Abstract
In the title complex, [Sn2(C7H7)4(C10H8N2O3)2(C2H5OH)2], the Sn(IV) atom is seven-coordinated in a distorted pentagonal–bipyramidal geometry by three O atoms and one N atom from the pyruvate benzoyl hydrazone ligand, one ethanol O atom and two axial C atoms from trans-benzyl groups, thus forming a dimeric molecule ( symmetry) via weak Sn—O interactions. The C atoms of one phenyl ring and the ethanol molecule are disordered over two sets of sites with site-occupancy factors of 0.57 (5):0.43 (5) and 0.79 (2):0.21 (2), respectively. Intermolecular O—H⋯O hydrogen bonds stabilize the crystal structure.
symmetry) via weak Sn—O interactions. The C atoms of one phenyl ring and the ethanol molecule are disordered over two sets of sites with site-occupancy factors of 0.57 (5):0.43 (5) and 0.79 (2):0.21 (2), respectively. Intermolecular O—H⋯O hydrogen bonds stabilize the crystal structure.
Related literature
For related structures, see: Sun & Hu (2007 ▶); Gielen et al. (2002 ▶).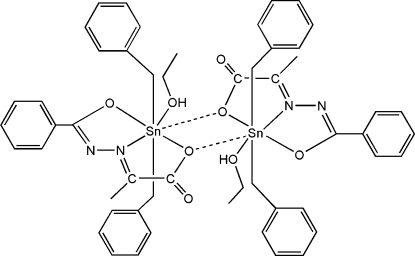
Experimental
Crystal data
[Sn2(C7H7)4(C10H8N2O3)2(C2H6O)2]
M r = 1102.42
Triclinic,
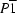
a = 8.7187 (18) Å
b = 11.385 (2) Å
c = 13.198 (3) Å
α = 96.170 (3)°
β = 93.728 (2)°
γ = 105.861 (3)°
V = 1246.8 (4) Å3
Z = 1
Mo Kα radiation
μ = 1.06 mm−1
T = 298 K
0.45 × 0.37 × 0.23 mm
Data collection
Siemens SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.647, T max = 0.793
6566 measured reflections
4356 independent reflections
3598 reflections with I > 2σ(I)
R int = 0.017
Refinement
R[F 2 > 2σ(F 2)] = 0.042
wR(F 2) = 0.116
S = 1.08
4356 reflections
298 parameters
H-atom parameters constrained
Δρmax = 1.04 e Å−3
Δρmin = −0.58 e Å−3
Data collection: SMART (Siemens, 1996 ▶); cell refinement: SAINT (Siemens, 1996 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811011937/lr2004sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811011937/lr2004Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected geometric parameters (Å, °).
| Sn1—C11 | 2.135 (6) |
| Sn1—O3 | 2.148 (3) |
| Sn1—C18 | 2.154 (6) |
| Sn1—N1 | 2.237 (4) |
| Sn1—O1 | 2.341 (3) |
| Sn1—O4 | 2.382 (4) |
| Sn1—O1i | 2.772 (3) |
| C11—Sn1—O3 | 97.42 (19) |
| C11—Sn1—C18 | 163.3 (2) |
| O3—Sn1—C18 | 94.77 (18) |
| C11—Sn1—N1 | 97.9 (2) |
| O3—Sn1—N1 | 70.83 (13) |
| C18—Sn1—N1 | 96.85 (18) |
| C11—Sn1—O1 | 88.59 (19) |
| O3—Sn1—O1 | 140.42 (12) |
| C18—Sn1—O1 | 89.30 (18) |
| N1—Sn1—O1 | 69.60 (12) |
| C11—Sn1—O4 | 84.9 (2) |
| O3—Sn1—O4 | 78.79 (13) |
| C18—Sn1—O4 | 86.28 (19) |
| N1—Sn1—O4 | 149.60 (14) |
| O1—Sn1—O4 | 140.79 (12) |
| C11—Sn1—O1i | 80.32 (18) |
| O3—Sn1—O1i | 154.13 (12) |
| C18—Sn1—O1i | 83.72 (16) |
| N1—Sn1—O1i | 135.04 (12) |
| O1—Sn1—O1i | 65.45 (12) |
| O4—Sn1—O1i | 75.34 (11) |
Symmetry code: (i) 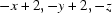 .
.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O4—H4⋯O2i | 0.82 | 1.82 | 2.624 (6) | 165 |
Symmetry code: (i) 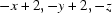 .
.
