Abstract
The P atom in the title compound, C23H25N4O4P, is in a slightly distorted tetrahedral coordination environment and the N atoms show sp 2 character. The phosphoryl group and the NH unit are syn with respect to each other. In the crystal, pairs of intermolecular N—H⋯O(P) hydrogen bonds form centrosymmetric dimers.
Related literature
For phosphorus compounds with general formula XP(O)[N(CH3)(CH2C6H5)]2, see: Gholivand et al. (2005 ▶). For bond lengths in a related structure, see: Sabbaghi et al. (2010 ▶). For hydrogen-bond motifs, see: Etter et al. (1990 ▶); Bernstein et al. (1995 ▶).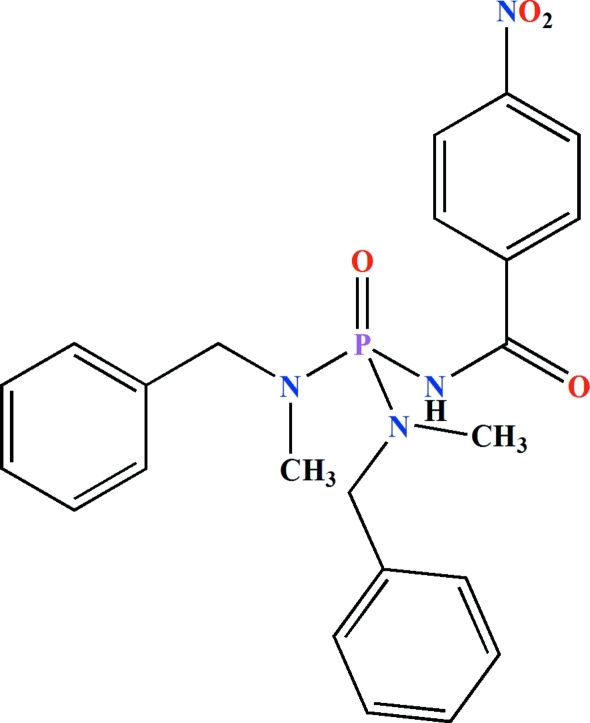
Experimental
Crystal data
C23H25N4O4P
M r = 452.44
Triclinic,

a = 8.3526 (5) Å
b = 11.8150 (5) Å
c = 12.2668 (4) Å
α = 77.184 (3)°
β = 81.289 (4)°
γ = 71.928 (4)°
V = 1117.70 (9) Å3
Z = 2
Cu Kα radiation
μ = 1.41 mm−1
T = 297 K
0.24 × 0.14 × 0.05 mm
Data collection
Oxford Diffraction Xcalibur Ruby Gemini diffractometer
Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2010 ▶) T min = 0.941, T max = 1.000
8669 measured reflections
4203 independent reflections
3779 reflections with I > 2σ(I)
R int = 0.025
Refinement
R[F 2 > 2σ(F 2)] = 0.035
wR(F 2) = 0.093
S = 1.05
4203 reflections
390 parameters
All H-atom parameters refined
Δρmax = 0.21 e Å−3
Δρmin = −0.26 e Å−3
Data collection: CrysAlis PRO (Oxford Diffraction, 2010 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SIR2004 (Burla et al., 2005 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811011275/ng5139sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811011275/ng5139Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N8—H8⋯O2i | 0.85 (2) | 2.07 (2) | 2.909 (2) | 169 (2) |
Symmetry code: (i)  .
.
Acknowledgments
Financial support from the Spanish Ministerio de Educacion y Ciencia (MAT2006–01997, MAT2010–15094 and the ‘Factoría de Cristalización’ Consolider Ingenio 2010) and FEDER funding is acknowledged.
supplementary crystallographic information
Comment
Some phosphoric triamide compounds of the general formula XP(O)[N(CH3)(CH2C6H5)]2 [X = Cl, C6H5C(O)NH & CCl3C(O)NH (Gholivand et al., 2005) have been structurally investigated. Here, we report on the synthesis and crystal structure of title compound (where X = 4-NO2C6H4C(O)NH).
