Abstract
In spite of research efforts to develop vaccines against the causative agent of human malaria, Plasmodium falciparum, effective control remains elusive. The predominant vaccine strategy focuses on targeting parasite blood stages in the vertebrate host. An alternative approach has been the development of transmission-blocking vaccines (TBVs). TBVs target antigens on parasite sexual stages that persist within the insect vector, anopheline mosquitoes, or target mosquito midgut proteins that are presumed to mediate parasite development. By blocking parasite development within the insect vector, TBVs effectively disrupt transmission and the resultant cascade of secondary infections. Using a mosquito midgut-specific mouse monoclonal antibody (MG96), we have partially characterized membrane-bound midgut glycoproteins in Anopheles gambiae and Anopheles stephensi. These proteins are present on the microvilli of midgut epithelial cells in both blood-fed and unfed mosquitoes, suggesting that the expression of the protein is not induced as a result of blood feeding. MG96 exhibits a dose-dependent blocking effect against Plasmodium yoelii development in An. stephensi. We achieved 100% blocking of parasite development in the mosquito midgut. Preliminary deglycosylation assays indicate that the epitope recognized by MG96 is a complex oligosaccharide. Future investigation of the carbohydrate epitope as well as gene identification should provide valuable insight into the possible mechanisms of ookinete attachment and invasion of mosquito midgut epithelial cells.
The life cycle of the malaria parasite itself is complex and involves asexual development within the vertebrate host as well as both sexual and asexual development within its primary insect vector, the Anopheles (Diptera: Culicidae) mosquito. Plasmodium gamete fertilization and the formation of ookinetes occur within the midgut of the Anopheles mosquito. This is followed by the crucial step of the escape through the peritrophic membrane that encloses the blood meal and invasion of midgut epithelial cells, which are key determinants for successful development and amplification of the parasite in the mosquito. For this reason, the midgut plays a special role as the first insect tissue barrier, and therefore the interaction between parasite and vector host at this site may provide promising molecular targets for vaccine intervention.
Most of the malaria vaccines currently under development target forms that are found in humans, such as preerythrocytic and erythrocytic stages of the parasite (2, 4). More recently, vaccines are being developed not only against the sexual stages (7, 13, 19, 26) but against the mosquito vector as well (1, 2, 9, 12, 17, 18, 23, 27). Whereas blood-stage vaccines operate within the vertebrate host to prevent the clinical manifestation of the disease, vaccines that target the vector stages operate within the mosquitoes to block transmission of the parasite from one vertebrate to another. Consequently, these vaccines are referred to as transmission-blocking vaccines (TBVs) (4). The success of a TBV depends on its ability to alter mosquito vector competence and, consequently, its vectorial capacity (21). In laboratory animals, membrane antigens of sexual stages, or more specifically those of the ookinete (7, 13, 19, 26, 29, 30), induce transmission-blocking immunity. Knockout of crucial genes that are expressed in the ookinete stage of the parasite was found to block its ability to develop as oocysts in the mosquito (6, 30). However, such efforts to understand ookinete biology present only one view of the intimate parasite-vector interaction in the gut lumen. To completely understand this interplay, the mosquito midgut proteins that are recognized by Plasmodium ookinetes in the mosquito midgut must be elucidated.
In this study, we used a mosquito midgut-specific mouse monoclonal antibody (MAb), MG96, to partially characterize a microvillus-associated, membrane-bound midgut glycoprotein in Anopheles gambiae and Anopheles stephensi. MG96 exhibited a dose-dependent blocking of the Plasmodium yoelii ookinete-to-oocyst transition.
MATERIALS AND METHODS
Biological material.
The An. stephensi (Liston) colony was maintained at the Biomedical Research Institute (Rockville, Md.) under standard rearing conditions (27°C, 80% relative humidity). An. gambiae (Giles) mosquitoes were raised under standard conditions as described above and were a generous gift from Nirbhay Kumar (Johns Hopkins University, Baltimore, Md.). Cohorts of 5- to 7-day-old female mosquitoes were either fed on uninfected mice or kept on a sugar diet. At 36 h after blood feeding, the midguts were removed from both blood-fed females and age-matched sugar-fed females and incubated in ice-cold phosphate-buffered saline (PBS). The dissected guts were transferred into mammalian protein extraction reagent (Pierce, Rockford, Ill.) with the addition of 2% ASCB8Ø (Calbiochem, San Diego, Calif.) and a 1:100 dilution of protease inhibitor cocktail (Sigma, St. Louis, Mo.). To extract protein, the sample was ground with a sterile pestle, subjected to repeated freeze-thaw cycles, and then spun down at 14,000 × g at 4°C. Mosquito carcass (minus midgut) protein extract was also prepared for the corresponding time point. Protein extracts from An. stephensi fourth-instar larvae, early-stage pupae, and 5- to 7-day-old males were prepared as described above. The protein concentration was determined by the bicinchoninic acid protein assay (Pierce). P. yoelii (17XNL, nonlethal strain, clone 1.1) sporozoites were isolated from infected An. stephensi mosquitoes (Naval Medical Research Center, Silver Spring, Md.) by density gradient centrifugation as previously described (20). The isolated sporozoites were then used to infect CD1 mice (Charles River Laboratories, Wilmington, Mass.). P. yoelii infection was maintained by blood passage to naive mice every week for a maximum of three passages.