Acknowledgments
We acknowledge financial support by the Clinical Laboratory of Liaocheng Hospital.
supplementary crystallographic information
Comment
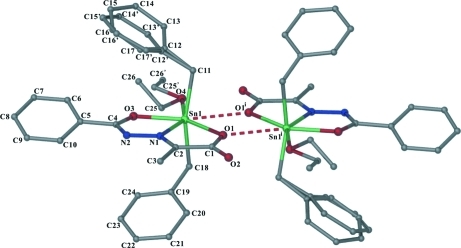
Organotin derivatives of carboxylic acid ligands have been extensively studied due to their biological activities (Gielen et al., 2002). In our ongoing studies with Schiff base organotin(IV) compounds, the title compound has been synthesized and we report herein its crystal structure. The molecular structure of the compound is shown in Fig.1. The atoms O1, O3, N1 and O4 are coplanar within 0.0120 Å, which form the equatorial plane. Furthermore, the angle of the axial C11—Sn1—C18 is 163.3 (3)°, which deviates from the linear angle of 180°. These data indicate that the tin atom of this complex is in a distorted octahedral configuration. The O1 atom of the carboxylate residue also binds the other tin atom, Sni, generating a Sn2O2 four-membered ring [symmetry code: 2 - x, 2 - y, -z]. The distances of Sn1–O1i 2.772 (4)Å are relatively longer than those of Sn1—O1 2.339 (4)Å (Table 1), but are comparable with those found in related seven-coordinate diorganotin systems (Sun et al., 2007). With weak interactions of Sn–O bonding, the structure of the title complex can be described as a dimer with crystallographically imposed 1 symmetry. and the coordination geometry of tin can be also described as a trans-C2SnO4N pentagonal bipyramid with the two benzyl groups occupying trans positions. The forming of the dimer leads to the shorter interaction between O and Oi, because the interaction of two monomers surpass the repelling effect of two O atoms. Otherwise, there exhibit the disorder at the C12 to C17 aromatic ring moiety and the C25, C26 atoms of the coordinated ethonal solvate molecule.
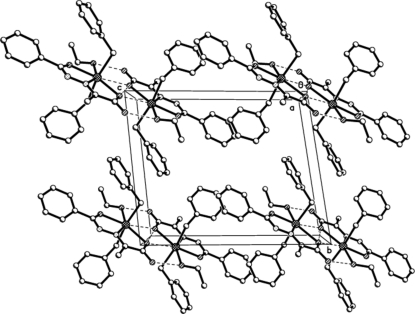
Each Sn atom is also coordinated by an ethanol molecule, the Sn1—O4 bond distance being 2.424 (3) Å, which is comparable with those in the analogous (Sun et al., 2007), due to the formation of intradimeric hydrogen bonds, O2–O4i (or O2i–O4) 2.624 (6)Å (Table 2). These hydrogen bonds contribute to the stability and compactness of the crystal structure (Fig. 2).
Experimental
Pyruvic acid benzoyldrazone (1 mmol) and sodium ethoxide (1 mmol) was added to the solution of dry benzene (20 ml) in a Schlenk flask and stirred for 0.5 h. Dibenzyltin dichloride (1 mmol) was then added and the reaction mixture was stirred for 12 h at 313 K and then filtered. The solvent was gradually removed by evaporation under vacuum until a solid product was obtained. The solid was then recrystallized from ethanol and colorless crystals suitable for X-ray diffraction were obtained. Elemental analysis, calculated for C26H28N2O4Sn: C 56.66, H 5.12, N 5.08; found: C 56.51, H 5.34, N 5.01%.
Refinement
The atoms C12, C13, C14, C15, C16 and C17 of the phenyl ring, C25 and C26 of the ethanol molecule were found to be disordered over two sites, and the ratio of the occupancy factors were refined to 0.57 (5):0.43 (5) and 0.79 (2):0.21 (2) for the phenyl ring C atoms and ethanol C atoms, respectively. The H atoms were positioned geometrically with aromatic C—H distances of 0.93 Å, and refined as riding on their parent atoms, with Uiso(H) = 1.2 Ueq(C, O). All other H atoms were also placed in idealized positions, with Uiso(H) = 1.5 Ueq(C, O).
Figures
Fig. 1.
The molecular structure of the compound, showing 50% probability displacement ellipsoids. H atoms have been omitted for clarity. Symmetry code: 2 - x, 2 - y, -z.
Fig. 2.
The crystal packing in a unit cell of the title complex, viewed along the b axis. H atoms have been omitted.