The asymmetric unit consists of a single molecule, shown in Fig. 1, of the title compound with no unusual bonding features. The P═O and P—N bond lengths are comparable to those in similar compounds like for example in P(O)[NHC(O)C6H4(4-NO2)][NHC6H11]2 (Sabbaghi et al., 2010). As can be expected, the N8—C26 bond length is shorter than the other N—C bonds in the molecule.
The phosphorus atom has a slightly distorted tetrahedral configuration, the bond angles around the P atom are in the range of 103.85 (6)° [N6—P1—N7] to 118.67 (6)° [O2—P1—N7]. The average of surrounding angles around the tertiary nitrogen atom N6 (119.7°) shows that it is bonded in an essentially planar geometry; whereas, the environment of N7 is slightly deviated from planarity (average of bond angles around N7 atom is equal to 117.3°). Furthermore, the angle C26—N8—P1 is 124.98 (10)°.
The oxygen atom of P═O group is a better H-acceptor than that of the C═ O counterpart; so, the hydrogen atom of the C(O)NHP(O) moiety is involving in an intermolecular –P═O···H—N– hydrogen bond (see Table 1) to form a centrosymmetric dimer [graph set: R22(8) (Etter et al., 1990; Bernstein et al., 1995)].
Experimental
4-NO2—C6H4C(O)NHP(O)Cl2 was prepared according to the procedure of literature (Sabbaghi et al., 2010). To a solution of (2 mmol) 4-NO2C6H4C(O)NHP(O)Cl2 in CH3CN (20 ml), a solution of N-methylbenzyl amine (8 mmol) in CH3CN (5 ml) was added dropwise at 273 K. After 4 h stirring, the solvent was removed in vacuum. Single crystals were obtained from a solution of title compound in C2H5OH after slow evaporation at room temperature. IR (KBr, cm-1): 3141, 2881, 1680, 1604, 1522, 1452, 1342, 1273, 1186, 1104, 1005, 949, 853, 793, 708.
Refinement
At the end of the refinement the highest peak in the electron density was 0.210 e Å -3, while the deepest hole was -0.260 e Å -3. All H atoms were sucessfully located by difference Fourier synthesis and isotropically refined.
Figures
Fig. 1.
An ORTEP style plot of title compound with the atom-labeling scheme. Ellipsoids are shown at the 50% probability level. H atoms are represented as small spheres of arbitrary radii.
Crystal data
| C23H25N4O4P | Z = 2 |
| Mr = 452.44 | F(000) = 476 |
| Triclinic, P1 | Dx = 1.344 Mg m−3 |
| Hall symbol: -P 1 | Cu Kα radiation, λ = 1.54180 Å |
| a = 8.3526 (5) Å | Cell parameters from 5608 reflections |
| b = 11.8150 (5) Å | θ = 3.7–70.5° |
