Abstract
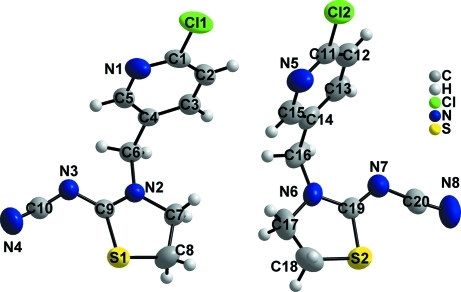
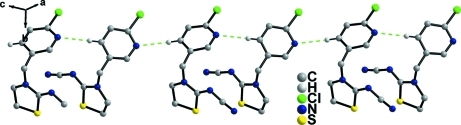
The asymmetric unit of the title compound, C10H9ClN4S, common name thiacloprid, comprises two molecules. In both molecules, the thiazolidine rings are almost planar (with r.m.s. deviations of 0.016 and 0.065 Å) and form dihedral angles of 73.36 (6) and 70.25 (8)° with the 2-chloropyridine rings. In the crystal, intermolecular C—H⋯N hydrogen bonds links the molecules into chains propagating in [ 01].
01].
Related literature
For background to the title compound, a member of the neonicotinoide class of insecticides, see Maienfisch et al. (2003 ▶). For the synthesis, see Ishimitsu et al., (1991 ▶)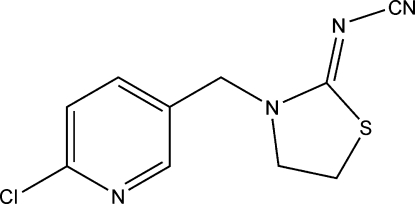
Experimental
Crystal data
C10H9ClN4S
M r = 252.73
Monoclinic,
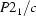
a = 7.1294 (14) Å
b = 35.469 (7) Å
c = 9.0211 (18) Å
β = 97.80 (3)°
V = 2260.1 (8) Å3
Z = 8
Mo Kα radiation
μ = 0.50 mm−1
T = 293 K
0.31 × 0.29 × 0.20 mm
Data collection
Rigaku R-AXIS RAPID diffractometer
Absorption correction: multi-scan (ABSCOR; Higashi, 1995 ▶) T min = 0.863, T max = 0.909
21869 measured reflections
5151 independent reflections
3505 reflections with I > 2σ(I)
R int = 0.046
Refinement
R[F 2 > 2σ(F 2)] = 0.048
wR(F 2) = 0.137
S = 1.08
5151 reflections
289 parameters
H-atom parameters constrained
Δρmax = 0.21 e Å−3
Δρmin = −0.29 e Å−3
Data collection: RAPID-AUTO (Rigaku, 1998 ▶); cell refinement: RAPID-AUTO; data reduction: CrystalClear (Rigaku/MSC, 2002 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811013316/kp2319sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811013316/kp2319Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯N5i | 0.93 | 2.55 | 3.459 (4) | 167 |
| C13—H13⋯N1ii | 0.93 | 2.49 | 3.408 (4) | 169 |
Symmetry codes: (i) 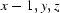 ; (ii)
; (ii) 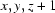 .
.
Acknowledgments
The authors thank the Special Funds for the Research of Scientific and Technological Innovative Talents of Harbin Municipal Science and Technology Bureau (2009RFXXG027), the Science and Technology Planning Project of Heilongjiang Province (GZ08A401) and Heilongjiang University for supporting this study.
supplementary crystallographic information
Comment
Thiacloprid is the common name of the title compound, which is neonicotinoide class of insecticide. High efficacy and flexible application methods make it well suited for modern integrated pest management programs in many cropping systems (Ishimitsu et al., 1991; Maienfisch et al., 2003). We report here the synthesis and crystal structure of thiacloprid.
The asymmetric unit comprises two molecules; the thiazolidine rings are almost planar, and form the dihedral angles with 2-chloropyridine rings of 73.36 (6) and 70.25 (8)°, respectively (Fig. 1).
In the crystal, the intermolecular C—H···N hydrogen bonds link the molecules to form a chain (Fig. 2, Table 1).