Crystal data
| [Sn2(C7H7)4(C10H8N2O3)2(C2H6O)2] | Z = 1 |
| Mr = 1102.42 | F(000) = 560 |
| Triclinic, P1 | Dx = 1.468 Mg m−3 |
| a = 8.7187 (18) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 11.385 (2) Å | Cell parameters from 3583 reflections |
| c = 13.198 (3) Å | θ = 2.6–27.3° |
| α = 96.170 (3)° | µ = 1.06 mm−1 |
| β = 93.728 (2)° | T = 298 K |
| γ = 105.861 (3)° | Block, colorless |
| V = 1246.8 (4) Å3 | 0.45 × 0.37 × 0.23 mm |
Data collection
| Siemens SMART CCD area-detector diffractometer | 4356 independent reflections |
| Radiation source: fine-focus sealed tube | 3598 reflections with I > 2σ(I) |
| graphite | Rint = 0.017 |
| phi and ω scans | θmax = 25.0°, θmin = 2.3° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −10→10 |
| Tmin = 0.647, Tmax = 0.793 | k = −13→12 |
| 6566 measured reflections | l = −15→15 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.042 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.116 | H-atom parameters constrained |
| S = 1.08 | w = 1/[σ2(Fo2) + (0.0509P)2 + 2.1936P] where P = (Fo2 + 2Fc2)/3 |
| 4356 reflections | (Δ/σ)max = 0.001 |
| 298 parameters | Δρmax = 1.04 e Å−3 |
| 0 restraints | Δρmin = −0.58 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Sn1 | 0.93329 (4) | 0.91659 (3) | 0.13554 (2) | 0.04906 (15) | |
| N1 | 0.7188 (5) | 0.9747 (4) | 0.1792 (3) | 0.0470 (9) | |
| N2 | 0.6493 (5) | 0.9324 (4) | 0.2637 (3) | 0.0512 (10) | |
| O1 | 0.8691 (4) | 1.0434 (3) | 0.0211 (2) | 0.0504 (8) | |
| O2 | 0.7086 (5) | 1.1605 (4) | −0.0098 (3) | 0.0724 (12) | |
| O3 | 0.8542 (4) | 0.8390 (3) | 0.2709 (2) | 0.0538 (9) | |
| O4 | 1.1210 (5) | 0.8036 (4) | 0.1661 (3) | 0.0688 (11) | |
| H4 | 1.1866 | 0.8112 | 0.1237 | 0.103* | |
| C1 | 0.7536 (6) | 1.0884 (5) | 0.0400 (4) | 0.0509 (12) | |
| C2 | 0.6661 (6) | 1.0494 (5) | 0.1312 (4) | 0.0506 (12) | |
| C3 | 0.5333 (7) | 1.0990 (6) | 0.1618 (4) | 0.0679 (16) | |
| H3A | 0.4925 | 1.0645 | 0.2212 | 0.102* | |
| H3B | 0.4492 | 1.0778 | 0.1066 | 0.102* | |
| H3C | 0.5720 | 1.1870 | 0.1774 | 0.102* | |
| C4 | 0.7284 (6) | 0.8633 (5) | 0.3039 (4) | 0.0537 (12) | |
| C5 | 0.6677 (6) | 0.8078 (5) | 0.3953 (4) | 0.0569 (13) | |
| C6 | 0.5814 (8) | 0.8628 (6) | 0.4586 (4) | 0.0730 (17) | |
| H6 | 0.5608 | 0.9355 | 0.4441 | 0.088* | |
| C7 | 0.5253 (9) | 0.8109 (7) | 0.5433 (5) | 0.0824 (19) | |
| H7 | 0.4656 | 0.8484 | 0.5851 | 0.099* | |
| C8 | 0.5554 (9) | 0.7072 (7) | 0.5662 (5) | 0.086 (2) | |
| H8 | 0.5173 | 0.6736 | 0.6240 | 0.104* | |
| C9 | 0.6420 (8) | 0.6504 (7) | 0.5052 (5) | 0.0817 (19) | |
| H9 | 0.6628 | 0.5783 | 0.5210 | 0.098* | |