Generation of MAbs.
The MAb MG96 (immunoglobulin G1 [IgG1] subclass) was produced at the Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Md. (I. Fields and M. Shahabuddin, unpublished data). Briefly, 6-week-old female BALB/c mice (Charles River Laboratories) were immunized by subcutaneous injection with Aedes aegypti midgut extracts in RIBI adjuvant (RIBI Immunochem Research, Hamilton, Mont.). A boost was given 21 days after the initial immunization, and a final boost immunization was given intravenously 14 days after the second boost. Generation of hybridoma cells was by the polyethylene glycol method with selection in hypoxanthine-aminopterin-thymidine medium (31). The resulting hybridomas were screened for midgut-specific antibodies by immunofluorescence assay with paraformaldehyde-fixed, Triton X-100-permeabilized Ae. aegypti midgut sections. The specificity for mosquito midgut was confirmed by Western blot analysis with midgut extracts and extracts of total carcasses. The isotype of MAb MG96 was determined with a mouse monoclonal typing kit (Pierce) according to the instructions provided by the manufacturer.
Immunoblotting.
Lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes. Blots were probed with MG96 antibodies from ascites at a 1:1,000 dilution. Bound antibodies were detected with an alkaline phosphatase-conjugated goat anti-mouse IgG antibody (Invitrogen, Carlsbad, Calif.), followed by a chemiluminescent reaction with the addition of CDP-Star substrate (Invitrogen). Protein loading and transfer efficiency were monitored by Coomassie blue staining and the use of prestained molecular weight markers (Pierce), respectively.
Immunofluorescence microscopy of midgut cryosections.
Whole mosquito midguts were dissected in cold PBS, transferred into Tissue Tek OCT embedding medium (Miles, Inc., Pittsburgh, Pa.), and frozen in a methyl-butane liquid nitrogen bath. Several sequential 7-μm sections were fixed in absolute methanol, air dried, and blocked with 10% bovine serum albumin. Each section was probed with a 1:500 dilution of MG96 for 1 h in a humidified chamber at 37°C. The slides were washed several times with PBS and then incubated with a fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG secondary antibody (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) in 0.02% Evans blue (as a general protein counterstain). After repeated washes, the slides were mounted with DAPI (4′,6′-diamidino-2-phenylindole) (a nucleus stain) mounting medium (Vector Labs, Burlingame, Calif.), and the staining patterns were assessed at magnifications of ×20 and ×40 with a Nikon phase-contrast ellipse E500 microscope. Photomicrographs were taken with a SPOT RT camera and SPOT RT software V3.2 (Diagnostic Instrument Inc., Sterling Heights, Mich.). A slide with an FITC-labeled isotype-matched MAb of irrelevant specificity was used as a control.
Immunoelectron microscopy.
The localization of the midgut protein was examined by immunoelectron microscopy by methods described previously, with some modifications (10). Briefly, unfed midguts from 8- to 10-day-old An. stephensi mosquitoes were fixed in PBS containing 1% paraformaldehyde and 0.1% glutaraldehyde for 24 h at room temperature. Ultrathin sections of guts embedded in LR-White resin (Polyscience, Inc., Warrington, Pa.) were mounted on nickel grids and immunostained with MG96. Antibody binding was visualized by using a 10-nm-colloidal gold-conjugated goat anti-mouse IgG (Ted Pella, Redding, Calif.). The grids were counterstained with uranyl acetate and examined with a JEOL 1200 EX transmission electron microscope. Controls for this experiment included the use of isotype-matched, nonspecific antibodies as the primary probe.
Characterization of the epitope recognized by MG96.
Chemical deglycosylation was carried out according to previously described protocols (33, 34) with modifications. Briefly, whole midgut lysates were electrophoretically separated on an SDS-polyacrylamide gel and blotted onto a polyvinylidene difluoride membrane. The membrane was divided into four identical sections and incubated in 50 mM sodium acetate wash buffer (pH 4.5). Sections were used as either control or experimental treatment groups. One experimental strip was incubated with 20 mM sodium periodate in 50 mM sodium acetate buffer (pH 4.5) at room temperature in the dark for 1 h. The other experimental strip was incubated in ice-cold 1 mM sodium periodate in the dark at 0°C on wet ice for 1 h. Controls strips were kept in wash buffer under the same conditions without the addition of sodium periodate. The strips were rinsed with three exchanges of wash buffer and then incubated with 50 mM sodium borohydride (in PBS) at room temperature for 30 min. The membranes were washed three times with PBS, followed by the standard detection of the antigens by MAbs as described for immunoblot analysis. For enzymatic deglycosylation, midgut lysates were boiled at 95°C for 10 min and then treated with either 0.5 mU of peptide N-glycosidase A (EC 3.5.1.52) or 1 mU of neuraminidase (EC 3.2.1.18) and 1 mU of O-glycosidase (EC 3.2.1.97/3.2.1.110) (Roche Molecular Biochemicals, Indianapolis, Ind.). The reaction proceeded overnight in deglycosylation buffer (10 mM Tris, 0.5% SDS, 2% 2-mercaptoethanol, 10 mM EDTA, 5% NP-40) at 37°C. Deglycosylated proteins were run on SDS-polyacrylamide gels and immunoblotted as described above.
