Abstract
Protective immunity against pulmonary tuberculosis (TB) is characterized by the formation in the lungs of granulomas consisting of macrophages and activated T cells producing tumor necrosis factor alpha and gamma interferon, both required for the activation of the phagocytes. In 90% of immunocompetent humans, this response controls the infection. To understand why immunity fails in the other 10%, we studied the lungs of six patients who underwent surgery for incurable TB. Histologic examination of different lung lesions revealed heterogeneous morphology and distribution of acid-fast bacilli; only at the surface of cavities, i.e., in granulomas with a patent connection to the airways, were there numerous bacilli. The mutation profile of the isolates suggested that a single founder strain of Mycobacterium tuberculosis may undergo genetic changes during treatment, leading to acquisition of additional drug resistance independently in discrete physical locales. Additional drug resistance was preferentially observed at the cavity surface. Cytokine gene expression revealed that failure to control the bacilli was not associated with a generalized suppression of cellular immunity, since cytokine mRNA was up regulated in all lesions tested. Rather, a selective absence of CD4+ and CD8+ T cells was noted at the luminal surface of the cavity, preventing direct T-cell-macrophage interactions at this site, probably allowing luminal phagocytes to remain permissive for bacillary growth. In contrast, in the perinecrotic zone of the granulomas, the two cell types colocalized and bacillary numbers were substantially lower, suggesting that in this microenvironment an efficient bacteriostatic or bactericidal phagocyte population was generated.
Mycobacterium tuberculosis is an extremely successful pathogen, spreading from individual to individual via the aerosolization of infectious nucleus droplets. The infectious particles are released from the lungs of patients with cavitary pulmonary disease through coughing. Once inhaled and phagocytosed by resident alveolar macrophages, the tubercle bacilli elicit the production of soluble effector molecules, including the cytokines tumor necrosis factor alpha and interleukin 12 (IL-12) and a large number of chemokines (14, 17, 26). These molecules regulate the development of the host cellular immune response that presumably controls the infection in the majority (90%) of immunocompetent individuals (3). Protective immunity is characterized by the formation of granulomas at the site of infection. The granulomas consist primarily of activated M. tuberculosis-infected macrophages and T cells. In the mouse model of M. tuberculosis infection, the maturation and maintenance of granulomas and the control of bacillary replication within macrophages is dependent upon the continued production of tumor necrosis factor alpha and gamma interferon (IFN-γ) by macrophages and activated T cells, respectively (2, 8, 12, 13), and the expression of inducible nitric oxide synthase (iNOS) in infected macrophages (5, 16). In mice, CD4+ T lymphocytes are the principal mediators of resistance to tuberculosis (TB); CD8+ T lymphocytes have been shown to contribute to this resistance (22). An important role for CD4+ T cells in protecting the human host from TB is underscored by the marked susceptibility to TB in patients with advanced human immunodeficiency virus-induced CD4+-T-cell depletion (9, 11, 28). In addition to cytokine production, human CD4+ and CD8+ T cells may directly induce the death of M. tuberculosis-infected macrophages, resulting in reduced viability of the bacilli (10, 24, 30).
In 10% of apparently immunocompetent persons, the infection is not contained by the host immune response. Progressive bacillary replication results in disease manifestations, tissue necrosis, and cavity formation (19). The host immune response directed at the infecting bacilli is believed to be the main cause of tissue necrosis, which may result from cytokine-mediated toxicity, as well as the release of activated proteolytic enzymes by macrophages (6, 7, 27). Most TB patients, however, respond to antibiotic treatment by clearance of the bacilli from the sputum, partial reversal of the granulomatous inflammatory process, and successful clinical cure. In patients who fail to respond to chemotherapy, including patients with multidrug-resistant (MDR) TB, chronic progressive disease may be observed. Pneumonectomy is sometimes employed in these patients in an attempt to reduce the bacillary load in the lungs, to achieve sputum conversion, and/or to reduce spread of the infection to the remaining healthy lung. In others, surgery may be performed to reduce the life-threatening complications of TB, such as severe hemoptysis (4, 32).
To better understand the etiology of progressive chronic TB, we studied the lungs of six patients who underwent surgery for incurable TB and/or complications related to their TB. The excised lung tissue from these patients provided a unique opportunity to study the pathogenic process that occurs during long-term antibiotic therapy and disease progression. By studying the histology, immunohistology, and host cellular immune response and by characterizing the bacterial populations present, we hoped to gain insight into the dynamics of the infectious process.
MATERIALS AND METHODS
Patients.
Six patients who underwent pneumonectomy for TB at Groote Schuur Hospital, Cape Town, South Africa, between January 2000 and December 2001 were studied. All subjects had been referred for the surgical management of treatment-refractory TB (n = 3) or the complications of post-TB lung disease (n = 3). All were residents of the Western Cape province of South Africa and had received TB treatment supervised by their local clinics or hospitals. Preoperative M. tuberculosis cultures from the diagnostic sputa and chest radiographs were performed on all subjects. The studies were approved by the institutional review boards of Rockefeller University and of the University of Medicine and Dentistry of New Jersey and by the ethics committee of the University of Cape Town.
Processing of lung tissues.
The resected lungs were immediately transferred to the biological safety level 3 facility for pathological dissection. TB lesions were identified macroscopically; ∼0.5 g of tissue from each lesion was snap frozen in liquid nitrogen for mRNA analysis; another 0.5 g from each lesion was homogenized and subjected to prolonged culture (up to 1 year) in mycobacterial growth medium (MGIT; Becton Dickinson, Sparks, Md.) and on Lowenstein-Jensen slants. The remainder of the lung was immersed in formalin and prepared for microscopic analysis of the selected lesions.