| C10 | 0.6986 (8) | 0.7022 (6) | 0.4190 (4) | 0.0717 (16) | |
| H10 | 0.7580 | 0.6644 | 0.3772 | 0.086* | |
| C11 | 0.8119 (8) | 0.7607 (5) | 0.0268 (4) | 0.0695 (16) | |
| H11A | 0.7330 | 0.7837 | −0.0161 | 0.083* | |
| H11B | 0.8892 | 0.7414 | −0.0170 | 0.083* | |
| C12 | 0.73 (9) | 0.65 (8) | 0.07 (6) | 0.08 (4) | 0.57 (5) |
| C13 | 0.804 (3) | 0.5516 (19) | 0.065 (3) | 0.084 (6) | 0.57 (5) |
| H13 | 0.8992 | 0.5572 | 0.0352 | 0.100* | 0.57 (5) |
| C14 | 0.723 (3) | 0.4437 (19) | 0.110 (2) | 0.087 (6) | 0.57 (5) |
| H14 | 0.7650 | 0.3766 | 0.1083 | 0.105* | 0.57 (5) |
| C15 | 0.582 (4) | 0.440 (4) | 0.156 (2) | 0.090 (9) | 0.57 (5) |
| H15 | 0.5369 | 0.3732 | 0.1897 | 0.108* | 0.57 (5) |
| C16 | 0.504 (4) | 0.533 (3) | 0.1544 (19) | 0.087 (8) | 0.57 (5) |
| H16 | 0.4062 | 0.5260 | 0.1805 | 0.105* | 0.57 (5) |
| C17 | 0.584 (7) | 0.638 (5) | 0.110 (3) | 0.080 (8) | 0.57 (5) |
| H17 | 0.5378 | 0.7020 | 0.1088 | 0.096* | 0.57 (5) |
| C12' | 0.71 (12) | 0.66 (11) | 0.08 (7) | 0.08 (5) | 0.43 (5) |
| C13' | 0.771 (4) | 0.562 (3) | 0.116 (3) | 0.085 (8) | 0.43 (5) |
| H13' | 0.8775 | 0.5638 | 0.1114 | 0.101* | 0.43 (5) |
| C14' | 0.668 (5) | 0.466 (4) | 0.159 (4) | 0.087 (12) | 0.43 (5) |
| H14' | 0.7032 | 0.4010 | 0.1792 | 0.105* | 0.43 (5) |
| C15' | 0.510 (7) | 0.472 (3) | 0.172 (3) | 0.085 (10) | 0.43 (5) |
| H15' | 0.4456 | 0.4140 | 0.2079 | 0.102* | 0.43 (5) |
| C16' | 0.447 (5) | 0.562 (4) | 0.133 (3) | 0.087 (9) | 0.43 (5) |
| H16' | 0.3407 | 0.5608 | 0.1378 | 0.105* | 0.43 (5) |
| C17' | 0.551 (9) | 0.654 (6) | 0.086 (4) | 0.080 (10) | 0.43 (5) |
| H17' | 0.5130 | 0.7144 | 0.0590 | 0.096* | 0.43 (5) |
| C18 | 1.1115 (7) | 1.0740 (5) | 0.2175 (4) | 0.0601 (14) | |
| H18A | 1.1875 | 1.0456 | 0.2581 | 0.072* | |
| H18B | 1.1696 | 1.1219 | 0.1684 | 0.072* | |
| C19 | 1.0435 (7) | 1.1550 (6) | 0.2859 (4) | 0.0598 (14) | |
| C20 | 1.0187 (8) | 1.2612 (6) | 0.2555 (5) | 0.0770 (17) | |
| H20 | 1.0463 | 1.2836 | 0.1919 | 0.092* | |
| C21 | 0.9518 (9) | 1.3352 (7) | 0.3206 (6) | 0.092 (2) | |
| H21 | 0.9346 | 1.4069 | 0.3007 | 0.110* | |
| C22 | 0.9123 (9) | 1.3008 (8) | 0.4137 (6) | 0.094 (2) | |
| H22 | 0.8676 | 1.3497 | 0.4569 | 0.113* | |
| C23 | 0.9365 (9) | 1.1978 (8) | 0.4443 (5) | 0.088 (2) | |
| H23 | 0.9088 | 1.1763 | 0.5081 | 0.105* | |
| C24 | 1.0019 (8) | 1.1246 (6) | 0.3818 (4) | 0.0721 (16) | |
| H24 | 1.0187 | 1.0537 | 0.4036 | 0.086* | |
| C25 | 1.2109 (15) | 0.7949 (13) | 0.2607 (7) | 0.075 (3) | 0.79 (2) |
| H25A | 1.1945 | 0.8530 | 0.3153 | 0.090* | 0.79 (2) |
| H25B | 1.3244 | 0.8163 | 0.2518 | 0.090* | 0.79 (2) |
| C26 | 1.1585 (19) | 0.6675 (12) | 0.2895 (11) | 0.110 (5) | 0.79 (2) |