Transmission blocking assay.
Six-week-old CD1 mice (Charles River Laboratories) were infected with P. yoelii by inoculation with blood-stage parasites. At 5 days postinfection, gametocytemic mice were exsanguinated and the cells were washed and resuspended in RPMI 1640 plus 10% heat-inactivated mouse serum at a 45% hematocrit. Protein G-purified MG96 at a final concentration of 100, 50, 25, or 5 μg/ml was delivered in 1 ml of infective blood into individual Parafilm-covered membrane feeders. Six-day-old An. stephensi mosquitoes, which had been starved for 36 h, were allowed to feed on the blood for 1 h. Infected blood without antibody treatment was used as a control. In addition, the IgG MAb PvNSV against Plasmodium vivax circumsporozoite protein, diluted to 100 μg/ml in infective blood, was included as a second control. Treatment and control groups were maintained at 22°C and 58% relative humidity. Infectivity was assessed at 10 days after blood feeding by dissecting midguts, staining with mercurochrome, and counting the number of oocysts per mosquito for a minimum of 20 mosquitoes. For each run, only those mosquitoes that showed egg maturation as a proxy for feeding to repletion were used for oocyst counts. Therefore, only guts from fully gravid females were included in the denominator in assessment of percent oocyst infection. All assays were repeated, or treatments were done in duplicates. The differences in the number of oocyst-infected guts between experimental and control groups were analyzed by the Mann-Whitney U test, and differences in the transmission-blocking effect across different antibody doses were analyzed by the Friedman test with STATVIEW 5.0 software (SAS Institute, Cary, N.C.).
RESULTS
MG96 recognizes distinct midgut proteins in both blood-fed and unfed An. stephensi and An. gambiae as determined by Western immunoblotting.
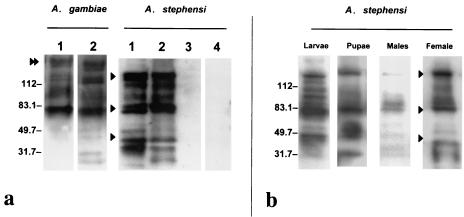
Although the hybridoma cell line MG96 was derived from mouse splenocytes that were reactive to Ae. aegypti midgut plasma membrane antigens, antibodies from this line were capable of binding to the midguts of Anopheles mosquitoes. A reproducible banding pattern was observed for midgut lysates from blood-fed and unfed female mosquito midguts in Western immunoblotting (Fig. 1a). MG96 recognized three primary protein double bands from An. gambiae and An. stephensi, at ∼40, ∼80, and ∼150 kDa, on reducing SDS-PAGE. On the other hand, only the ∼80- and ∼150-kDa double peptide bands were observed from a midgut lysate of blood-fed Ae. aegypti (data not shown). The banding pattern showing multiple secondary bands in addition to the primary double bands strongly suggests that the midgut protein is glycosylated. Separation of the protein products under nonreducing conditions greatly reduced the presence of secondary bands but maintained the three primary doublets (data not shown). The three primary doublets observed for An. stephensi suggest that the midgut protein is polymeric. A similar but not identical banding pattern was observed for midgut lysates from An. gambiae-fed and unfed females, with the addition of a ∼200-kDa protein band. The presence of this band consistently differentiates the two banding patterns of the two vector species. Recognition of the protein doublets in unfed midguts indicates that expression is not induced by the ingestion of a blood meal. To understand the recognition specificity of MG96, a protein lysate from An. stephensi carcasses minus midguts was compared to both fed and unfed midguts by Western blotting. The results confirmed that the banding pattern is found specifically in the midguts of anopheline mosquitoes (Fig. 1a). An isotype-matched MAb control did not reveal any nonspecific recognition of midgut antigens.
FIG. 1.
Immunological reactivity of MG96 to protein lysates from An. stephensi and An. gambiae. (a) Lanes 1 to 3, Western immunoblot analysis of blood-fed (lanes 1) and unfed (lanes 2) midguts and of carcasses minus midguts (lane 3) from 11-day-old mosquitoes. Lane 4, An. stephensi unfed midgut probed with an isotype-matched MAb of irrelevant specificity. (b) Western immunoblot of whole-body lysates from An. stephensi fourth-instar larvae and pupae, as well as midguts of males probed with MG96. Molecular masses are indicated in kilodaltons at the left. Arrowheads indicate the protein doublets at ∼150, ∼80 and ∼40 kDa. The double arrowhead highlights the presence of a unique protein band in An. gambiae.
In addition, MG96 was capable of recognizing both the ∼150- and ∼80-kDa protein bands from whole-body protein extracts from a mixed-sex population of fourth-instar larvae and pupae of An. stephensi (Fig. 1b). Similarly, faint bands corresponding to the ∼150- and ∼80-kDa molecules were also recognized by MG96 in whole-body protein lysates from An. stephensi males.
Localization of midgut antigens by immunofluoresence microscopy.