Experimental
The title compound was synthesised according the reference (Ishimitsu et al., 1991). A mixture of 2-cyanoiminothiazolidine (12.7 g, 0.1 mol), 2-chloro-5-pyridylmethyl-chloride (17.4 g, 0.1 mol), and K2CO3 (41.4 g, 0.3 mol) in 150 mL of DMF was heated to 323 K and kept stirring for 7 h. After filtered under reduced pressure, the DMF solution was distilled off. A total of 20.2 g (80.2%) title compound was obtained after the recrystallisation from ethyl acetate (15 mL). The suitable colourless block crystal was picked out for the single crystal X-ray diffaction measurement.
Refinement
H atoms bound to C atoms were placed in calculated positions and treated as riding on their parent atoms, with C—H = 0.93 Å (aromatic); C—H = 0.97 Å (methylene), and with Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
The molecular structure of the title compound showing displacement ellipsoids at the 50% probability level for non-H atoms.
Fig. 2.
A partial packing view, showing the hydrogen bonding chain. Dashed lines indicate the hydrogen bonds, no involving H atoms have been omitted for clarity.
Crystal data
| C10H9ClN4S | F(000) = 1040 |
| Mr = 252.73 | Dx = 1.486 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 12991 reflections |
| a = 7.1294 (14) Å | θ = 3.1–27.5° |
| b = 35.469 (7) Å | µ = 0.50 mm−1 |
| c = 9.0211 (18) Å | T = 293 K |
| β = 97.80 (3)° | Block, colourless |
| V = 2260.1 (8) Å3 | 0.31 × 0.29 × 0.20 mm |
| Z = 8 |
Data collection
| Rigaku R-AXIS RAPID diffractometer | 5151 independent reflections |
| Radiation source: fine-focus sealed tube | 3505 reflections with I > 2σ(I) |
| graphite | Rint = 0.046 |
| ω scans | θmax = 27.5°, θmin = 3.1° |
| Absorption correction: multi-scan (ABSCOR; Higashi, 1995) | h = −8→9 |
| Tmin = 0.863, Tmax = 0.909 | k = −45→45 |
| 21869 measured reflections | l = −11→11 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.048 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.137 | H-atom parameters constrained |
| S = 1.08 | w = 1/[σ2(Fo2) + (0.0595P)2 + 0.4995P] where P = (Fo2 + 2Fc2)/3 |
| 5151 reflections | (Δ/σ)max < 0.001 |
| 289 parameters | Δρmax = 0.21 e Å−3 |
| 0 restraints | Δρmin = −0.29 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.8652 (3) | 0.04805 (6) | 0.2994 (3) | 0.0528 (6) | |
| C2 | 0.7778 (4) | 0.05431 (7) | 0.4241 (3) | 0.0564 (6) | |
| H2 | 0.8315 | 0.0461 | 0.5183 | 0.068* | |