| H26A | 1.0507 | 0.6509 | 0.3080 | 0.165* | 0.79 (2) |
| H26B | 1.2283 | 0.6609 | 0.3466 | 0.165* | 0.79 (2) |
| H26C | 1.1629 | 0.6091 | 0.2324 | 0.165* | 0.79 (2) |
| C25' | 1.120 (7) | 0.719 (5) | 0.238 (3) | 0.082 (12) | 0.21 (2) |
| H25C | 1.0292 | 0.6468 | 0.2190 | 0.099* | 0.21 (2) |
| H25D | 1.1083 | 0.7569 | 0.3053 | 0.099* | 0.21 (2) |
| C26' | 1.274 (7) | 0.681 (4) | 0.241 (4) | 0.110 (18) | 0.21 (2) |
| H26D | 1.2485 | 0.5930 | 0.2267 | 0.165* | 0.21 (2) |
| H26E | 1.3307 | 0.7075 | 0.3082 | 0.165* | 0.21 (2) |
| H26F | 1.3391 | 0.7184 | 0.1911 | 0.165* | 0.21 (2) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Sn1 | 0.0480 (2) | 0.0633 (3) | 0.0411 (2) | 0.02061 (17) | 0.01282 (14) | 0.01142 (15) |
| N1 | 0.040 (2) | 0.063 (3) | 0.039 (2) | 0.0126 (19) | 0.0118 (17) | 0.0094 (19) |
| N2 | 0.042 (2) | 0.069 (3) | 0.042 (2) | 0.012 (2) | 0.0105 (18) | 0.010 (2) |
| O1 | 0.0480 (19) | 0.069 (2) | 0.0437 (18) | 0.0262 (17) | 0.0145 (15) | 0.0146 (16) |
| O2 | 0.086 (3) | 0.099 (3) | 0.061 (2) | 0.058 (3) | 0.033 (2) | 0.037 (2) |
| O3 | 0.052 (2) | 0.068 (2) | 0.0473 (19) | 0.0206 (18) | 0.0159 (16) | 0.0149 (17) |
| O4 | 0.070 (3) | 0.096 (3) | 0.059 (2) | 0.042 (2) | 0.0245 (19) | 0.030 (2) |
| C1 | 0.050 (3) | 0.063 (3) | 0.045 (3) | 0.025 (3) | 0.008 (2) | 0.008 (2) |
| C2 | 0.047 (3) | 0.065 (3) | 0.044 (3) | 0.020 (2) | 0.011 (2) | 0.010 (2) |
| C3 | 0.062 (4) | 0.093 (4) | 0.062 (3) | 0.037 (3) | 0.023 (3) | 0.018 (3) |
| C4 | 0.057 (3) | 0.064 (3) | 0.041 (3) | 0.016 (3) | 0.011 (2) | 0.011 (2) |
| C5 | 0.051 (3) | 0.075 (4) | 0.044 (3) | 0.011 (3) | 0.012 (2) | 0.016 (3) |
| C6 | 0.076 (4) | 0.094 (5) | 0.052 (3) | 0.021 (4) | 0.023 (3) | 0.020 (3) |
| C7 | 0.083 (5) | 0.105 (5) | 0.058 (4) | 0.017 (4) | 0.027 (3) | 0.019 (4) |
| C8 | 0.085 (5) | 0.106 (6) | 0.061 (4) | 0.003 (4) | 0.021 (3) | 0.029 (4) |
| C9 | 0.083 (5) | 0.085 (5) | 0.073 (4) | 0.007 (4) | 0.017 (4) | 0.030 (4) |
| C10 | 0.073 (4) | 0.084 (4) | 0.058 (3) | 0.017 (3) | 0.015 (3) | 0.018 (3) |
| C11 | 0.082 (4) | 0.070 (4) | 0.054 (3) | 0.018 (3) | 0.009 (3) | 0.004 (3) |
| C12 | 0.09 (11) | 0.07 (8) | 0.06 (9) | 0.02 (6) | 0.00 (5) | 0.01 (6) |
| C13 | 0.099 (12) | 0.076 (10) | 0.074 (14) | 0.024 (8) | −0.004 (10) | 0.010 (10) |
| C14 | 0.103 (13) | 0.078 (11) | 0.075 (14) | 0.018 (9) | −0.004 (10) | 0.011 (9) |
| C15 | 0.10 (3) | 0.083 (19) | 0.077 (12) | 0.013 (17) | −0.002 (17) | 0.016 (12) |
| C16 | 0.103 (19) | 0.079 (19) | 0.073 (14) | 0.015 (16) | −0.002 (11) | 0.015 (12) |
| C17 | 0.09 (2) | 0.075 (16) | 0.066 (19) | 0.014 (13) | 0.001 (14) | 0.011 (12) |