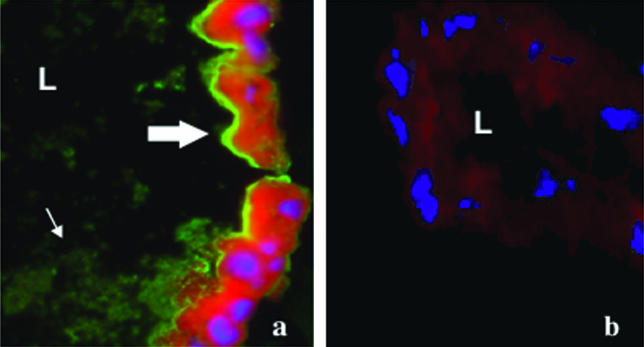
To further assess the specificity of MG96 for gut antigens, midgut cross-sections from 11-day-old unfed and blood-fed Anopheles were immunostained with MG96. Intense fluorescent staining was observed throughout the apical, luminal brush border of the midgut epithelia from both fed (Fig. 2a) and unfed (data not shown) mosquitoes. The sucrose cryoprotection step (14) was omitted from our protocol, and this may have contributed in part to the shearing of the microvilli, owing to the pattern of staining among particles within the gut lumen itself. Immunofluorescence microscopy with FITC-labeled isotype-matched IgG as a control showed insignificant background fluorescence (Fig. 2b).
FIG. 2.
(a) Localization of midgut antigens by immunofluorescence staining of midgut cryosections from an 11-day-old non-blood-fed An. gambiae midgut cryosection probed with MAb MG96 demonstrates staining along the microvilli (thick arrow) and glycocalyx (thin arrow) on the luminal side of midgut epithelia. (b) An isotype-matched IgG was used to stain an An. gambiae midgut cryosection as a control. Evans blue (red) was used as a counterstain against general cellular protein, and DAPI staining (blue) was used to identify nuclei. L, lumen of the midgut. Magnification, ×40.
Subcellular localization of midgut antigens by immunoelectron microscopy.
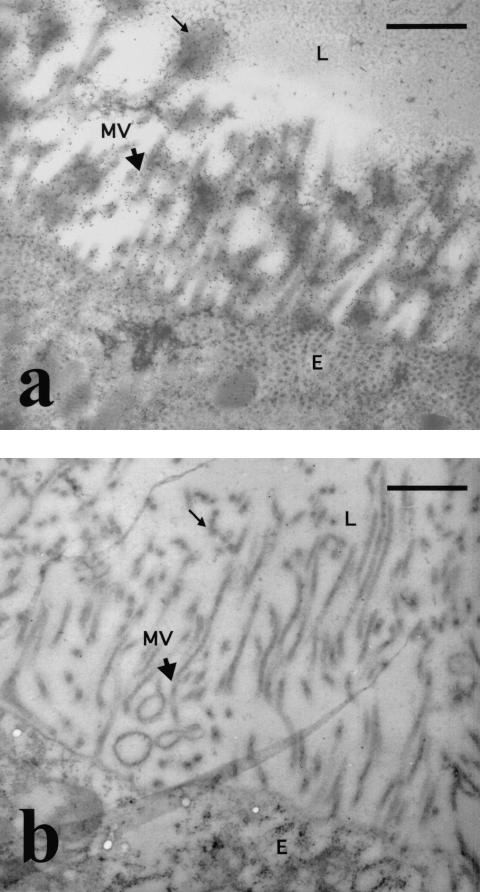
Immunoelectron microscopy was performed to determine more precisely the subcellular localization of midgut antigens recognized by MG96 in An. stephensi (Fig. 3a). Gold particle labeling showed that MG96 was preferentially associated with the apical microvilli and the microvillus network in cross-sections of the gut epithelial cell. Labeling of the cell cytoplasm was also observed and is presumed to be evidence of antigen transport to the cell surface. Immunostaining with an isotype-matched control antibody to a serial cross-section of the midgut showed no background labeling of either extracellular or intracellular components (Fig. 3b).
FIG. 3.
Immunoelectron microscopy of unfed An. stephensi midguts. (a) Cross-section of the midgut showing the association of MG96 with the extracellular microvilli (arrowhead) and glycocalyx (thin arrow) on the apical end of the midgut epithelial cell. (b) Cross-section of the midgut following staining with an isotype-matched control MAb. MVN, microvillus network; MV, microvilli; E, epithelial lining; L, lumens Bars, 1 μm. Magnification, ×15,000.
Effect of MG96 on P. yoelii development in An. stephensi mosquitoes.
In the initial series of experimental trials, mosquitoes were provided an infective blood meal that contained a 1:500 dilution of MG96 from ascites. Transmission-blocking activity of MG96 was assessed as the number of infected mosquitoes (i.e., mosquitoes having oocysts in the midguts at 10 days postinfection) among treatment groups. The results showed a reduction in the number of P. yoelii-infected midguts at 10 days postinfection, with 8% of mosquitoes remaining infected among treatment groups (n = 75) compared to 40% remaining infected among controls (n = 43). This difference was found to be statistically significant by using the Mann-Whitney U test at α = 0.05 (P = 0.03). To determine the spectrum of transmission blocking induced by MG96, serial dilutions of purified antibody were made and added to infective blood meals. We achieved complete elimination of oocyst development at a 100-μg/ml concentration of MG96 (P < 0.05). In addition, the trend in the blocking effect was observed to be dose dependent (Table 1). However, the difference in treatment effect between any two doses was not statistically significant (Friedman test, P > 0.05). These assay results were reproducible across three trials.