Tissue sections (2 μm thick) were stained with hematoxylin and eosin or with carbolfuchsin (Ziehl-Neelsen) to visualize acid-fast bacilli (AFB). The number of mycobacteria in each area of the section was quantified, using a 40× objective, as none, scanty (individual bacilli found in each granuloma), moderate (1 to 10 bacilli in each granuloma), or numerous (>10 bacilli in clumps found in each field examined). For immunohistology, additional sections were collected on charged glass slides (Superfrost/Plus; Fisher Scientific, Pittsburgh, Pa.), deparaffinized, rehydrated in alcohol. and submitted to antigen retrieval by being boiled in 0.1 M citrate buffer, pH 7.0 (CD3, CD8, CD68, or TIA-1), or in 0.1 M EDTA buffer, pH 7.0 (CD4), for 20 min using a microwave oven. The phenotypes of the cellular infiltrates in the tissue sections were determined by using monoclonal antibodies against a pan-T-cell marker, CD3 (Ventana, Tucson, Ariz.), at a dilution of 1:100; against the T-cell subsets CD4 (Nova Castra, New Castle upon Tyne, United Kingdom) at a dilution of 1:20 and CD8 (Dako, Carpinteria, Calif.) at a dilution of 1:20; and against the cytotoxic cell marker TIA-1 (Coulter Inc., Hialeah, Fla.) at a dilution of 1:300. KP1 (CD68) antibody (Dako) was used as a marker of histiocytes and macrophages (dilution, 1:500). Reactions were carried out in an automated immunostainer (Ventana) using an immunoperoxidase-diaminobenzidene kit (Ventana) (29).
Real-time quantitative PCR (TaqMan).
To evaluate the expression level of the IL-2, IL-12, IFN-γ, and iNOS genes in the lung lesions, quantitative reverse transcription (RT)-PCR was performed with real-time TaqMan technology (Sequence Detection System model 7700; Perkin-Elmer, Wellesley, Mass.). Gene-specific primers and 6-carboxy-fluorescein probes were designed using Primer Express software and synthesized by Perkin-Elmer. RT-PCR was carried out with the TaqMan RT-PCR core Reagents kit (PE Applied Biosystems, Foster City, Calif.). Briefly, 5 ng of RNA extracted from the lung tissues was reverse transcribed and amplified in TaqMan EZ buffer containing 300 μmol each of dATP, dCTP, and dGTP/liter, 600 μmol of dUTP/liter, 3 mmol of manganese acetate/liter, 0.1 U of DNA polymerase/μl, 0.01 U of AmpErase uracil N-glycosylase/μl, 200 nmol of each primer/liter, and 100 nmol of each detection probe/liter. The thermal-cycling conditions were as follows: 2 min at 50°C (initial step), 30 min at 60°C (RT), 5 min at 95°C (deactivation of uracil N-glycosylase), 40 cycles of 15 s at 95°C (denaturation), and 1 min at 60°C (annealing and extension). Sequence-specific amplification was detected as 6-carboxy-fluorescein fluorescence exceeding the threshold limit (10 times the standard deviation of the baseline) during the amplification cycle. Gene-specific mRNA was quantified using standard curves established from PCR amplifications of serial dilutions of known mRNA levels. Samples were assayed at 10 to 3.2 ng per reaction per well. Amplification of the gene for human acidic ribosomal protein was performed on all samples tested to control for variability in the amount of RNA. The quantity of cDNA for each experimental gene was normalized to the amount of human acidic ribosomal protein in each sample. Levels of gene-specific messages were graphed as normalized message units as determined from the standard curve. A no-template control was included in each amplification reaction to control for contaminating templates. For valid sample analysis, the fluorescence intensity in the no-template control was required to be zero.
RFLP analysis and gene sequencing.
Restriction fragment length polymorphism (RFLP) analysis of M. tuberculosis isolated from sputa, as well as various lung lesions, was performed as described previously (31). Briefly, M. tuberculosis was harvested from Lowenstein-Jensen slants, and DNA was extracted and digested with PvuII restriction endonuclease. DNA fragments were separated by agarose gel electrophoresis, transferred by Southern blotting, and hybridized with labeled IS6110 probe. Bands were visualized by enhanced chemiluminescence and analyzed with Whole Band Analyzer software, version 3.4 (BioImage; RM Luton, Inc., Jackson, Miss.). Direct DNA sequencing of rpoB, katG, inhA (mabA), pncA, embB, rpsL, rrs, and gyrA was performed on all isolates as described previously (25).
RESULTS
Patients.
Six patients who underwent pulmonary resection for complications arising from TB were studied. All six patients had unilateral lung disease, all with (almost) complete destruction of the affected lung (Fig. 1). Three patients (two of whom had MDR TB) underwent surgery for chronic sputum-positive TB despite 18 to 24 months of supervised multidrug therapy (Table 1). The other three patients, who were culture negative at the time of sputum sampling, all had histories of pulmonary TB. These patients had presented with hemoptysis and other symptoms suggestive of relapse and had received empirical treatment for TB for 7 to 15 months. Lung resection in these patients was performed to relieve ongoing hemoptysis thought to have arisen from a focus in the destroyed lung. Sputum cultures from these three patients at the time of presentation, however, failed to yield M. tuberculosis (Table 1). All patients received TB therapy up to the time of surgery, and none of the patients were coinfected with human immunodeficiency virus.