| c = 12.2668 (4) Å | µ = 1.41 mm−1 |
| α = 77.184 (3)° | T = 297 K |
| β = 81.289 (4)° | Prismatic, colorless |
| γ = 71.928 (4)° | 0.24 × 0.14 × 0.05 mm |
| V = 1117.70 (9) Å3 |
Data collection
| Oxford Diffraction Xcalibur Ruby Gemini diffractometer | 4203 independent reflections |
| Radiation source: Enhance (Cu) X-ray Source | 3779 reflections with I > 2σ(I) |
| graphite | Rint = 0.025 |
| Detector resolution: 10.2673 pixels mm-1 | θmax = 70.6°, θmin = 3.7° |
| ω scans | h = −10→10 |
| Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2010) | k = −12→14 |
| Tmin = 0.941, Tmax = 1.000 | l = −14→14 |
| 8669 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.035 | All H-atom parameters refined |
| wR(F2) = 0.093 | w = 1/[σ2(Fo2) + (0.049P)2 + 0.1381P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max = 0.001 |
| 4203 reflections | Δρmax = 0.21 e Å−3 |
| 390 parameters | Δρmin = −0.26 e Å−3 |
| 0 restraints | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0073 (5) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| P1 | 0.37947 (4) | 0.43216 (3) | 0.68154 (3) | 0.03514 (12) | |
| O2 | 0.39241 (13) | 0.55008 (8) | 0.61457 (8) | 0.0441 (2) | |
| O3 | 0.40102 (16) | 0.16957 (10) | 0.70306 (10) | 0.0570 (3) | |
| O4 | 1.0449 (2) | −0.09851 (14) | 0.30155 (13) | 0.0866 (5) | |
| O5 | 0.9874 (2) | −0.24087 (12) | 0.42737 (14) | 0.0867 (5) | |
| N6 | 0.18214 (15) | 0.43519 (10) | 0.71762 (10) | 0.0422 (3) | |
| N7 | 0.46802 (15) | 0.38121 (10) | 0.80040 (10) | 0.0415 (3) | |
| N8 | 0.48310 (16) | 0.32916 (10) | 0.59984 (10) | 0.0404 (3) | |
| N9 | 0.9666 (2) | −0.13399 (13) | 0.38622 (13) | 0.0594 (4) | |
| C10 | −0.08796 (18) | 0.59948 (13) | 0.70707 (13) | 0.0455 (3) | |
| C11 | −0.2562 (2) | 0.61378 (15) | 0.69736 (16) | 0.0559 (4) | |
| C12 | −0.3838 (2) | 0.69259 (18) | 0.7548 (2) | 0.0693 (6) | |
| C13 | −0.3441 (3) | 0.75656 (19) | 0.82172 (19) | 0.0718 (6) | |
| C14 | −0.1776 (3) | 0.7439 (2) | 0.8315 (2) | 0.0717 (5) | |
| C15 | −0.0499 (2) | 0.66649 (16) | 0.77376 (17) | 0.0587 (4) | |
| C16 | 0.0512 (2) | 0.51473 (15) | 0.64516 (14) | 0.0494 (4) | |
| C17 | 0.1226 (2) | 0.34870 (15) | 0.80655 (16) | 0.0537 (4) | |
| C18 | 0.3946 (3) | 0.44794 (17) | 0.89219 (14) | 0.0563 (4) | |
| C19 | 0.6514 (2) | 0.32321 (15) | 0.80428 (14) | 0.0484 (3) | |
| C20 | 0.69385 (18) | 0.22357 (14) | 0.90560 (12) | 0.0451 (3) | |
| C21 | 0.7774 (2) | 0.2365 (2) | 0.98905 (15) | 0.0615 (4) | |