TABLE 1.
Effect of MAb MG96 on P. yoelii development in An. stephensi mosquito midguts
| Treatment group | Dose (μg/ml) | % Reduction in infected midgutsa | No. of infected mosquitoes/number dissected (% infected) | P valueb |
|---|---|---|---|---|
| Control (no MAb) | 0 | 0 | 14/40 (35) | |
| Control MAb | 100 | 0 | 23/60 (38) | |
| MG96 | 10 | 74 | 2/20 (10) | 0.03 |
| 50 | 85 | 1/18 (6) | 0.04 | |
| 100 | 100 | 0/38 (0) | <0.01 |
Percent reduction in the number of infected midguts, i.e., 1 − (percent infected in treatment/percent infected in control with MAb) × 100. Results are from representative experiments from three trials.
Mann-Whitney U test, nonparametric analysis, α = 0.05.
The epitope recognized by MG96 has a carbohydrate component.
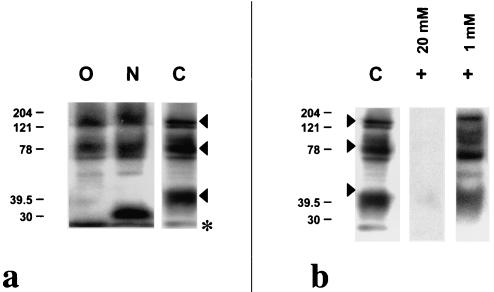
To understand the banding pattern observed on Western immunoblots, a partial characterization of the epitope was ascertained through a combination of enzyme and chemical deglycosylation assays. Treatment of mosquito midgut lysates with PNGase A, an N-linked oligosaccharide-Asn-specific exoglycosidase, slightly altered the MG96 recognition pattern. A mobility shift was expressly observed for the ∼40-kDa double band. However, there was no modification in the MG96 recognition of the two higher-molecular-mass bands (Fig. 4a). Initial treatment with a nonspecific neuraminidase to remove terminal neuraminic acid, followed by treatment with O-glycosidase, an O-linked Galβ(1, 3)GalNAc-α-Ser/Thr-specific endoglycosidase, completely altered the staining profile of the ∼40-kDa band (Fig. 4a). As with treatment with PNGase A, staining of the ∼150- and ∼80-kDa bands remained unaffected. This observation may have been due to presumably incomplete digestion, as the enzyme rate of hydrolysis may be much lower for larger glycoproteins, thereby requiring a more protracted incubation period in a denaturing reaction buffer. Incomplete denaturing of the protein secondary structures may not have revealed masked or obscured glycosidase recognition sites. Treatment with 1 mM sodium periodate at 0°C preferentially cleaves terminal neuraminic acid on both N- and O-linked branching oligosaccharides, whereas treatment with 20 mM sodium periodate cleaves off all oligosaccharides from the protein backbone. The MG96 recognition profile was unaffected following treatment with 1 mM sodium periodate; however, treatment with 20 mM sodium periodate completely eliminated recognition by MG96 on a Western blot (Fig. 4b).
FIG. 4.
Western immunoblot analysis of An. stephensi midgut lysates following enzyme and chemical deglycosylation. (a) Lysates that were treated with 1 mU of neuraminidase plus 1 mU of O-glycosidase (lane O) or with 0.5 mU of PNGase A (lane N) were probed with MAb MG96 and compared to control lysates that were subjected to the same treatment conditions without enzyme (lane C). (b) Western immunoblot analysis of An. stephensi midgut lysates probed with MG96 following chemical deglycosylation with 1 or 20 mM sodium periodate compared to control. Molecular masses are indicated in kilodaltons at the left. Arrowheads indicate the protein doublets at ∼150, ∼80, and ∼40 kDa on untreated midgut lysate. The asterisk indicates the migration front.
DISCUSSION
MAb MG96, generated against the Ae. aegypti midgut, shows cross-reactivity with midgut antigens from An. stephensi and An. gambiae. This phenomenon is, in itself, particularly interesting within an evolutionary context, especially since it is believed that Culicinae and Anophelinae diverged over 200 million years ago (3). However, from a functional perspective, the most intriguing observation is that the antibody is capable of inducing transmission blocking in An. stephensi when ingested with an infective blood meal. At a concentration of 100 μg/ml, complete nullification of both oocyst development and salivary gland infection was achieved. While we saw a decreasing trend in the transmission-blocking effect with increasing dilutions of the antibody, the difference between the proportions of mosquito guts that remained infected at different doses was not statistically significant. This highlights the efficiency of the blocking effect of MG96, since low concentrations of this MAb can still effectively block parasite development in the mosquito midgut. The mechanism by which this antibody obstructs the natural progress of infection by Plasmodium is not yet understood. However, it is evident that MG96 recognizes midgut antigens that are specifically located along the brush border that lines the apical side of gut epithelial cells facing the lumen. Since the peritrophic matrix, which had enclosed the blood meal, had been completely removed to ensure a clean specimen, the presence of this glycoprotein on the luminal side of the peritrophic matrix (24, 31, 35, 36) could not be completely ascertained by our assays. The staining pattern demonstrated by indirect fluorescent-antibody assay and immunoelectron microscopy does suggest that the glycoprotein is present in the glycocalyx network attached to the microvilli. The apical side of gut epithelial cells is the first to confront invading ookinetes. The disruption of the ookinete-to-oocyst transition may therefore occur at either the level of attachment to the glycocalyx or to the microvillus itself (36) or at the level of a more downstream event following invasion, e.g., interference of cell signaling and/or cell functioning.