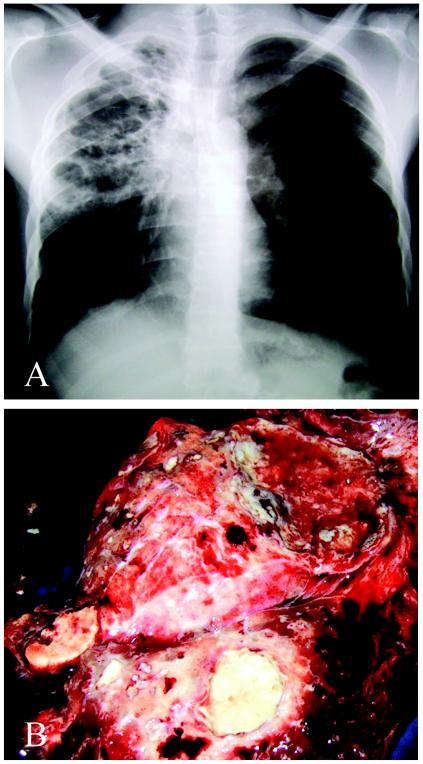
FIG. 1.
Radiogram of the lungs of patient 2 before surgery (A) and the resected lung at the time of sample collection (B). The right lung is almost fully destroyed. It contains areas with cavitations, fibrosis, nodules, and granulomas with central necrosis.
TABLE 1.
Characteristics of patients
| Patient no. | Age (yr) | Genderb | Treatment
at
surgery
|
Sputumd
|
Indication for surgery | Macroscopic descriptione | Caseationf | Cavitationf | Fibrosisf | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drugsc | Mo | Smear | Culture | ||||||||
| 1 | 19 | F | R H Z E S | 18 | Pos | Pos | Persistent TB | Destroyed L lung | Y | Y | Y |
| 2a | 17 | M | R H Z E S Et Th Km Of | 18 | Pos | Pos | Persistent TB | Destroyed R lung | Y | Y | Y |
| 3a | 35 | M | R H Z E Et Th Km Of Cl | 24 | Pos | Pos | Persistent TB | Destroyed L lung | Y | Y | Y |
| 4 | 54 | M | R H Z E | 7 | Neg | Neg | Hemoptysis | Destroyed L lung | N | Y | Y |
| 5 | 59 | F | R H Z E | 9 | Neg | Neg | Hemoptysis | Destroyed L lung | N | Y | Y |
| 6 | 45 | M | R H Z E | 15 | Neg | Neg | Hemoptysis | Destroyed L lung | N | Y | Y |
MDR TB.
M, male; F, female.
R, rifampin; H, isoniazid; Z, pyrazinamide; E, ethambutol; S, streptomycin; E, ethionamide; Th, thiacetazone; Km, kanamycin; Of, ofloxacin; Cl, clofazamine.
At the time of surgery. Pos, positive; Neg, negative.
R, right; L, left.
Y, yes; N, no.
Presence of mycobacteria in lesions obtained from the resected lungs.
The lungs of all six patients revealed evidence of TB. The various areas of the lungs examined contained diverse lesions with differing extents of pathology, including cavitating granulomas, fibrosis, smaller nodules, closed necrotic noncavitating granulomas, and bronchiectasis. Macroscopic caseation (Fig. 1) was observed only in patients with active TB (patients 1 to 3 [Table 1]). Dissection of the lungs allowed us to identify and sample a number of macroscopically distinct lesions (two to six per patient) and, in some cases, lung tissue which appeared uninvolved (Table 2). Each lesion was examined microscopically, characterized histologically, assigned a semiquantitative enumeration for the abundance of AFB, and categorized according to its continuity with the airways. AFB were almost exclusively observed in the lesions of patients with sputum-positive disease. M. tuberculosis was cultured from all lesions of sputum-positive patients. Bacilli were not cultured from any of the lesions of the sputum-negative subjects, including those with scanty AFB.
TABLE 2.
Characteristics of lesions and lung tissue
| Patient no. | Specimen or sitea | Histopathologic
examination
|
Bacteriology
|
Genotypic
analysis of selected drug
targetsb
|
mRNA expression
of:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lesion | Airway | AFB | Culture | rpoB | katG | inhA | pncA | embB | rpsL | rrs | gyrA | IL-2 | IL-12 | IFN-γ | iNOS | ||
| 1 | Sputum | Numerous | M. tuberculosis | WT | WT | WT | WT | WT | WT | NDc | WT | ||||||
| LUL | Cavity wall (caseous necrosis) | Open | Numerous | M. tuberculosis | WT | WT | WT | WT | WT | K43R | ND | WT | 120 | 190 | 5,400 | ND | |
| LLL | Cavity wall (caseous necrosis) | Open | Numerous | ND | 100 | 220 | 6,300 | ND | |||||||||
| LLL | Granuloma (fibrocaseous) | Closed | Scanty | M. tuberculosis | WT | WT | WT | WT | WT | WT | ND | WT | 85 | 415 | 5,000 | ND | |
| LN | Granuloma (nonnecrotic) | Closed | None | ND | 80 | 340 | 8,700 | ND | |||||||||
| 2 | Sputum | Numerous | M. tuberculosis | S531L | WT | C15T | WT | M306V | K88R | ND | WT | ||||||
| RUL | Cavity wall (caseous necrosis) | Open | Moderate | M. tuberculosis | S531L | WT | C15T | WT | M306V | K88R | ND | D89N | 107 | 244 | 3,149 | 46 | |
| RLL | Cavity wall (caseous necrosis) | Open | Numerous | M. tuberculosis | S531L | WT | C15T | WT | M306V | K88R | ND | D94G | 89 | 345 | 1,630 | 621 | |
| RLL | Cavity wall (caseous necrosis) | Open | Scanty | M. tuberculosis | S531L | WT | C15T | WT | M306V | K88R | ND | D89N | 28 | 136 | 1,465 | 21 | |
| RML | Small nodule (caseous) | Closed | Scanty | M. tuberculosis | S531L | WT | C15T | WT | M306V | K88R | ND | WT | 32 | 240 | 1,196 | 90 | |
| RLL | Infarction (coagulative necrosis) | Closed | Scanty | M. tuberculosis | S531L | WT | C15T | WT | M306V | K88R | ND | WT | 13 | 89 | 7,243 | 135 | |
| 3 | Sputum | Numerous | M. tuberculosis | S531L | S315T | WT | 173 (T del) | M306V | K43R | WT | D94G | ||||||