| C22 | 0.8215 (3) | 0.1426 (3) | 1.08008 (17) | 0.0796 (6) | |
| C23 | 0.7830 (3) | 0.0367 (2) | 1.08917 (18) | 0.0742 (6) | |
| C24 | 0.7001 (3) | 0.02349 (18) | 1.00715 (18) | 0.0697 (5) | |
| C25 | 0.6558 (3) | 0.11582 (16) | 0.91598 (16) | 0.0592 (4) | |
| C26 | 0.48804 (18) | 0.20940 (12) | 0.62439 (11) | 0.0402 (3) | |
| C27 | 0.61200 (18) | 0.12548 (12) | 0.55456 (11) | 0.0392 (3) | |
| C28 | 0.7086 (2) | 0.16247 (13) | 0.45913 (12) | 0.0478 (4) | |
| C29 | 0.8241 (2) | 0.07733 (14) | 0.40268 (13) | 0.0526 (4) | |
| C30 | 0.8402 (2) | −0.04361 (13) | 0.44417 (13) | 0.0461 (3) | |
| C31 | 0.7468 (2) | −0.08292 (14) | 0.53841 (15) | 0.0543 (4) | |
| C32 | 0.6313 (2) | 0.00252 (14) | 0.59370 (15) | 0.0515 (4) | |
| H11 | −0.278 (3) | 0.569 (2) | 0.6490 (18) | 0.069 (6)* | |
| H12 | −0.499 (4) | 0.701 (2) | 0.745 (2) | 0.092 (7)* | |
| H13 | −0.432 (3) | 0.809 (2) | 0.860 (2) | 0.087 (7)* | |
| H14 | −0.154 (3) | 0.786 (2) | 0.882 (2) | 0.093 (8)* | |
| H15 | 0.067 (3) | 0.6575 (19) | 0.7821 (18) | 0.070 (6)* | |
| H16A | 0.104 (2) | 0.5613 (17) | 0.5845 (17) | 0.054 (5)* | |
| H16B | 0.001 (3) | 0.4617 (19) | 0.6165 (17) | 0.061 (5)* | |
| H17A | 0.041 (3) | 0.3951 (19) | 0.8615 (18) | 0.067 (6)* | |
| H17B | 0.218 (3) | 0.292 (2) | 0.843 (2) | 0.076 (6)* | |
| H17C | 0.070 (3) | 0.303 (2) | 0.7758 (19) | 0.076 (6)* | |
| H18A | 0.276 (3) | 0.490 (2) | 0.885 (2) | 0.084 (7)* | |
| H18B | 0.444 (3) | 0.512 (3) | 0.892 (2) | 0.096 (8)* | |
| H18C | 0.407 (3) | 0.394 (2) | 0.964 (2) | 0.088 (7)* | |
| H19A | 0.702 (3) | 0.3836 (19) | 0.8089 (17) | 0.061 (5)* | |
| H19B | 0.693 (2) | 0.2926 (17) | 0.7372 (17) | 0.057 (5)* | |
| H21 | 0.803 (3) | 0.312 (2) | 0.9826 (19) | 0.074 (6)* | |
| H22 | 0.878 (4) | 0.154 (3) | 1.137 (3) | 0.117 (10)* | |
| H23 | 0.810 (3) | −0.029 (3) | 1.148 (2) | 0.096 (8)* | |
| H24 | 0.672 (3) | −0.051 (2) | 1.009 (2) | 0.078 (6)* | |
| H25 | 0.600 (3) | 0.1054 (19) | 0.8612 (19) | 0.069 (6)* | |
| H28 | 0.698 (2) | 0.2437 (19) | 0.4309 (17) | 0.061 (5)* | |
| H29 | 0.894 (3) | 0.101 (2) | 0.3376 (19) | 0.070 (6)* | |
| H31 | 0.766 (3) | −0.165 (2) | 0.5660 (18) | 0.069 (6)* | |
| H32 | 0.565 (3) | −0.0228 (19) | 0.6597 (18) | 0.066 (6)* | |
| H8 | 0.533 (2) | 0.3600 (17) | 0.5403 (17) | 0.049 (5)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| P1 | 0.0438 (2) | 0.02572 (18) | 0.03071 (18) | −0.00681 (13) | 0.00261 (13) | −0.00327 (12) |