| C3 | 0.6087 (4) | 0.07309 (7) | 0.4038 (3) | 0.0541 (6) | |
| H3 | 0.5443 | 0.0777 | 0.4850 | 0.065* | |
| C4 | 0.5334 (3) | 0.08520 (5) | 0.2624 (3) | 0.0417 (5) | |
| C5 | 0.6313 (4) | 0.07628 (6) | 0.1459 (3) | 0.0525 (6) | |
| H5 | 0.5795 | 0.0835 | 0.0500 | 0.063* | |
| C6 | 0.3545 (3) | 0.10860 (6) | 0.2362 (3) | 0.0486 (5) | |
| H6A | 0.2856 | 0.1025 | 0.1390 | 0.058* | |
| H6B | 0.2744 | 0.1024 | 0.3115 | 0.058* | |
| C7 | 0.4452 (4) | 0.16785 (7) | 0.3852 (3) | 0.0586 (6) | |
| H7A | 0.5677 | 0.1592 | 0.4331 | 0.070* | |
| H7B | 0.3516 | 0.1622 | 0.4507 | 0.070* | |
| C8 | 0.4506 (6) | 0.20899 (8) | 0.3572 (4) | 0.0831 (10) | |
| H8A | 0.5573 | 0.2202 | 0.4200 | 0.100* | |
| H8B | 0.3354 | 0.2207 | 0.3811 | 0.100* | |
| C9 | 0.4189 (3) | 0.16902 (6) | 0.1222 (3) | 0.0422 (5) | |
| C10 | 0.4177 (3) | 0.17589 (7) | −0.1287 (3) | 0.0545 (6) | |
| C11 | 1.3337 (3) | 0.05498 (7) | 0.7709 (3) | 0.0558 (6) | |
| C12 | 1.2138 (4) | 0.04665 (8) | 0.8736 (3) | 0.0618 (6) | |
| H12 | 1.2387 | 0.0268 | 0.9406 | 0.074* | |
| C13 | 1.0549 (4) | 0.06898 (8) | 0.8737 (3) | 0.0609 (7) | |
| H13 | 0.9712 | 0.0646 | 0.9423 | 0.073* | |
| C14 | 1.0214 (3) | 0.09783 (7) | 0.7712 (3) | 0.0523 (6) | |
| C15 | 1.1509 (4) | 0.10300 (8) | 0.6735 (3) | 0.0665 (7) | |
| H15 | 1.1280 | 0.1222 | 0.6033 | 0.080* | |
| C16 | 0.8483 (3) | 0.12283 (9) | 0.7672 (4) | 0.0678 (8) | |
| H16A | 0.7818 | 0.1234 | 0.6660 | 0.081* | |
| H16B | 0.7636 | 0.1121 | 0.8315 | 0.081* | |
| C17 | 0.8939 (5) | 0.19245 (10) | 0.7102 (4) | 0.0840 (10) | |
| H17A | 0.7664 | 0.1963 | 0.6595 | 0.101* | |
| H17B | 0.9753 | 0.1866 | 0.6353 | 0.101* | |
| C18 | 0.9605 (6) | 0.22673 (9) | 0.7904 (4) | 0.0853 (10) | |
| H18A | 0.8799 | 0.2478 | 0.7556 | 0.102* | |
| H18B | 1.0887 | 0.2325 | 0.7728 | 0.102* | |
| C19 | 0.9155 (3) | 0.17075 (7) | 0.9595 (3) | 0.0464 (5) | |
| C20 | 0.9267 (3) | 0.15741 (8) | 1.2059 (3) | 0.0593 (6) | |
| Cl1 | 1.08715 (10) | 0.02601 (2) | 0.31970 (11) | 0.0811 (3) | |
| Cl2 | 1.53924 (11) | 0.02813 (2) | 0.76846 (10) | 0.0809 (2) | |
| N1 | 0.7967 (3) | 0.05776 (6) | 0.1620 (3) | 0.0592 (5) | |
| N2 | 0.3963 (2) | 0.14899 (5) | 0.2425 (2) | 0.0432 (4) | |
| N3 | 0.4000 (3) | 0.15412 (5) | −0.0121 (2) | 0.0504 (5) | |
| N4 | 0.4288 (4) | 0.19221 (7) | −0.2375 (3) | 0.0777 (7) | |
| N5 | 1.3080 (3) | 0.08234 (7) | 0.6720 (3) | 0.0658 (6) | |
| N6 | 0.8957 (3) | 0.16118 (6) | 0.8155 (2) | 0.0540 (5) | |