| LUL | Cavity wall (caseous necrosis) | Open | Moderate | M. tuberculosis | S531L | S315T | WT | 173 (T del) | M306V | K43R | WT | D94G | 80 | 199 | 7,536 | 207 | |
| LLL | Cavity wall (caseous necrosis) | Open | Scanty | M. tuberculosis | S531L | S315T | WT | 173 (T del) | M306V | K43R | WT | D94G | 92 | 290 | 715 | 119 | |
| LLL | Fibrotic nodule (liquefaction) | Open | Moderate | M. tuberculosis | S531L | S315T | WT | 173 (T del) | M306V | K43R | G1484T | D94G | 80 | 388 | 1,142 | 129 | |
| LUL | Granuloma (caseous necrosis) | Closed | Moderate | M. tuberculosis | S531L | S315T | WT | 173 (T del) | M306V | K43R | WT | D94G | 175 | 421 | 1,861 | 38 | |
| LLL | Miliary nodules (caseous) | Closed | Scanty | M. tuberculosis | S531L | S315T | WT | 173 (T del) | M306V | K43R | WT | D94G | 64 | 78 | 202 | 181 | |
| LLL | Normal lung tissue | Scanty | M. tuberculosis | S531L | WT | WT | 173 (T del) | WT | K43R | WT | ND | 64 | 138 | 605 | 274 | ||
| 4 | Sputum | None | Negative | ||||||||||||||
| LUL | Granuloma (nonnecrotic) | Closed | None | Negative | 101 | 197 | 857 | 96 | |||||||||
| LUL | Granuloma (noncaseous) | Closed | Scanty | Negative | 313 | 289 | 1,201 | 103 | |||||||||
| LLL | Normal lung tissue | None | Negative | 78 | 107 | 362 | 373 | ||||||||||
| 5 | Sputum | None | Negative | ||||||||||||||
| LUL | Cavity wall (no necrosis) | Open | None | Negative | 168 | 409 | 2,407 | 102 | |||||||||
| LLL | Granuloma (fibrotic) | Closed | None | Negative | 76 | 325 | 2,066 | 14 | |||||||||
| 6 | Sputum | None | Negative | ||||||||||||||
| LUL | Cavity wall (no necrosis) | Open | None | Negative | 87 | 234 | 1,666 | 145 | |||||||||
| LUL | Granuloma (caseous) | Closed | None | Negative | 159 | 385 | 2,541 | 115 | |||||||||
| LLL | Miliary nodules (caseous) | Closed | Scanty | Negative | 90 | 152 | 1,321 | 270 | |||||||||
LUL and RUL, Left and right upper lobe; LN, lymph node.
WT, wild type; boldface indicates changes in drug resistance compared to that in other lung lesions.
ND, not done.
Genetic analysis of M. tuberculosis cultures obtained from lesions of resected lungs.
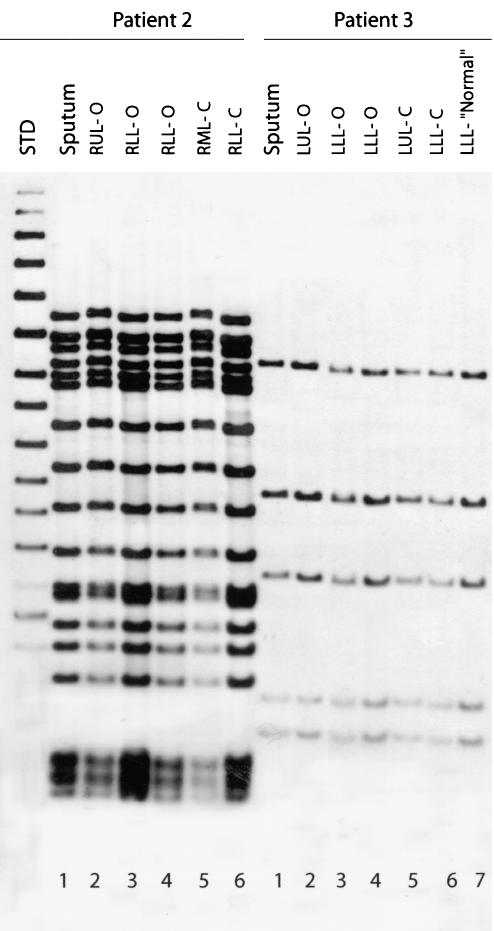
Genetic analysis was carried out on all isolates cultured from preoperative sputum samples and lung lesions. IS6110-based RFLP analysis revealed a homogeneous bacterial population and no evidence of mixed infection in each of the three patients with active disease (Fig. 2). In contrast, sequence analyses of several genes implicated in drug resistance revealed heterogeneity in the resistance-associated alleles among the isolates recovered from different lung lesions of the same patient (Table 2). For example, the isolate cultured from the upper-lobe cavity of patient 1 contained the AAG→AGG K43R resistance mutation in the genetic target of streptomycin (rpsL), whereas the wild-type gene was present in the isolate obtained from sputum, as well as a closed lower-lobe granuloma (Table 2). Moreover, in the lung from patient 2, two mutations in the fluoroquinolone resistance-associated gene gyrA, GAC→AAC and GAC→GGC (D89N and D94G, respectively), were found in all three isolates obtained from open lesions, whereas the bacilli from the sputum and the two closed lesions bore the wild-type gyrA allele. Most strikingly, patient 3 had three discrete bacillary populations identified, with different alleles of the resistance-associated genes. Apparently normal lung tissue from this patient contained few M. tuberculosis cells, and these bore the wild-type alleles in the katG (a target for INH), embB (a target for EMB), and rrs (a target for aminoglycosides) genes. Bacteria isolated from the sputum of patient 3 and from four pathological sites in the lung had identical katG (S315T) and embB (M306V) mutations but were wild type for rrs. A sixth site contained bacilli that, in addition to the katG and embB mutations, had acquired a resistance mutation (G1484T) in the rrs gene. The mutation profiles of these isolates suggest that the acquisition of drug resistance is a dynamic process whereby an initial infecting strain may spread from one pulmonary site to another, becoming the founder for acquisition of additional antibiotic resistance at secondary sites. Interestingly, in all three patients, the additionally acquired drug resistance mutations were seen in M. tuberculosis cultured from open lesions, i.e., lesions connected to an airway where the bacilli were numerous (Table 2).