| O2 | 0.0586 (6) | 0.0298 (5) | 0.0380 (5) | −0.0110 (4) | 0.0061 (4) | −0.0035 (4) |
| O3 | 0.0742 (7) | 0.0362 (5) | 0.0535 (6) | −0.0178 (5) | 0.0162 (5) | −0.0060 (5) |
| O4 | 0.0973 (11) | 0.0691 (9) | 0.0630 (9) | 0.0154 (8) | 0.0114 (8) | −0.0189 (7) |
| O5 | 0.1171 (13) | 0.0377 (7) | 0.0854 (10) | 0.0103 (7) | −0.0052 (9) | −0.0200 (7) |
| N6 | 0.0434 (6) | 0.0352 (6) | 0.0402 (6) | −0.0065 (5) | 0.0002 (5) | −0.0006 (5) |
| N7 | 0.0460 (6) | 0.0371 (6) | 0.0354 (6) | −0.0048 (5) | −0.0009 (5) | −0.0064 (5) |
| N8 | 0.0530 (7) | 0.0292 (5) | 0.0337 (6) | −0.0098 (5) | 0.0060 (5) | −0.0044 (4) |
| N9 | 0.0701 (9) | 0.0463 (8) | 0.0522 (8) | 0.0072 (6) | −0.0137 (7) | −0.0185 (6) |
| C10 | 0.0418 (7) | 0.0386 (7) | 0.0505 (8) | −0.0079 (6) | −0.0075 (6) | 0.0004 (6) |
| C11 | 0.0500 (9) | 0.0452 (8) | 0.0674 (10) | −0.0159 (7) | −0.0141 (8) | 0.0087 (8) |
| C12 | 0.0398 (9) | 0.0600 (11) | 0.0866 (14) | −0.0080 (8) | −0.0010 (8) | 0.0178 (10) |
| C13 | 0.0578 (11) | 0.0591 (11) | 0.0732 (12) | 0.0025 (9) | 0.0135 (9) | −0.0011 (10) |
| C14 | 0.0686 (12) | 0.0629 (11) | 0.0768 (13) | −0.0026 (9) | −0.0034 (10) | −0.0243 (10) |
| C15 | 0.0469 (9) | 0.0555 (10) | 0.0723 (11) | −0.0054 (7) | −0.0089 (8) | −0.0198 (8) |
| C16 | 0.0514 (8) | 0.0469 (8) | 0.0468 (8) | −0.0072 (7) | −0.0101 (7) | −0.0086 (7) |
| C17 | 0.0516 (9) | 0.0425 (8) | 0.0578 (10) | −0.0134 (7) | 0.0105 (8) | −0.0012 (7) |
| C18 | 0.0698 (11) | 0.0528 (10) | 0.0400 (8) | −0.0051 (8) | −0.0041 (7) | −0.0146 (7) |
| C19 | 0.0449 (8) | 0.0500 (9) | 0.0451 (8) | −0.0141 (7) | −0.0012 (6) | 0.0005 (7) |
| C20 | 0.0377 (7) | 0.0486 (8) | 0.0418 (7) | −0.0068 (6) | −0.0007 (5) | −0.0036 (6) |
| C21 | 0.0593 (10) | 0.0786 (13) | 0.0509 (9) | −0.0310 (9) | −0.0070 (7) | −0.0029 (8) |
| C22 | 0.0693 (12) | 0.1179 (19) | 0.0511 (10) | −0.0358 (12) | −0.0214 (9) | 0.0091 (11) |
| C23 | 0.0635 (11) | 0.0782 (14) | 0.0599 (11) | −0.0073 (10) | −0.0144 (9) | 0.0174 (10) |
| C24 | 0.0835 (14) | 0.0454 (10) | 0.0674 (12) | −0.0087 (9) | −0.0101 (10) | 0.0039 (8) |
| C25 | 0.0723 (11) | 0.0496 (9) | 0.0524 (9) | −0.0128 (8) | −0.0143 (8) | −0.0037 (7) |
| C26 | 0.0502 (8) | 0.0306 (6) | 0.0365 (7) | −0.0097 (6) | −0.0020 (6) | −0.0034 (5) |
| C27 | 0.0493 (7) | 0.0294 (6) | 0.0370 (7) | −0.0073 (5) | −0.0076 (6) | −0.0056 (5) |
| C28 | 0.0675 (10) | 0.0283 (7) | 0.0388 (7) | −0.0052 (6) | −0.0007 (7) | −0.0035 (6) |