| N7 | 0.9051 (3) | 0.14604 (6) | 1.0652 (2) | 0.0551 (5) | |
| N8 | 0.9439 (4) | 0.16423 (9) | 1.3319 (3) | 0.0864 (8) | |
| S1 | 0.47264 (10) | 0.216369 (16) | 0.16494 (8) | 0.05802 (19) | |
| S2 | 0.95422 (12) | 0.21907 (2) | 0.98475 (9) | 0.0698 (2) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0496 (12) | 0.0361 (10) | 0.0750 (18) | 0.0031 (10) | 0.0159 (12) | 0.0066 (11) |
| C2 | 0.0653 (14) | 0.0536 (13) | 0.0499 (15) | 0.0087 (12) | 0.0069 (12) | 0.0066 (11) |
| C3 | 0.0656 (14) | 0.0505 (12) | 0.0483 (14) | 0.0063 (11) | 0.0159 (11) | 0.0021 (11) |
| C4 | 0.0476 (11) | 0.0336 (9) | 0.0458 (13) | −0.0015 (9) | 0.0138 (9) | 0.0020 (8) |
| C5 | 0.0664 (14) | 0.0486 (12) | 0.0450 (14) | 0.0081 (11) | 0.0160 (11) | 0.0053 (10) |
| C6 | 0.0465 (11) | 0.0424 (11) | 0.0580 (15) | −0.0045 (9) | 0.0113 (10) | 0.0002 (10) |
| C7 | 0.0753 (16) | 0.0539 (13) | 0.0462 (14) | −0.0019 (12) | 0.0061 (12) | −0.0049 (11) |
| C8 | 0.133 (3) | 0.0574 (16) | 0.060 (2) | 0.0008 (18) | 0.0185 (19) | −0.0117 (14) |
| C9 | 0.0367 (10) | 0.0416 (10) | 0.0476 (13) | 0.0033 (8) | 0.0029 (9) | 0.0007 (9) |
| C10 | 0.0555 (13) | 0.0566 (13) | 0.0501 (15) | 0.0046 (11) | 0.0032 (11) | 0.0001 (12) |
| C11 | 0.0568 (13) | 0.0556 (13) | 0.0579 (16) | −0.0027 (11) | 0.0177 (12) | −0.0152 (12) |
| C12 | 0.0708 (16) | 0.0605 (15) | 0.0575 (17) | −0.0074 (13) | 0.0214 (13) | −0.0025 (12) |
| C13 | 0.0601 (14) | 0.0736 (16) | 0.0541 (16) | −0.0178 (13) | 0.0257 (12) | −0.0177 (13) |
| C14 | 0.0495 (12) | 0.0619 (14) | 0.0465 (14) | −0.0090 (11) | 0.0106 (10) | −0.0205 (11) |
| C15 | 0.0728 (17) | 0.0727 (17) | 0.0591 (18) | 0.0088 (14) | 0.0279 (14) | 0.0006 (13) |
| C16 | 0.0482 (13) | 0.0861 (19) | 0.0685 (19) | −0.0033 (13) | 0.0062 (12) | −0.0297 (15) |
| C17 | 0.096 (2) | 0.112 (3) | 0.0459 (17) | 0.009 (2) | 0.0141 (15) | 0.0145 (17) |
| C18 | 0.106 (2) | 0.0733 (19) | 0.080 (2) | 0.0222 (18) | 0.0239 (19) | 0.0220 (17) |
| C19 | 0.0389 (10) | 0.0567 (13) | 0.0438 (13) | 0.0051 (10) | 0.0068 (9) | −0.0056 (10) |
| C20 | 0.0484 (12) | 0.0786 (17) | 0.0525 (16) | 0.0114 (12) | 0.0125 (11) | 0.0098 (13) |
| Cl1 | 0.0568 (4) | 0.0582 (4) | 0.1311 (8) | 0.0141 (3) | 0.0232 (4) | 0.0113 (4) |
| Cl2 | 0.0785 (5) | 0.0756 (5) | 0.0938 (6) | 0.0180 (4) | 0.0307 (4) | −0.0044 (4) |
| N1 | 0.0684 (13) | 0.0531 (11) | 0.0623 (15) | 0.0100 (10) | 0.0310 (11) | 0.0084 (10) |
| N2 | 0.0454 (9) | 0.0407 (9) | 0.0437 (11) | 0.0020 (8) | 0.0068 (8) | −0.0008 (8) |