The distinct multiple-banding profile observed on Western blots was found to be present in the midguts of both unfed and blood-fed adult female mosquitoes. This multiple-banding profile was also observed in protein lysates from males, mixed-sex pupae, and fourth-instar larvae. However, the banding patterns were not identical. Males and aquatic life stages do not engage in blood feeding. This implies that the midgut proteins recognized by MG96 may not have anything to do with blood feeding and may in fact be found in other tissues outside the midgut. A comparison of unfed adult female mosquito carcasses with the midguts removed to unfed midguts alone confirmed the tissue specificity of the observed banding pattern. Moreover, whether these antigens are present only in the guts of larvae and pupae remains to be seen. The fact that these proteins are present across different life stages, sexes, and tissues strongly suggests an integral, albeit poorly understood, functional role in mosquito development and survival.
The detection of multiple protein bands by Western analysis is not uncommon (11, 28, 31, 34). Posttranslational modification of proteins, through the addition of carbohydrate moieties, has been shown to affect the apparent molecular weight of glycoproteins analyzed by SDS-PAGE. Glycosylation contributes as much as 50 to 80% of the molecular weight of mature glycoproteins in mammals (5, 8, 28) and up to 40% in invertebrates (5, 16). In addition, it has been shown that the glycosylation profile can influence MAb binding to glycoproteins (5, 28). Enzyme and chemical deglycosylation assays revealed that MG96 recognizes a midgut epitope in which carbohydrates are a major component. MG96 recognition of the gut epitope is influenced by N-linked oligosaccharides, as prolonged treatment with PNGase A modified the antibody recognition profile. PNGase A is an N-glycosidase that hydrolyzes an N4-(acetyl-β-d-glucosaminyl) asparagine (Asn) residue (11). It can also cleave fucose linked α to Asn-N-acetylglucosamine (GlcNAc). In contrast to N-glycosidases, O-glycosidases specifically cleave the Gal(β1,3)GalNAc disaccharide attached to serine and threonine residues (11). Treatment of midgut lysate with this enzyme eliminated MG96 recognition of the ∼40-kDa band, suggesting that an O-linked carbohydrate may be a component of the MG96 epitope. Periodate treatment of An. stephensi midgut lysates completely abolished recognition by MG96, thereby confirming the importance of carbohydrates to the MG96 epitope structure.
These results suggest that the epitope is complex and may be composed primarily of O-linked carbohydrates. However, N-linked carbohydrates and the peptide backbone may also contribute to a lesser degree. Sustained recognition by MG96 of the ∼40-kDa band following PNGase A treatment may be due to the presence of O-linked oligosaccharides that partially maintain the integrity of the epitope. Inefficient enzyme digestion of proximal N- and O-linked oligosaccharides may have contributed to sustained recognition of the two higher-molecular-weight molecules. Another possibility would be that the epitope is composed of both carbohydrate and protein. This is not an uncommon phenomenon, as there are several examples of antibodies that recognize epitopes consisting of protein carbohydrate linkages, e.g., antibodies to M and N glycophorins on erythrocyte membranes (5, 28), or a combination of peptides and carbohydrates (28). For example, in the development of antibodies to human epithelial mucin MUC1, the specificities of several MAbs were shown to be contingent upon the addition of a Gal(β1,3)GalNAc disaccharide (13). It is difficult to ascertain from these assays which of these explanations is the most reasonable. Only an in-depth analysis of sequentially detached carbohydrates would allow us to gain insight into the essential carbohydrate moieties that contribute to the complexity of this midgut epitope.
The recognition of a carbohydrate epitope helps clarify our observations of multiple bands on SDS-PAGE. One possible explanation is that the midgut protein is multimeric. Another possibility is that the ∼40-kDa doublet represents a polypeptide heterodimer captured in its nascent form as it cycles through the Golgi compartments. For both of these possibilities, a common denominator is requisite. MG96 may therefore recognize a carbohydrate epitope, a result of distinct branching structures of several oligosaccharide side chains, which is shared among different multimers of the same protein or among various glycoproteins. Several studies have shown that insect midgut proteins in particular are heavily O glycosylated (15, 16, 32, 33). Although several different glycoproteins are present in the midgut of Anopheles, only a limited repertoire of carbohydrate moieties are added onto proteins as they cycle through the Golgi apparatus (33). This limited repertoire is presumably due to the nature of the mosquito gut environment. In mammals, specific tissues and organs display distinct glycan patterns in accordance with their primary function. For example, heavily O-glycosylated proteins such as intestinal mucins have characteristic GalNAc-based glycan structures that protect the gut epithelial cells from the harsh proteolytic environment (11). O-linked glycoproteins that harbor similar GalNAc oligosaccharide chains covalently attached to serine and threonine repeat residues are classified as mucin-type glycoproteins (11). The AgMUC1 (from An. gambiae) (25) and AeIMUC1 (from Ae. aegypti) (22) mucin-like proteins have been described to be midgut-specific O-glycosylated proteins that may be involved in parasite recognition of the midgut. It remains to be seen whether MG96 can recognize O-linked carbohydrate epitopes on these and other mucin-like proteins that may be conserved across different arthropod vectors. If so, we can then determine whether or not the transmission-blocking effect of anticarbohydrate antibodies can indeed translate to other insect vector-pathogen systems.