| C29 | 0.0698 (10) | 0.0393 (8) | 0.0377 (7) | −0.0044 (7) | 0.0022 (7) | −0.0058 (6) |
| C30 | 0.0548 (8) | 0.0353 (7) | 0.0434 (8) | 0.0017 (6) | −0.0126 (6) | −0.0128 (6) |
| C31 | 0.0673 (10) | 0.0279 (7) | 0.0617 (10) | −0.0059 (7) | −0.0064 (8) | −0.0069 (7) |
| C32 | 0.0605 (9) | 0.0320 (7) | 0.0558 (9) | −0.0105 (6) | 0.0018 (7) | −0.0042 (6) |
Geometric parameters (Å, °)
| P1—O2 | 1.4787 (10) | C17—H17C | 0.95 (2) |
| P1—N6 | 1.6319 (13) | C18—H18A | 0.97 (3) |
| P1—N7 | 1.6420 (12) | C18—H18B | 0.96 (3) |
| P1—N8 | 1.6910 (11) | C18—H18C | 0.97 (3) |
| O3—C26 | 1.2163 (18) | C19—C20 | 1.510 (2) |
| O4—N9 | 1.206 (2) | C19—H19A | 0.95 (2) |
| O5—N9 | 1.219 (2) | C19—H19B | 0.95 (2) |
| N6—C17 | 1.462 (2) | C20—C25 | 1.380 (3) |
| N6—C16 | 1.4684 (19) | C20—C21 | 1.382 (2) |
| N7—C18 | 1.4663 (19) | C21—C22 | 1.391 (3) |
| N7—C19 | 1.474 (2) | C21—H21 | 0.96 (2) |
| N8—C26 | 1.3686 (18) | C22—C23 | 1.364 (4) |
| N8—H8 | 0.85 (2) | C22—H22 | 0.96 (3) |
| N9—C30 | 1.4729 (19) | C23—C24 | 1.365 (3) |
| C10—C11 | 1.383 (2) | C23—H23 | 0.93 (3) |
| C10—C15 | 1.385 (2) | C24—C25 | 1.383 (3) |
| C10—C16 | 1.509 (2) | C24—H24 | 0.97 (2) |
| C11—C12 | 1.395 (3) | C25—H25 | 0.92 (2) |
| C11—H11 | 0.95 (2) | C26—C27 | 1.5035 (19) |
| C12—C13 | 1.367 (3) | C27—C28 | 1.385 (2) |
| C12—H12 | 0.96 (3) | C27—C32 | 1.391 (2) |
| C13—C14 | 1.373 (3) | C28—C29 | 1.388 (2) |
| C13—H13 | 0.94 (3) | C28—H28 | 0.93 (2) |
| C14—C15 | 1.389 (3) | C29—C30 | 1.377 (2) |
| C14—H14 | 0.96 (3) | C29—H29 | 0.96 (2) |
| C15—H15 | 0.97 (2) | C30—C31 | 1.369 (2) |
| C16—H16A | 0.96 (2) | C31—C32 | 1.383 (2) |
| C16—H16B | 1.00 (2) | C31—H31 | 0.92 (2) |
| C17—H17A | 1.01 (2) | C32—H32 | 0.96 (2) |
| C17—H17B | 0.96 (2) | ||
| O2—P1—N6 | 110.89 (6) | H18A—C18—H18B | 104 (2) |
| O2—P1—N7 | 118.67 (6) | N7—C18—H18C | 110.9 (15) |
| N6—P1—N7 | 103.85 (6) | H18A—C18—H18C | 110 (2) |
| O2—P1—N8 | 105.24 (6) | H18B—C18—H18C | 108 (2) |
| N6—P1—N8 | 113.52 (6) | N7—C19—C20 | 112.83 (12) |
| N7—P1—N8 | 104.82 (6) | N7—C19—H19A | 107.8 (12) |
| C17—N6—C16 | 113.89 (13) | C20—C19—H19A | 107.4 (12) |
| C17—N6—P1 | 125.48 (11) | N7—C19—H19B | 108.0 (11) |
| C16—N6—P1 | 119.62 (10) | C20—C19—H19B | 110.6 (12) |
| C18—N7—C19 | 112.51 (13) | H19A—C19—H19B | 110.2 (17) |
| C18—N7—P1 | 117.29 (10) | C25—C20—C21 | 118.07 (16) |
| C19—N7—P1 | 122.11 (10) | C25—C20—C19 | 121.29 (15) |
| C26—N8—P1 | 124.98 (10) | C21—C20—C19 | 120.60 (16) |