| C12' | 0.09 (16) | 0.07 (10) | 0.06 (12) | 0.02 (8) | 0.00 (8) | 0.01 (8) |
| C13' | 0.098 (16) | 0.079 (14) | 0.072 (17) | 0.018 (11) | −0.004 (13) | 0.015 (14) |
| C14' | 0.10 (3) | 0.08 (2) | 0.074 (19) | 0.011 (18) | 0.00 (2) | 0.018 (15) |
| C15' | 0.10 (3) | 0.08 (2) | 0.074 (15) | 0.020 (19) | −0.003 (15) | 0.017 (15) |
| C16' | 0.10 (2) | 0.081 (18) | 0.073 (16) | 0.014 (14) | −0.001 (14) | 0.012 (12) |
| C17' | 0.09 (3) | 0.075 (19) | 0.07 (3) | 0.014 (15) | 0.001 (19) | 0.011 (15) |
| C18 | 0.053 (3) | 0.076 (4) | 0.053 (3) | 0.020 (3) | 0.008 (2) | 0.009 (3) |
| C19 | 0.052 (3) | 0.072 (4) | 0.051 (3) | 0.013 (3) | 0.004 (2) | 0.004 (3) |
| C20 | 0.075 (4) | 0.084 (5) | 0.070 (4) | 0.023 (4) | 0.004 (3) | 0.000 (3) |
| C21 | 0.089 (5) | 0.090 (5) | 0.093 (5) | 0.027 (4) | −0.001 (4) | −0.004 (4) |
| C22 | 0.084 (5) | 0.104 (6) | 0.087 (5) | 0.023 (4) | 0.015 (4) | −0.021 (5) |
| C23 | 0.080 (5) | 0.100 (6) | 0.070 (4) | 0.010 (4) | 0.017 (4) | −0.009 (4) |
| C24 | 0.069 (4) | 0.082 (4) | 0.058 (3) | 0.012 (3) | 0.011 (3) | 0.001 (3) |
| C25 | 0.073 (7) | 0.090 (8) | 0.069 (6) | 0.026 (6) | 0.015 (5) | 0.025 (5) |
| C26 | 0.127 (11) | 0.117 (9) | 0.099 (9) | 0.049 (8) | 0.005 (8) | 0.033 (7) |
| C25' | 0.09 (3) | 0.08 (3) | 0.07 (2) | 0.03 (3) | 0.00 (2) | 0.02 (2) |
| C26' | 0.13 (4) | 0.12 (3) | 0.10 (3) | 0.05 (3) | 0.01 (3) | 0.03 (2) |
Geometric parameters (Å, °)
| Sn1—C11 | 2.135 (6) | C15—C16 | 1.40 (4) |
| Sn1—O3 | 2.148 (3) | C15—H15 | 0.9300 |
| Sn1—C18 | 2.154 (6) | C16—C17 | 1.41 (6) |
| Sn1—N1 | 2.237 (4) | C16—H16 | 0.9300 |
| Sn1—O1 | 2.341 (3) | C17—H17 | 0.9300 |
| Sn1—O4 | 2.382 (4) | C12'—C13' | 1.4 (12) |
| Sn1—O1i | 2.772 (3) | C12'—C17' | 1.4 (9) |
| N1—C2 | 1.276 (6) | C13'—C14' | 1.41 (4) |
| N1—N2 | 1.373 (5) | C13'—H13' | 0.9300 |
| N2—C4 | 1.310 (7) | C14'—C15' | 1.41 (6) |
| O1—C1 | 1.276 (6) | C14'—H14' | 0.9300 |
| O2—C1 | 1.233 (6) | C15'—C16' | 1.42 (4) |
| O3—C4 | 1.293 (6) | C15'—H15' | 0.9300 |
| O4—C25' | 1.42 (4) | C16'—C17' | 1.42 (9) |
| O4—C25 | 1.458 (10) | C16'—H16' | 0.9300 |
| O4—H4 | 0.8200 | C17'—H17' | 0.9300 |
| C1—C2 | 1.508 (7) | C18—C19 | 1.485 (8) |
| C2—C3 | 1.481 (7) | C18—H18A | 0.9700 |
| C3—H3A | 0.9600 | C18—H18B | 0.9700 |
| C3—H3B | 0.9600 | C19—C20 | 1.380 (9) |
| C3—H3C | 0.9600 | C19—C24 | 1.392 (8) |
| C4—C5 | 1.483 (7) | C20—C21 | 1.403 (9) |
| C5—C10 | 1.364 (8) | C20—H20 | 0.9300 |
| C5—C6 | 1.372 (8) | C21—C22 | 1.366 (10) |
| C6—C7 | 1.376 (8) | C21—H21 | 0.9300 |
| C6—H6 | 0.9300 | C22—C23 | 1.345 (11) |
| C7—C8 | 1.338 (10) | C22—H22 | 0.9300 |