FIG. 2.
IS6110 Southern blot hybridization patterns of M. tuberculosis isolates recovered from multiple anatomical sites in the lungs of patients 2 and 3. The six isolates from patient 2 were recovered from sputum (lane 1), right upper lobe open (RUL-O) (lane 2), right lower lobe open (RLL-O) (lane 3), right lower lobe open (lane 4), right middle lobe closed (RML-C) (lane 5), and right lower lobe closed (RLL-C) (lane 6). The seven isolates from patient 3 were recovered from sputum (lane 1), left upper lobe open (LUL-O) (lane 2), left lower lobe open (LLL-O) (lane 3), left lower lobe open (lane 4), left upper lobe closed (LUL-C) (lane 5), left lower lobe closed (LLL-C) (lane 6), and left lower lobe normal (LLL-“Normal”) (lane 7) (Table 2). Lane STD, molecular weight standard.
Cytokine and iNOS gene expression in lesions of resected lungs.
The different lung samples obtained from all six patients were evaluated for expression of mRNA for IL-2, IL-12, IFN-γ, and iNOS. Cytokine and iNOS gene expression was observed in all lesions from patients with active TB, as well as from those who had post-tuberculous, culture-negative lung disease (Table 2). No apparent correlation of the levels of expression of the different genes with the type of lesion or the presence of AFB was observed. Rather, our results showed variable levels of immune activation in the tissue samples obtained from the different types of lesions of all subjects studied. This heterogeneity of cytokine expression suggests that the relatively large tissue fragments collected for study (∼0.5 g each) contained a mixture of microenvironments rather than a single specific histologic microenvironment (see below). Our results also suggested that failure to control the growth of the bacilli was not associated with a global suppression of cellular immunity in the lungs of the three patients with chronic sputum-positive disease. However, the absolute levels of immune activation in the lungs of these patients could not be determined, since we did not know the baseline level of immune activation that would be induced in the lungs of normal or infected individuals who do not develop active disease.
Histopathologic analysis of lesions of resected lungs.
Examination of histopathology sections of the various lesions revealed heterogeneous cellular architecture, most prominent in the layered cavitating and noncavitating granulomas seen in the lungs of patients with sputum-positive disease. Cross-sections of these lesions (examined from the inside of the cavity luman outward) revealed striking cellular accumulation at the luminal surface of the cavity consisting of numerous mononuclear cells essentially surrounded by a layer of acellular caseous necrotic material (Fig. 3A, B, and C). Subtending the acellular necrotic layer there was granulomatous-fibrotic tissue with a mixed mononuclear-cell infiltrate consisting of Langhans-type giant cells, sheets of epithelioid macrophages, and many scattered lymphocytes (Fig. 3E and F).
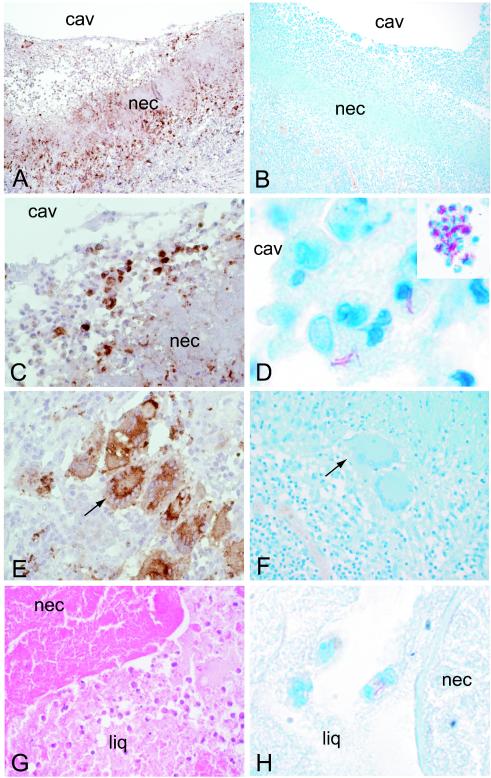
FIG. 3.
Localization of macrophages and AFB in resected lungs. Macrophages, stained with CD68 (A, C, and E) and AFB stained with carbolfuchsin (B, D, and F) in the left upper-lobe cavity wall of the open lesion of patient 1 are shown. CD68 staining is seen at the cavity (cav) surface (A and C), within the necrotic area (nec) (A and C), and in the granulomatous area below the necrotic area (E). AFB are seen predominantly within macrophages at the cavity surface (D) and not in the Langhans cells or macrophages of the granulomatous tissue (F). The inset in panel D shows AFB in cells at the luminal surface of the right-lower-lobe cavity of patient 2. AFB are also seen in macrophages within the liquefied (liq) material adjacent to the necrotic area (H) of the left-lower-lobe fibrotic nodule undergoing breakdown (liquefaction) in patient 3. Magnifications, ×10 (A and B), ×40 (C, E, F, and G), ×80 (D, inset), and ×200 (D and H).