| C26—N8—H8 | 122.7 (12) | C20—C21—C22 | 120.3 (2) |
| P1—N8—H8 | 112.4 (12) | C20—C21—H21 | 118.3 (14) |
| O4—N9—O5 | 123.56 (15) | C22—C21—H21 | 121.4 (14) |
| O4—N9—C30 | 118.42 (14) | C23—C22—C21 | 121.0 (2) |
| O5—N9—C30 | 118.01 (16) | C23—C22—H22 | 120.3 (19) |
| C11—C10—C15 | 118.31 (16) | C21—C22—H22 | 118.7 (19) |
| C11—C10—C16 | 121.02 (15) | C22—C23—C24 | 119.00 (19) |
| C15—C10—C16 | 120.65 (14) | C22—C23—H23 | 124.1 (17) |
| C10—C11—C12 | 120.54 (18) | C24—C23—H23 | 116.9 (17) |
| C10—C11—H11 | 116.3 (13) | C23—C24—C25 | 120.7 (2) |
| C12—C11—H11 | 123.1 (13) | C23—C24—H24 | 121.8 (14) |
| C13—C12—C11 | 120.40 (18) | C25—C24—H24 | 117.5 (14) |
| C13—C12—H12 | 121.6 (16) | C20—C25—C24 | 120.95 (19) |
| C11—C12—H12 | 118.0 (16) | C20—C25—H25 | 119.4 (13) |
| C12—C13—C14 | 119.66 (19) | C24—C25—H25 | 119.6 (14) |
| C12—C13—H13 | 119.4 (15) | O3—C26—N8 | 121.84 (13) |
| C14—C13—H13 | 120.9 (16) | O3—C26—C27 | 120.11 (12) |
| C13—C14—C15 | 120.3 (2) | N8—C26—C27 | 117.97 (12) |
| C13—C14—H14 | 117.8 (16) | C28—C27—C32 | 119.72 (13) |
| C15—C14—H14 | 121.8 (17) | C28—C27—C26 | 124.63 (12) |
| C10—C15—C14 | 120.80 (18) | C32—C27—C26 | 115.62 (13) |
| C10—C15—H15 | 119.7 (13) | C27—C28—C29 | 120.19 (14) |
| C14—C15—H15 | 119.5 (13) | C27—C28—H28 | 121.6 (12) |
| N6—C16—C10 | 112.55 (12) | C29—C28—H28 | 118.2 (12) |
| N6—C16—H16A | 108.2 (11) | C30—C29—C28 | 118.48 (15) |
| C10—C16—H16A | 109.3 (11) | C30—C29—H29 | 120.0 (13) |
| N6—C16—H16B | 107.3 (12) | C28—C29—H29 | 121.5 (13) |
| C10—C16—H16B | 108.7 (12) | C31—C30—C29 | 122.66 (14) |
| H16A—C16—H16B | 110.8 (16) | C31—C30—N9 | 118.90 (14) |
| N6—C17—H17A | 108.4 (12) | C29—C30—N9 | 118.42 (15) |
| N6—C17—H17B | 109.0 (14) | C30—C31—C32 | 118.49 (14) |
| H17A—C17—H17B | 110.9 (18) | C30—C31—H31 | 120.1 (13) |
| N6—C17—H17C | 110.5 (14) | C32—C31—H31 | 121.3 (14) |
| H17A—C17—H17C | 110.8 (18) | C31—C32—C27 | 120.47 (16) |
| H17B—C17—H17C | 107 (2) | C31—C32—H32 | 120.0 (13) |
| N7—C18—H18A | 110.9 (15) | C27—C32—H32 | 119.6 (13) |
| N7—C18—H18B | 113.1 (17) | ||
| O2—P1—N6—C17 | −162.93 (13) | N7—C19—C20—C21 | −112.67 (17) |
| N7—P1—N6—C17 | −34.39 (15) | C25—C20—C21—C22 | 0.2 (3) |
| N8—P1—N6—C17 | 78.85 (14) | C19—C20—C21—C22 | −177.67 (17) |
| O2—P1—N6—C16 | 29.27 (13) | C20—C21—C22—C23 | −0.2 (3) |
| N7—P1—N6—C16 | 157.80 (11) | C21—C22—C23—C24 | 0.1 (3) |
| N8—P1—N6—C16 | −88.96 (12) | C22—C23—C24—C25 | 0.1 (3) |