| C7—H7 | 0.9300 | C23—C24 | 1.369 (9) |
| C8—C9 | 1.368 (10) | C23—H23 | 0.9300 |
| C8—H8 | 0.9300 | C24—H24 | 0.9300 |
| C9—C10 | 1.394 (8) | C25—C26 | 1.49 (2) |
| C9—H9 | 0.9300 | C25—H25A | 0.9700 |
| C10—H10 | 0.9300 | C25—H25B | 0.9700 |
| C11—C12 | 1.5 (9) | C26—H26A | 0.9600 |
| C11—C12' | 1.5 (12) | C26—H26B | 0.9600 |
| C11—H11A | 0.9700 | C26—H26C | 0.9600 |
| C11—H11B | 0.9700 | C25'—C26' | 1.52 (7) |
| C12—C17 | 1.4 (6) | C25'—H25C | 0.9700 |
| C12—C13 | 1.4 (7) | C25'—H25D | 0.9700 |
| C13—C14 | 1.45 (3) | C26'—H26D | 0.9600 |
| C13—H13 | 0.9300 | C26'—H26E | 0.9600 |
| C14—C15 | 1.41 (3) | C26'—H26F | 0.9600 |
| C14—H14 | 0.9300 | ||
| C11—Sn1—O3 | 97.42 (19) | C17—C12—C11 | 121 (10) |
| C11—Sn1—C18 | 163.3 (2) | C13—C12—C11 | 117 (10) |
| O3—Sn1—C18 | 94.77 (18) | C12—C13—C14 | 116 (10) |
| C11—Sn1—N1 | 97.9 (2) | C12—C13—H13 | 122.0 |
| O3—Sn1—N1 | 70.83 (13) | C14—C13—H13 | 122.0 |
| C18—Sn1—N1 | 96.85 (18) | C15—C14—C13 | 120 (2) |
| C11—Sn1—O1 | 88.59 (19) | C15—C14—H14 | 120.0 |
| O3—Sn1—O1 | 140.42 (12) | C13—C14—H14 | 120.0 |
| C18—Sn1—O1 | 89.30 (18) | C16—C15—C14 | 123 (3) |
| N1—Sn1—O1 | 69.60 (12) | C16—C15—H15 | 118.3 |
| C11—Sn1—O4 | 84.9 (2) | C14—C15—H15 | 118.3 |
| O3—Sn1—O4 | 78.79 (13) | C15—C16—C17 | 116 (3) |
| C18—Sn1—O4 | 86.28 (19) | C15—C16—H16 | 122.0 |
| N1—Sn1—O4 | 149.60 (14) | C17—C16—H16 | 122.0 |
| O1—Sn1—O4 | 140.79 (12) | C12—C17—C16 | 122 (10) |
| C11—Sn1—O1i | 80.32 (18) | C12—C17—H17 | 118.8 |
| O3—Sn1—O1i | 154.13 (12) | C16—C17—H17 | 118.8 |
| C18—Sn1—O1i | 83.72 (16) | C13'—C12'—C17' | 120 (10) |
| N1—Sn1—O1i | 135.04 (12) | C13'—C12'—C11 | 122.0 |
| O1—Sn1—O1i | 65.45 (12) | C17'—C12'—C11 | 119.0 |
| O4—Sn1—O1i | 75.34 (11) | C12'—C13'—C14' | 121 (10) |
| C2—N1—N2 | 120.3 (4) | C12'—C13'—H13' | 119.7 |
| C2—N1—Sn1 | 121.9 (3) | C14'—C13'—H13' | 119.7 |
| N2—N1—Sn1 | 117.7 (3) | C13'—C14'—C15' | 118 (3) |
| C4—N2—N1 | 109.7 (4) | C13'—C14'—H14' | 120.9 |
| C1—O1—Sn1 | 117.0 (3) | C15'—C14'—H14' | 120.9 |
| C4—O3—Sn1 | 115.9 (3) | C14'—C15'—C16' | 122 (4) |
| C25'—O4—C25 | 41 (2) | C14'—C15'—H15' | 118.9 |
| C25'—O4—Sn1 | 128.5 (18) | C16'—C15'—H15' | 118.9 |
| C25—O4—Sn1 | 130.5 (5) | C17'—C16'—C15' | 118 (4) |
| C25'—O4—H4 | 119.4 | C17'—C16'—H16' | 121.1 |
| C25—O4—H4 | 104.5 | C15'—C16'—H16' | 121.1 |
| Sn1—O4—H4 | 111.7 | C16'—C17'—C12' | 121 (10) |
| O2—C1—O1 | 125.3 (5) | C16'—C17'—H17' | 119.7 |
| O2—C1—C2 | 118.0 (4) | C12'—C17'—H17' | 119.7 |
| O1—C1—C2 | 116.7 (4) | C19—C18—Sn1 | 113.5 (4) |
| N1—C2—C3 | 124.2 (5) | C19—C18—H18A | 108.9 |
| N1—C2—C1 | 114.7 (4) | Sn1—C18—H18A | 108.9 |