| N3 | 0.0559 (11) | 0.0496 (10) | 0.0450 (12) | 0.0029 (9) | 0.0045 (9) | −0.0002 (9) |
| N4 | 0.0968 (18) | 0.0831 (17) | 0.0537 (15) | 0.0063 (14) | 0.0119 (13) | 0.0126 (13) |
| N5 | 0.0701 (14) | 0.0680 (13) | 0.0662 (15) | 0.0064 (11) | 0.0349 (12) | −0.0018 (11) |
| N6 | 0.0504 (10) | 0.0702 (13) | 0.0413 (11) | 0.0095 (10) | 0.0058 (8) | −0.0082 (9) |
| N7 | 0.0581 (11) | 0.0601 (12) | 0.0485 (13) | 0.0040 (10) | 0.0129 (9) | 0.0025 (9) |
| N8 | 0.0813 (17) | 0.133 (2) | 0.0470 (15) | 0.0174 (16) | 0.0147 (12) | 0.0044 (15) |
| S1 | 0.0681 (4) | 0.0420 (3) | 0.0615 (4) | −0.0052 (3) | −0.0003 (3) | 0.0019 (3) |
| S2 | 0.0892 (5) | 0.0566 (4) | 0.0649 (5) | 0.0004 (3) | 0.0145 (4) | −0.0053 (3) |
Geometric parameters (Å, °)
| C1—N1 | 1.314 (3) | C11—N5 | 1.314 (4) |
| C1—C2 | 1.376 (4) | C11—C12 | 1.375 (4) |
| C1—Cl1 | 1.752 (2) | C11—Cl2 | 1.750 (3) |
| C2—C3 | 1.368 (3) | C12—C13 | 1.383 (4) |
| C2—H2 | 0.9300 | C12—H12 | 0.9300 |
| C3—C4 | 1.383 (3) | C13—C14 | 1.378 (4) |
| C3—H3 | 0.9300 | C13—H13 | 0.9300 |
| C4—C5 | 1.375 (3) | C14—C15 | 1.373 (4) |
| C4—C6 | 1.513 (3) | C14—C16 | 1.516 (4) |
| C5—N1 | 1.340 (3) | C15—N5 | 1.340 (4) |
| C5—H5 | 0.9300 | C15—H15 | 0.9300 |
| C6—N2 | 1.463 (3) | C16—N6 | 1.454 (3) |
| C6—H6A | 0.9700 | C16—H16A | 0.9700 |
| C6—H6B | 0.9700 | C16—H16B | 0.9700 |
| C7—N2 | 1.451 (3) | C17—N6 | 1.459 (4) |
| C7—C8 | 1.482 (4) | C17—C18 | 1.461 (5) |
| C7—H7A | 0.9700 | C17—H17A | 0.9700 |
| C7—H7B | 0.9700 | C17—H17B | 0.9700 |
| C8—S1 | 1.782 (3) | C18—S2 | 1.781 (4) |
| C8—H8A | 0.9700 | C18—H18A | 0.9700 |
| C8—H8B | 0.9700 | C18—H18B | 0.9700 |
| C9—N3 | 1.312 (3) | C19—N7 | 1.305 (3) |
| C9—N2 | 1.325 (3) | C19—N6 | 1.331 (3) |
| C9—S1 | 1.754 (2) | C19—S2 | 1.746 (2) |
| C10—N4 | 1.152 (3) | C20—N8 | 1.152 (4) |
| C10—N3 | 1.324 (3) | C20—N7 | 1.321 (3) |
| N1—C1—C2 | 125.2 (2) | C14—C13—C12 | 119.5 (2) |
| N1—C1—Cl1 | 115.5 (2) | C14—C13—H13 | 120.2 |
| C2—C1—Cl1 | 119.3 (2) | C12—C13—H13 | 120.2 |
| C3—C2—C1 | 117.2 (2) | C15—C14—C13 | 117.3 (2) |
| C3—C2—H2 | 121.4 | C15—C14—C16 | 121.5 (3) |
| C1—C2—H2 | 121.4 | C13—C14—C16 | 121.1 (3) |
| C2—C3—C4 | 120.0 (2) | N5—C15—C14 | 124.7 (3) |
| C2—C3—H3 | 120.0 | N5—C15—H15 | 117.6 |
| C4—C3—H3 | 120.0 | C14—C15—H15 | 117.6 |
| C5—C4—C3 | 117.4 (2) | N6—C16—C14 | 112.62 (19) |
| C5—C4—C6 | 120.7 (2) | N6—C16—H16A | 109.1 |