Bacilli, apparently cell associated, were detected in large numbers at the cavity surface (Fig. 3D). The area of acellular necrotic material had few, if any, visible AFB. The granulomatous-fibrotic layer, with abundant macrophages and giant cells, was essentially devoid of visible AFB (Fig. 3F). In addition, no AFB were seen in alveolar macrophages residing within airspaces of the residual functional lung (not shown). In closed (noncavitary) necrotizing granulomas, small to moderate numbers of AFB were observed in macrophages infiltrating the necrotic areas, most prominently where breakdown was occurring (Fig. 3G and H). Thus, in the three patients with sputum-positive disease, AFB were most numerous at the luminal surfaces of the cavities, i.e., in granulomas with a patent connection to the airways.
In comparison, in the patients who were sputum negative, the surfaces of the cavities appeared inactive, with reepithelization over fibrotic tissue (not shown). Multiple mononuclear cells, including multinucleated giant cells, epithelioid macrophages, and lymphocytes, were seen in the granulomatous-fibrotic tissue, despite the absence of any visible AFB and the failure to grow bacilli from this tissue. The extensive cellular immune response may explain the high levels of expression of iNOS and cytokine genes in these lesions, suggesting the persistence of antigen in the absence of intact visible and/or culturable AFB (Table 2).
Immunohistologic localization of macrophages and T lymphocytes.
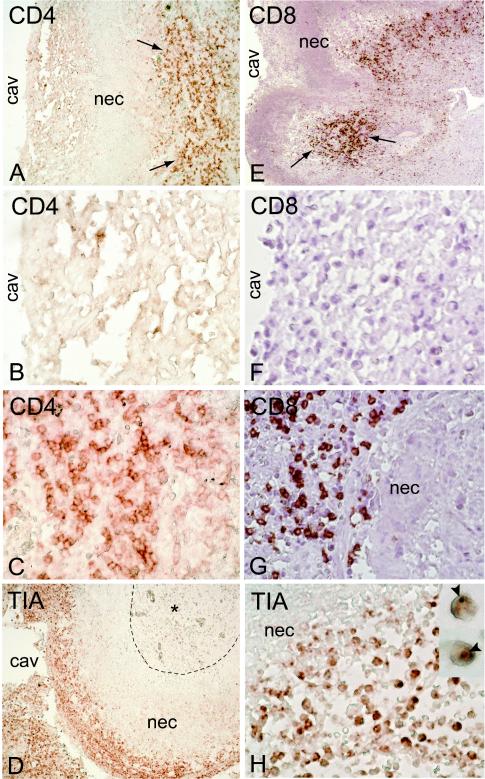
Immunohistologic examination of lung sections revealed that CD68+ macrophages were most abundant in two areas of the cavitating granulomas: (i) at the luminal surface of the cavity and (ii) at the other side of the acellular necrotic area in the granulomatous-fibrotic zone (Fig. 3A and E). The necrotic area was itself diffusely stained CD68+, suggesting that remnants of necrotic macrophages still expressed residual antigen. In addition, scattered CD68+ macrophages were seen among the fibroblasts of fibrotic areas, and large numbers of alveolar macrophages were seen within the airspaces (not shown). Contrary to dogma that assumes that at the cavity surface bacilli grow in the extracellular necrotic matter, we observed cells with macrophage morphology (that stained CD68+ in serial sections) that appeared to be infected with multiple bacilli (Fig. 3D and inset). Staining for the presence of CD3+ CD4+ and CD3+ CD8+ T lymphocytes revealed an abundance of these cells within the granulomatous-fibrotic layer and in lymphoid aggregates of the granuloma (Fig. 4A, C, E, and G). Scattered T lymphocytes were seen within the fibrotic areas and in the airspaces (not shown). In contrast, a striking absence of CD3+, CD4+, and CD8+ T cells was noted in the acellular necrotic zone, as well as at the luminal surface of the cavity (Fig. 4A and E). This area, however, contained large numbers of CD3−, CD4−, CD8− mononuclear cells with lymphoid morphology that stained for the presence of cytotoxic granules (TIA+ cells) (Fig. 4D and H). Taken together, these results suggest that the luminal surface of the cavity represents a microenvironment within the lung in which macrophages and T cells are not colocalized, thereby preventing direct T-cell-macrophage interactions at those sites. In contrast, only millimeters away, at the other side of the necrotic zone (Fig. 4D), another microenvironment exists in which the two cell types are colocalized and free to interact directly, resulting in an efficient immune response capable of inhibiting mycobacterial replication.
FIG. 4.
Localization of lymphoid cells in lesions of resected lungs. CD4+ (A, B, and C) and CD8+ (E, F, and G) T cells are seen in the granulomatous-fibrotic areas of the lung (arrows) but not in the necrotic (nec) zone or at the cavity (cav) surface. CD4− CD8− cytotoxic cells (TIA-1+) are seen at the cavity surface but not in the necrotic zone (D) and are less frequent in the granulomatous area (*). These cells contain cytotoxic granules that stain TIA-1+ (H, inset). Magnifications, ×4 (A, D, and E), ×40 (B, C, F, G, and H), and ×100 (H, inset).
DISCUSSION
In this paper, we present data supporting the idea that in the lungs of patients affected by TB, a single founder strain of M. tuberculosis may undergo mutagenesis during treatment, leading to the acquisition of drug resistance independently in discrete physical locales, resulting in parallel evolution of heterogeneous subpopulations of drug-resistant bacilli. We also show that the lung of a chronic TB patient contains a diversity of microanatomical niches created by the different immunological processes occurring independently at these sites. Such anatomical and immunological variability appears to be associated with discrete genetic (mutated) subpopulations of bacilli. We observe that the acquisition of new drug resistance mutations is preferentially localized to the microenvironment where bacillary growth seems most active, in macrophages residing at the luminal surfaces of the cavities. At these sites, the macrophages probably remain inactivated and thus permissive for bacillary growth. The observation that the bacilli are growing inside macrophages at the cavity surface is contrary to common dogma, which presumes that bacillary replication at this site is extracellular.