| C3—C2—C1 | 121.1 (5) | C19—C18—H18B | 108.9 |
| C2—C3—H3A | 109.5 | Sn1—C18—H18B | 108.9 |
| C2—C3—H3B | 109.5 | H18A—C18—H18B | 107.7 |
| H3A—C3—H3B | 109.5 | C20—C19—C24 | 118.6 (6) |
| C2—C3—H3C | 109.5 | C20—C19—C18 | 120.8 (5) |
| H3A—C3—H3C | 109.5 | C24—C19—C18 | 120.6 (6) |
| H3B—C3—H3C | 109.5 | C19—C20—C21 | 119.8 (7) |
| O3—C4—N2 | 125.9 (4) | C19—C20—H20 | 120.1 |
| O3—C4—C5 | 117.2 (5) | C21—C20—H20 | 120.1 |
| N2—C4—C5 | 116.9 (5) | C22—C21—C20 | 119.2 (8) |
| C10—C5—C6 | 118.8 (5) | C22—C21—H21 | 120.4 |
| C10—C5—C4 | 120.7 (5) | C20—C21—H21 | 120.4 |
| C6—C5—C4 | 120.5 (5) | C23—C22—C21 | 121.5 (7) |
| C5—C6—C7 | 120.3 (7) | C23—C22—H22 | 119.3 |
| C5—C6—H6 | 119.8 | C21—C22—H22 | 119.3 |
| C7—C6—H6 | 119.8 | C22—C23—C24 | 120.1 (7) |
| C8—C7—C6 | 120.8 (7) | C22—C23—H23 | 119.9 |
| C8—C7—H7 | 119.6 | C24—C23—H23 | 119.9 |
| C6—C7—H7 | 119.6 | C23—C24—C19 | 120.8 (7) |
| C7—C8—C9 | 120.4 (6) | C23—C24—H24 | 119.6 |
| C7—C8—H8 | 119.8 | C19—C24—H24 | 119.6 |
| C9—C8—H8 | 119.8 | O4—C25—C26 | 110.9 (11) |
| C8—C9—C10 | 119.1 (7) | O4—C25—H25A | 109.5 |
| C8—C9—H9 | 120.5 | C26—C25—H25A | 109.5 |
| C10—C9—H9 | 120.5 | O4—C25—H25B | 109.5 |
| C5—C10—C9 | 120.6 (6) | C26—C25—H25B | 109.5 |
| C5—C10—H10 | 119.7 | H25A—C25—H25B | 108.1 |
| C9—C10—H10 | 119.7 | O4—C25'—C26' | 111 (4) |
| C12—C11—C12' | 8(10) | O4—C25'—H25C | 109.5 |
| C12—C11—Sn1 | 116 (10) | C26'—C25'—H25C | 109.5 |
| C12'—C11—Sn1 | 112 (10) | O4—C25'—H25D | 109.5 |
| C12—C11—H11A | 108.3 | C26'—C25'—H25D | 109.5 |
| C12'—C11—H11A | 104.2 | H25C—C25'—H25D | 108.1 |
| Sn1—C11—H11A | 108.2 | C25'—C26'—H26D | 109.5 |
| C12—C11—H11B | 108.2 | C25'—C26'—H26E | 109.5 |
| C12'—C11—H11B | 119.0 | H26D—C26'—H26E | 109.5 |
| Sn1—C11—H11B | 108.2 | C25'—C26'—H26F | 109.5 |
| H11A—C11—H11B | 107.4 | H26D—C26'—H26F | 109.5 |
| C17—C12—C13 | 122 (10) | H26E—C26'—H26F | 109.5 |
Symmetry codes: (i) −x+2, −y+2, −z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O4—H4···O2i | 0.82 | 1.82 | 2.624 (6) | 165 |
Symmetry codes: (i) −x+2, −y+2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LR2004).
References
- Gielen, M., Vanbellinghen, C., Gelan, J. & Willem, R. (2002). Bull. Soc. Chim. Belg. 97, 873–878.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Siemens (1996). SMART and SAINT Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Sun, L.-N. & Hu, C.-W. (2007). Acta Cryst. E63, m1832–m1833.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811011937/lr2004sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811011937/lr2004Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report