| C3—C4—C6 | 121.9 (2) | C14—C16—H16A | 109.1 |
| N1—C5—C4 | 124.1 (2) | N6—C16—H16B | 109.1 |
| N1—C5—H5 | 118.0 | C14—C16—H16B | 109.1 |
| C4—C5—H5 | 118.0 | H16A—C16—H16B | 107.8 |
| N2—C6—C4 | 111.60 (17) | N6—C17—C18 | 109.7 (3) |
| N2—C6—H6A | 109.3 | N6—C17—H17A | 109.7 |
| C4—C6—H6A | 109.3 | C18—C17—H17A | 109.7 |
| N2—C6—H6B | 109.3 | N6—C17—H17B | 109.7 |
| C4—C6—H6B | 109.3 | C18—C17—H17B | 109.7 |
| H6A—C6—H6B | 108.0 | H17A—C17—H17B | 108.2 |
| N2—C7—C8 | 108.2 (2) | C17—C18—S2 | 108.1 (2) |
| N2—C7—H7A | 110.1 | C17—C18—H18A | 110.1 |
| C8—C7—H7A | 110.1 | S2—C18—H18A | 110.1 |
| N2—C7—H7B | 110.1 | C17—C18—H18B | 110.1 |
| C8—C7—H7B | 110.1 | S2—C18—H18B | 110.1 |
| H7A—C7—H7B | 108.4 | H18A—C18—H18B | 108.4 |
| C7—C8—S1 | 108.5 (2) | N7—C19—N6 | 122.2 (2) |
| C7—C8—H8A | 110.0 | N7—C19—S2 | 125.99 (19) |
| S1—C8—H8A | 110.0 | N6—C19—S2 | 111.81 (18) |
| C7—C8—H8B | 110.0 | N8—C20—N7 | 174.3 (3) |
| S1—C8—H8B | 110.0 | C1—N1—C5 | 116.1 (2) |
| H8A—C8—H8B | 108.4 | C9—N2—C7 | 116.01 (19) |
| N3—C9—N2 | 122.1 (2) | C9—N2—C6 | 122.68 (19) |
| N3—C9—S1 | 125.52 (18) | C7—N2—C6 | 120.61 (19) |
| N2—C9—S1 | 112.34 (17) | C9—N3—C10 | 119.3 (2) |
| N4—C10—N3 | 174.2 (3) | C11—N5—C15 | 115.9 (2) |
| N5—C11—C12 | 125.0 (2) | C19—N6—C16 | 121.3 (2) |
| N5—C11—Cl2 | 115.7 (2) | C19—N6—C17 | 115.6 (2) |
| C12—C11—Cl2 | 119.3 (2) | C16—N6—C17 | 122.3 (2) |
| C11—C12—C13 | 117.5 (3) | C19—N7—C20 | 119.1 (2) |
| C11—C12—H12 | 121.3 | C9—S1—C8 | 91.61 (12) |
| C13—C12—H12 | 121.3 | C19—S2—C18 | 92.62 (14) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···N5i | 0.93 | 2.55 | 3.459 (4) | 167 |
| C13—H13···N1ii | 0.93 | 2.49 | 3.408 (4) | 169 |
Symmetry codes: (i) x−1, y, z; (ii) x, y, z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: KP2319).
References
- Higashi, T. (1995). ABSCOR Rigaku Corporation, Tokyo, Japan.
- Ishimitsu, K., Suzuki, J., Ohishi, H., Yamada, T., Hatano, R., Takakusa, N. & Mitsui, J. (1991). WO Patent 91/04965.
- Maienfisch, P., Haettenschwiler, J., Rindlisbacher, A., Decock, A., Wellmann, H. & Kayser, H. (2003). Chimia, 57, 710–714.
- Rigaku (1998). RAPID-AUTO Rigaku Corporation, Tokyo, Japan.
- Rigaku/MSC (2002). CrystalClear Rigaku/MSC Inc., The Woodlands, Texas, USA.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811013316/kp2319sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811013316/kp2319Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report