| O2—P1—N7—C18 | 67.60 (14) | C21—C20—C25—C24 | −0.1 (3) |
| N6—P1—N7—C18 | −56.00 (14) | C19—C20—C25—C24 | 177.82 (17) |
| N8—P1—N7—C18 | −175.36 (13) | C23—C24—C25—C20 | −0.1 (3) |
| O2—P1—N7—C19 | −78.73 (13) | P1—N8—C26—O3 | 8.3 (2) |
| N6—P1—N7—C19 | 157.67 (12) | P1—N8—C26—C27 | −168.53 (10) |
| N8—P1—N7—C19 | 38.30 (13) | O3—C26—C27—C28 | 175.52 (15) |
| O2—P1—N8—C26 | −173.12 (12) | N8—C26—C27—C28 | −7.6 (2) |
| N6—P1—N8—C26 | −51.67 (14) | O3—C26—C27—C32 | −6.7 (2) |
| N7—P1—N8—C26 | 60.98 (14) | N8—C26—C27—C32 | 170.15 (14) |
| C15—C10—C11—C12 | −0.9 (2) | C32—C27—C28—C29 | 0.0 (2) |
| C16—C10—C11—C12 | −179.86 (15) | C26—C27—C28—C29 | 177.69 (15) |
| C10—C11—C12—C13 | −0.2 (3) | C27—C28—C29—C30 | −0.3 (3) |
| C11—C12—C13—C14 | 0.6 (3) | C28—C29—C30—C31 | 0.2 (3) |
| C12—C13—C14—C15 | −0.1 (3) | C28—C29—C30—N9 | −178.13 (15) |
| C11—C10—C15—C14 | 1.5 (3) | O4—N9—C30—C31 | 177.97 (17) |
| C16—C10—C15—C14 | −179.55 (18) | O5—N9—C30—C31 | −2.8 (2) |
| C13—C14—C15—C10 | −1.0 (3) | O4—N9—C30—C29 | −3.7 (2) |
| C17—N6—C16—C10 | 65.91 (18) | O5—N9—C30—C29 | 175.58 (17) |
| P1—N6—C16—C10 | −124.93 (13) | C29—C30—C31—C32 | 0.2 (3) |
| C11—C10—C16—N6 | −132.44 (15) | N9—C30—C31—C32 | 178.54 (15) |
| C15—C10—C16—N6 | 48.6 (2) | C30—C31—C32—C27 | −0.5 (3) |
| C18—N7—C19—C20 | 66.18 (18) | C28—C27—C32—C31 | 0.4 (2) |
| P1—N7—C19—C20 | −146.04 (12) | C26—C27—C32—C31 | −177.47 (15) |
| N7—C19—C20—C25 | 69.5 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N8—H8···O2i | 0.85 (2) | 2.07 (2) | 2.909 (2) | 169 (2) |
Symmetry codes: (i) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: NG5139).
References
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., De Caro, L., Giacovazzo, C., Polidori, G. & Spagna, R. (2005). J. Appl. Cryst. 38, 381–388.
- Etter, M. C., MacDonald, J. C. & Bernstein, J. (1990). Acta Cryst. B46, 256–262. [DOI] [PubMed]
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Gholivand, K., Pourayoubi, M., Shariatinia, Z. & Mostaanzadeh, H. (2005). Polyhedron, 24, 655–662.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Oxford Diffraction (2010). CrysAlis PRO Oxford Diffraction Ltd, Abingdon, England.
- Sabbaghi, F., Pourayoubi, M., Toghraee, M. & Divjakovic, V. (2010). Acta Cryst. E66, o344. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536811011275/ng5139sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811011275/ng5139Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