Lack of macrophage activation to a bacteriostatic state may be due to the selective exclusion of CD3+ CD4+ and CD3+ CD8+ T cells from the lumen of the cavity (Fig. 4). The underlying mechanism for the exclusion of CD3+ T cells from the cavity surface is unknown. Interestingly, this exclusion appears to be selective: there is a relative enrichment at this site for CD68+ macrophages, as well as a population of as yet undefined TIA-1+ cytotoxic lymphoid cells. The anti-TIA-1 is a monoclonal antibody that recognizes the 17-kDa granule membrane protein (GMP-17) expressed predominantly in the granules of CD8+ αβ T-cell receptor (TCR) natural killer (NK) cells, as well as γδ TCR+ cells and some CD4+ αβ TCR+ cells (1, 18, 20). Although the role of GMP-17 remains obscure, the protein is known to translocate to the cytotoxic-T-cell surface after fusion of the granules with the cell membrane and to have sequence homology with calcium channel proteins (20). TIA-1+ cells have been shown to kill target cells by two distinct mechanisms, Fas receptor-mediated apoptosis and granule exocytosis (15, 21, 23). However, in the present study, there was no direct evidence of any cytotoxic activity of these cells, nor do the cells appear to activate the adjacent macrophages to a bacteriostatic-bactericidal phenotype. As expected, in the necrotic layer between the luminal surface of the cavity and the perinecrotic granulomatous-fibrotic zone, CD68+ macrophages and TIA-1+ cytotoxic cells, as well as CD3+ CD4+ and CD3+ CD8+ T cells, are not seen. In contrast, where macrophages and T cells are colocalized and potentially in close contact with each other, as observed in the granulomatous-fibrotic areas, the macrophages are morphologically activated (multinucleated giant cells or epithelioid) and few if any bacilli are present. The phenotypic and functional (cytokine response) identification of the TIA-1+ leukocytes seen at the surfaces of the cavities in patients with active TB must await the immunohistologic probing of unfixed frozen tissue that can be performed only in a specially equipped biological safety level 3 facility.
The growth of M. tuberculosis is well known to occur in proportion to oxygen tension (33). Thus, another factor contributing to the florid bacterial growth seen at the luminal surface of the cavity could be improved access to oxygen in this microenvironment. The extent of bacterial growth appears to follow an intuitive pattern with respect to oxygen concentration; the necrotic and deeper fibrotic regions are anticipated to be largely anoxic and therefore free of visible AFB and almost sterile. However, our inability to visualize the AFB does not exclude the possibility that a few viable nonreplicating bacilli remain within the macrophages in these sites. If and when breakdown and liquefaction occur at these sites, the bacilli may start growing and become more numerous, as seen in Fig. 3G and H. The extent to which oxygen concentration and immune pressure combine to suppress bacterial growth at sites distal from the cavity lumen remains to be determined.
The presence of discrete populations of bacteria in patients in which they are presumed to have acquired drug resistance during therapy has not been carefully studied. Our results suggest that relying on drug susceptibility tests of organisms isolated from patient sputa may not provide an accurate representation of the bacterial susceptibility in all subpopulations within the lung. An examination of the levels of resistance (MICs of drugs) of the bacilli isolated from different sites in the lung would provide useful information for directing therapeutic options. Because quantification of the bacterial subpopulations and their absolute resistance levels was not performed in this study, it is not possible for us to make specific therapeutic recommendations. However, our observations suggest the possibility that a careful analysis of resistance levels and bacillary population size might lead to a recommendation in some cases to continue therapy with a first-line or even a second-line drug in the face of resistance to a given drug in the sputum isolate.
These studies provide a preliminary analysis of the immunological and bacterial attributes in the lungs of human patients during the dynamic process of tuberculous-lesion evolution. Continued study of lung tissues from patients with active TB will provide important benchmarks for validation of animal models of disease and may suggest alternative therapeutic strategies for the treatment of chronic and MDR TB.
Acknowledgments
We thank Liana Tsenova for help with the micrographs and Sabrina Dalton for help in preparing the manuscript.