We have described a potential TBV target carbohydrate receptor on midgut glycoproteins that may serve as an attachment site for ookinetes. Experiments are currently under way to identify the gene(s) encoding these glycoproteins. More importantly, future research will focus on characterizing the carbohydrate epitope and determining its role in the ookinete-to-oocyst transition in the mosquito midgut.
Acknowledgments
We are grateful to Tom Mitchell for generously providing the An. stephensi mosquitoes, to Joan Buenconsejo for assistance with statistical analysis, and to Albert Mulenga and Patricia Strickler for reviews of the manuscript.
This work was supported by grants from NIAID/NIH.
Editor: B. B. Finlay
REFERENCES
- 1.Almeida, A. P. G., and P. F. Billingsley. 1998. Induced immunity against the mosquito Anopheles stephensi Liston (Diptera: Culicidae): effects on mosquito survival and fecundity. Int. J. Parasitol. 28:1721-1731. [DOI] [PubMed] [Google Scholar]
- 2.Beier, J. C. 1998. Malaria parasite development in mosquitoes. Annu. Rev. Entomol. 43:519-543. [DOI] [PubMed] [Google Scholar]
- 3.Besansky, N. J., V. Finnerty, and F. H. Collins. 1992. A molecular genetic perspective on mosquitoes. Adv. Genet. 30:123-184. [DOI] [PubMed] [Google Scholar]
- 4.Carter, R. 2001. Transmission blocking malaria vaccines. Vaccine 19:2309-2314. [DOI] [PubMed] [Google Scholar]
- 5.Corfield, A. P., and A. K. Shukla. 2001. Mucins: vital components of the mucosal defensive barrier. Genom./Proteom. Technol. 3:20-23. [Google Scholar]
- 6.Dessens, J. T., A. L. Beetsma, G. Dimopoulos, K. Wengelnik, A. Crisanti, F. C. Kafatos, and R. E. Sinden. 1999. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 18:6221-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffy, P. E., and D. C. Kaslow. 1997. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect. Immun. 65:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueroa, J. V., G. M. Buening, D A. Kinden, and T. J. Green. 1990. Identification of common surface antigens among Babesia bigemina isolates by using monoclonal antibodies. Parasitology 100:161-175. [DOI] [PubMed] [Google Scholar]
- 9.Foy, B. D., T. Magalhaes, W. E. Injera, I. Sutherland, M. Davenport, A. Thanawastien, D. Ripley, L. Cardenas-Freytag, and J. C. Beier. 2003. Induction of mosquitocidal activity in mice immunized with Anopheles gambiae midgut cDNA. Infect. Immun. 71:2032-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaywee, J., J. B. Sacci, Jr., S. Radulovic, M. S. Beier, and A. F. Azad. 2003. Subcellular localization of rickettsial invasion protein, InvA. Am. J. Trop. Med. Hyg. 6891:92-96. [PubMed] [Google Scholar]
- 11.Hanisch, F. G. 2001. O-glycosylation of the mucin type. Biol. Chem. 382:143-149. [DOI] [PubMed] [Google Scholar]
- 12.Hatfield, P. 1988. Anti-mosquito antibodies and their effects on feeding, fecundity and mortality of Aedes aegypti. Med. Vet. Entomol. 2:339-345. [DOI] [PubMed] [Google Scholar]
- 13.Hisaeda, H., A. W. Stowers, T. Tsuboi, W. E. Collins, J. S. Sattabongkot, and N. Suwanabun. 2000. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect. Immun. 68:6618-6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holt, S. J., E. E. Hobbiger, E. Eluned, and G. L. S. Pawan. 1960. Preservation of the integrity of rat tissues for cytochemical staining purposes. J. Biophys. Biochem. Cytol. 7:383-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramerov, A. A., N. P. Arbatsky, Y. A. Rozovsky, E. A. Mikhaleva, O. O. Polesskaya, V. A. Gvozdev, and V. N. Shibaev. 1996. Mucin-type glycoprotein from Drosophila melanogaster embryonic cells: characterization of the carbohydrate component. FEBS Lett. 378:213-218. [DOI] [PubMed] [Google Scholar]
- 16.Kramerov, A. A., E. A. Mikhaleva, Y. M. Rozovsky, T. V. Pochechueva, N. A. Baikova, E. L. Arsenjeva, and V. A. Gvozdev. 1997. Insect mucin-type glycoprotein: immunodetection of the O-glycoyslated epitope in Drosophila melanogaster cells and tissues. Insect Biochem. Mol. Biol. 27:513-521. [DOI] [PubMed] [Google Scholar]
- 17.Lal, A. A., P. S. Patterson, J. B. Sacci, J. A. Vaughan, C. Paul, W. E. Collins, R. A. Wirtz, and A. F. Azad. 2001. Anti-mosquito midgut antibodies block development of Plasmodium falciparum and Plasmodium vivax in multiple species of Anopheles mosquitoes and reduce vector fecundity and survivorship. Proc. Natl. Acad. Sci. USA 98:5228-5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lal, A. A., M. E. Schriefer, J. B. Sacci, I. F. Goldman, V. Louis-Wileman, W. E. Collins, and A. F. Azad. 1994. Inhibition of malaria parasite development in mosquitoes by anti-mosquito midgut antibodies. Infect. Immun 62:316-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langer, R. C., F. Li, and J. M. Vinetz. 2001. Identification of novel Plasmodium gallinaceum zygote- and ookinete-expressed proteins as targets for blocking malaria transmission. Infect. Immun. 70:102-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau, A. O. T., J. B. Sacci, and A. F. Azad. 2000. Retrieving parasite specific liver stage gene products in Plasmodium yoelii infected livers using differential display. Mol. Biochem. Parasitol. 111:143-151. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald, G. 1957. The epidemiology and control of malaria. Oxford University Press, London, United Kingdom.
