. J. Infect. Dis. 162:768-772. [DOI] [PubMed] [Google Scholar]
- 29.Robinson, G. L. 1968. The laboratory diagnosis of human parasitic amoeba. Trans. R. Soc. Trop. Med. Hyg. 62:285-294. [DOI] [PubMed] [Google Scholar]
- 30.Salata, R. A., R. D. Pearson, and J. I. Ravdin. 1985. Interaction of human leukocytes with Entamoeba histolytica: killing of virulent amoeba by activated macrophage. J. Clin. Investig. 76:491-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sargeant, P. G. 1978. The reliability of Entamoeba histolytica zymodeme in clinical diagnosis. Parasitol. Today. 3:40-43. [DOI] [PubMed] [Google Scholar]
- 32.Soong, G., K. Kain, M. Abd-Alla, T. F. H. G. Jackson, and J. I. Ravdin. 1995. A recombinant cysteine-rich section of Entamoeba histolytica galactose-inhibitable lectin is efficacious as a subunit vaccine in the gerbil model of amebic liver abscess. J. Infect. Dis. 171:645-651. [DOI] [PubMed] [Google Scholar]
- 33.Stanley, S. L. 2000. Prevention and potential of new interventions, p. 137-162. In J. I. Ravdin (ed.), Amebiasis. Imperial College Press, London, England.
- 34.Zaki, M., S. G. Reddy, T. F. H. G. Jackson, J. I. Ravdin, and G. C. Clark. 2003. Genotyping of Entamoeba species in South Africa: diversity, stability, and transmission patterns within families. J. Infect. Dis. 187:1860-1869. [DOI] [PubMed] [Google Scholar]