These studies were supported in part by the Fogarty grant AITRP W00231 (to Frank A. Post and Linda-Gail Bekker) and NIH grants AI 22616 and AI 54338 (to Gilla Kaplan). Funding was also provided by DACST, South Africa.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Anderson, P., C. Nagler-Anderson, C. O'Brien, H. Levine, S. Watkins, H. S. Slayter, M. L. Blue, and S. F. Schlossman. 1990. A monoclonal antibody reactive with a 15-kDa cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J. Immunol. 144:574-582. [PubMed] [Google Scholar]
- 2.Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. [PubMed] [Google Scholar]
- 3.Bloom, B. R., and C. J. Murray. 1992. Tuberculosis: commentary on a reemergent killer. Science 257:1055-1064. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg, H. M., W. J. Burman, R. E. Chaisson, C. L. Daley, S. C. Etkind, L. N. Friedman, P. Fujiwara, M. Grzemska, P. C. Hopewell, M. D. Iseman, R. M. Jasmer, V. Koppaka, R. I. Menzies, R. J. O'Brien, R. R. Reves, L. B. Reichman, P. M. Simone, J. R. Starke, and A. A. Vernon. 2003. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167:603-662. [DOI] [PubMed] [Google Scholar]
- 5.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, J. C., A. Wysocki, K. M. Tchou-Wong, N. Moskowitz, Y. Zhang, and W. N. Rom. 1996. Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax 51:306-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condos, R., W. N. Rom, Y. M. Liu, and N. W. Schluger. 1998. Local immune responses correlate with presentation and outcome in tuberculosis. Am. J. Respir. Crit. Care Med. 157:729-735. [DOI] [PubMed] [Google Scholar]
- 8.Cooper, A. M., and J. L. Flynn. 1995. The protective immune response to Mycobacterium tuberculosis. Curr. Opin. Immunol. 7:512-516. [DOI] [PubMed] [Google Scholar]
- 9.Daley, C. L., P. M. Small, G. F. Schecter, G. K. Schoolnik, R. A. McAdam, W. R. Jacobs, Jr., and P. C. Hopewell. 1992. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N. Engl. J. Med. 326:231-235. [DOI] [PubMed] [Google Scholar]
- 10.Dieli, F., M. Troye-Blomberg, J. Ivanyi, J. J. Fournie, M. Bonneville, M. A. Peyrat, G. Sireci, and A. Salerno. 2000. Vγ9/Vδ2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur. J. Immunol. 30:1512-1519. [DOI] [PubMed] [Google Scholar]
- 11.Di Perri, G., M. Cruciani, M. C. Danzi, R. Luzzati, G. De Checchi, M. Malena, S. Pizzighella, R. Mazzi, M. Solbiati, E. Concia, et al. 1989. Nosocomial epidemic of active tuberculosis among HIV-infected patients. Lancet ii:1502-1504. [PubMed] [Google Scholar]
- 12.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 14.Henderson, R. A., S. C. Watkins, and J. L. Flynn. 1997. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J. Immunol. 159:635-643. [PubMed] [Google Scholar]
- 15.Kagi, D., F. Vignaux, B. Ledermann, K. Burki, V. Depraetere, S. Nagata, H. Hengartner, and P. Golstein. 1994. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 265:528-530. [DOI] [PubMed] [Google Scholar]
- 16.MacMicking, J. D., R. J. North, R. LaCourse, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manca, C., L. Tsenova, C. E. Barry III, A. Bergtold, S. Freeman, P. A. Haslett, J. M. Musser, V. H. Freedman, and G. Kaplan. 1999. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J. Immunol. 162:6740-6746. [PubMed] [Google Scholar]
- 18.Matutes, E., E. Coelho, M. J. Aguado, R. Morilla, A. Crawford, K. Owusu-Ankomah, and D. Catovsky. 1996. Expression of TIA-1 and TIA-2 in T cell malignancies and T cell lymphocytosis. J. Clin. Pathol. 49:154-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medlar, E. M. 1955. Necropsy studies of human pulmonary tuberculosis. Am. Rev. Tuberc. 71:29-55. [Google Scholar]
- 20.Medley, Q. G., N. Kedersha, S. O'Brien, Q. Tian, S. F. Schlossman, M. Streuli, and P. Anderson. 1996. Characterization of GMP-17, a granule membrane protein that moves to the plasma membrane of natural killer cells following target cell recognition. Proc. Natl. Acad. Sci. USA 93:685-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meehan, S. M., R. T. McCluskey, M. Pascual, F. I. Preffer, P. Anderson, S. F. Schlossman, and R. B. Colvin. 1997. Cytotoxicity and apoptosis in human renal allografts: identification, distribution, and quantitation of cells with a cytotoxic granule protein GMP-17 (TIA-1) and cells with fragmented nuclear DNA. Lab. Investig. 76:639-649. [PubMed] [Google Scholar]
- 22.Mogues, T., M. E. Goodrich, L. Ryan, R. LaCourse, and R. J. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagata, S., and P. Golstein. 1995. The Fas death factor. Science 267:1449-1456. [DOI] [PubMed] [Google Scholar]
- 24.Oddo, M., T. Renno, A. Attinger, T. Bakker, H. R. MacDonald, and P. R. Meylan. 1998. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J. Immunol. 160:5448-5454. [PubMed] [Google Scholar]
- 25.Ramaswamy, S., and J. M. Musser. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber. Lung Dis. 79:3-29. [DOI] [PubMed] [Google Scholar]
- 26.Roach, D. R., A. G. Bean, C. Demangel, M. P. France, H. Briscoe, and W. J. Britton. 2002. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 168:4620-4627. [DOI] [PubMed] [Google Scholar]
- 27.Schluger, N. W., and W. N. Rom. 1998. The host immune response to tuberculosis. Am. J. Respir. Crit. Care Med. 157:679-691. [DOI] [PubMed] [Google Scholar]
- 28.Selwyn, P. A., D. Hartel, V. A. Lewis, E. E. Schoenbaum, S. H. Vermund, R. S. Klein, A. T. Walker, and G. H. Friedland. 1989. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N. Engl. J. Med. 320:545-550. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui, M. R., A. L. Moreira, Y. Negesse, G. A. Taye, W. A. Hanekom, P. A. Haslett, S. Britton, and G. Kaplan. 2002. Local nerve damage in leprosy does not lead to an impaired cellular immune response or decreased wound healing in the skin. J. Infect. Dis. 186:260-265. [DOI] [PubMed] [Google Scholar]
- 30.Stenger, S., R. J. Mazzaccaro, K. Uyemura, S. Cho, P. F. Barnes, J. P. Rosat, A. Sette, M. B. Brenner, S. A. Porcelli, B. R. Bloom, and R. L. Modlin. 1997. Differential effects of cytolytic T cell subsets on intracellular infection. Science 276:1684-1687. [DOI] [PubMed] [Google Scholar]
- 31.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, et al. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Leuven, M., M. De Groot, K. P. Shean, U. O. von Oppell, and P. A. Willcox. 1997. Pulmonary resection as an adjunct in the treatment of multiple drug-resistant tuberculosis. Ann. Thorac. Surg. 63:1368-1373. [PubMed] [Google Scholar]
- 33.Wayne, L. G. 1977. Synchronized replication of Mycobacterium tuberculosis. Infect. Immun. 17:528-530. [DOI] [PMC free article] [PubMed] [Google Scholar]