- 22.Morlais, I., and D. W. Severson. 2001. Identification of a polymorphic mucin-like Gene expressed in the midgut of the mosquito, Aedes aegypti, using an integrated bulked segregant and differential display analysis. Genetics 158:1125-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramasamy, M. S., R. Ramasamy, B. H. Kay, and C. Kidson. 1988. Anti-mosquito antibodies decrease the reproductive capacity of Aedes aegypti. Med. Vet. Entomol. 2:87-93. [DOI] [PubMed] [Google Scholar]
- 24.Shahabuddin, M., and A. Costero. 2001. Spatial distribution of factors that determine sporogonic development of malaria parasites in mosquitoes. Insect Biochem. Mol. Biol. 31:231-240. [DOI] [PubMed] [Google Scholar]
- 25.Shen, Z., G. Dimopoulos, F. C. Kafatos, and M. Jacobs-Lorena. 1999. A cell surface mucin specifically expressed in the midgut of the malaria mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 96:5610-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stowers, A. W., D. B. Keister, O. Muratova, and D. C. Kaslow. 2000. A region of Plasmodium falciparum antigen Pfs25 that is the target of highly potent transmission-blocking antibodies. Infect. Immun. 68:5530-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutherland, G. B., and A. B. Ewen. 1974. Fecundity decrease in mosquitoes ingesting blood from specifically sensitized mammals. J. Insect Physiol. 20:655-660. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi, H., K. Kato, K. Dendai-Nagai, F. G. Hanisch, H. Lausen, and T. Irimura. 2002. The epitope recognized by the unique anti-MUC1 monoclonal antibody MY.1E12 involves sialylα2-3galactosylβ1-3N-acetylgalactosaminide linked to a distinct threonine residue in the MUC1 tandem repeat. J. Immunol. Methods 270:199-209. [DOI] [PubMed] [Google Scholar]
- 29.Templeton, T. J., and D. C. Kaslow. 1999. Identification of additional members defines a Plasmodium falciparum gene superfamily which includes Pfs48/45 and Pfs230. Mol. Biochem. Parasitol. 101:223-227. [DOI] [PubMed] [Google Scholar]
- 30.Tsai, Y. L., R. E. Hayward, R. C. Langer, D. A. Fidock, and J. M. Vinetz. 2001. Disruption of Plasmodium falciparum chitinase markedly impairs parasite invasion of mosquito midgut. Infect. Immun. 69:4048-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, P., J. T. Conrad, and M. Shahabuddin. 2001. Localization of midgut-specific protein antigens from Aedes aegypti (Diptera: Culicidae) using monoclonal antibodies. J. Med. Entomol. 2:223-230. [DOI] [PubMed] [Google Scholar]
- 32.Wang, P., and R. R. Granados. 1997. Molecular cloning and sequencing of a novel invertebrate intestinal mucin cDNA. J. Biol. Chem. 272:16663-16669. [DOI] [PubMed] [Google Scholar]
- 33.Wilkins, S., and P. F. Billingsley. 2001. Partial characterization of oligosaccharides expressed on midgut microvillar glycoproteins of the mosquito, Anopheles stephensi Liston. Insect Biochem. Mol. Biol. 31:937-948. [DOI] [PubMed] [Google Scholar]
- 34.Woodward, M. P., W. W. Young, Jr., and R. A. Bloodgood. 1985. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J. Immunol. Methods 78:143-153. [DOI] [PubMed] [Google Scholar]
- 35.Zieler, H., C. F. Garon, E. R. Fischer, and M. Shahabuddin. 2000. A tubular network associated with the brush-border surface of the Aedes aegypti midgut: implications for pathogen transmission by mosquitoes. J. Exp Biol. 203:1599-1611. [DOI] [PubMed] [Google Scholar]
- 36.Zieler, H., J. P. Nawrocki, and M. Shahabuddin. 1999. Plasmodium gallinaceum ookinetes adhere specifically to the Aedes aegypti by interaction with a carbohydrate ligand. J. Exp. Biol. 202:485-495. [DOI] [PubMed] [Google Scholar]