Abstract
Protective immunity in mice to the infective third-stage larvae (L3) of Strongyloides stercoralis was shown to be dependent on immunoglobulin M (IgM), complement activation, and granulocytes. The objectives of the present study were to determine whether IgG was also a protective antibody isotype and to define the specificity and the mechanism by which IgG functions. Purified IgG recovered from mice 3 weeks after a booster immunization with live L3 was shown to transfer high levels of protective immunity to naïve mice. IgG transferred into mice treated to block complement activation or to eliminate granulocytes failed to kill the challenge larvae. Transfer of immune IgG into IL-5 knockout (KO) mice, which are deficient in eosinophils, resulted in larval attrition, while transfer into FcRγ KO mice did not result in larval killing. These findings suggest that IgG from mice immunized with live L3 requires complement activation and neutrophils for killing of L3 through an antibody-dependent cellular cytotoxicity (ADCC) mechanism. This is in contrast to the results of investigations using IgM from mice immunized with live L3 and IgG from mice immunized with larval antigens soluble in deoxycholate in which protective immunity was shown to be ADCC independent. Western blot analyses with immune IgM and IgG identified few antigens recognized by all protective antibody isotypes. Results from immunoelectron microscopy demonstrated that the protective antibodies bound to different regions in the L3. It was therefore concluded that while IgM and IgG antibodies are both protective against larval S. stercoralis, they recognize different antigens and utilize different killing mechanisms.
Large multicellular organisms like Strongyloides stercoralis present a significant challenge to the immune system, and the mechanisms by which this parasite is controlled and eliminated by the immune response remain poorly defined. In humans with severe strongyloidiasis, a significant decrease in the immunoglobulin M (IgM) and IgG levels was observed compared to the levels found in people with asymptomatic and mildly symptomatic infections. Eosinophil levels were also lower in people with severe strongyloidiasis than in individuals with asymptomatic or symptomatic infections. These findings suggest that antibody and eosinophils play a role in protective immunity to larval S. stercoralis in humans (5). In Erythrocebus patas monkeys and dogs infected with S. stercoralis, elevated anti-larval IgG titers were observed; however, it was not determined whether the elevated antibody titers were related to decreases in parasite survival (9, 12). Jirds can also support the complete S. stercoralis life cycle. It was determined that after a single primary infection, jirds eliminated the challenge infection within 24 h via a mechanism dependent on cell contact and a factor found in serum (27).
To gain an understanding of how the immune response eliminates the larval stages of S. stercoralis, a mouse model was developed. In this model it was demonstrated that immunization of mice with live infective third-stage larvae (L3) induced protective immunity which resulted in the elimination of approximately 90% of the challenge larvae within 24 h (1). Serum collected from mice 1 week after a booster immunization could passively transfer immunity into naïve mice (4). Parasite-specific IgG1, IgM, and IgA titers were elevated in the immunized mice (1); however, it was shown that IgM was the only antibody isotype capable of participating in parasite elimination at that time. IgM-mediated killing of the L3 was dependent on cell contact, the presence of granulocytes, and complement activation (4). Furthermore, it was shown that IgM from immunized mice could passively transfer immunity to naïve IL-5 knockout (KO) mice, which are deficient in eosinophils (20), thus suggesting that neutrophils were the required granulocyte for the IgM-dependent killing mechanism (15).
IgG and not IgM was protective against larval S. stercoralis in mice immunized with L3 antigens solubilized in deoxycholate (DOC) (16). This observation suggested that IgG could function in the killing of S. stercoralis L3 and that the development of a protective IgG response was dependent on how the mice were immunized. IgG has been shown to be responsible for immunity-dependent protection in other nematode infections, including Strongyloides ratti (25), Nematospiroides dubius (41), Trichinella spiralis (3), and Brugia pahangi (24). The goal of the present study was therefore to determine whether and when IgG from mice immunized with live L3 functioned as a component of the larval killing process. In addition, comparisons were made between the mechanisms used by IgM and IgG to kill the larvae. Finally, the molecular and morphological targets used by IgM and IgG were identified.
MATERIALS AND METHODS
Animals and parasites.
BALB/cByJ mice and C57BL/6J mice 6 to 8 weeks of age were purchased from Jackson Laboratories (Bar Harbor, Maine). IL-5 KO and FcRγ KO mice (both on a C57BL/6 background) were produced in our breeding colony in the Laboratory Animal Sciences facility at Thomas Jefferson University (Philadelphia, Penn.). IL-5 KO mice were a generous gift from Manfred Kopf of the Basel Institute for Immunology (20), and FcRγ KO mice were a generous gift from Jeffery Ravetch of Rockefeller University (37). All mice were housed in microisolator boxes (Lab Products Inc., Maywood, N.J.) and kept in a climate-controlled environment.
S. stercoralis L3 were harvested from the feces of an infected laboratory dog as previously described (33). L3 were washed five times with a 1:1 mixture of NCTC-135 and IMDM supplemented with 100 U of penicillin (Gibco, Grand Island, N.Y.)/ml, 0.1 mg of streptomycin (Gibco)/ml, and 0.1 mg of gentamicin (Gibco)/ml.
Live immunization and challenge.
Mice were immunized with 5,000 live L3 on day 0 and received a similar booster immunization on day 14. Immunized mice received challenge infections of 50 S. stercoralis L3 (contained in diffusion chambers) at various time points after the booster immunization. Diffusion chambers were made from 14-mm-diameter Lucite rings covered with 2.0-μm-pore-size polycarbonate Isopore membranes (Millipore, Bedford, Ma.). The rings are glued together with a 1:1 mixture of 1,2-dichloroethane (Sigma, St. Louis, Mo.) and acryloid resin (Rohm and Haas Co., Philadelphia, Pa.). The membranes are attached to the rings with cyanoacrylate (Super Glue, Hollis, N.Y.). The completed diffusion chambers were then sterilized by exposure to 100% ethylene oxide gas followed by 12 h of aeration. Diffusion chambers containing the challenge L3 were placed in a subcutaneous pocket on the rear flank of the mice. At 24 h later, the diffusion chambers were recovered and larval survival was determined on the basis of morphology and mobility of the worms. Host cells found in the diffusion chambers were centrifuged onto slides through the use of a Cytospin 3 apparatus (Shandon, Pittsburgh, Pa.) and then stained for differential counts with DiffQuik (Baxter Healthcare, Miami, Fla.).
Preparation and immunization with soluble antigen.
Mice were immunized with larval antigens solubilized in DOC as previously described (17). Briefly, S. stercoralis L3 were incubated in a petri dish for 20 min with a 1% solution of low-melt agarose (Type I-A Low EEO; Sigma). Phosphate-buffered saline (PBS) supplemented with antibiotics (100 U of penicillin/ml, 0.1 mg of streptomycin/ml, and 0.1 mg of gentamicin/ml) was added, and larvae that migrated into the PBS were harvested and stored at −80°C in 50% glycerol-PBS. L3 were homogenized on ice in PBS supplemented with a protease inhibitor cocktail (Sigma) and 50 mM EDTA and then sonicated. DOC-soluble L3 antigens were obtained first by extraction from the L3 with PBS and then by extraction of the remaining pellet with 20 mM Tris-HCl-0.5% DOC in the presence of protease inhibitors; the extracted antigens were then dialyzed overnight in PBS. DOC antigens (DOC Ag) were then passed through a 0.2-μm-pore-size syringe filter, and protein concentrations were determined with a Micro BCA protein assay (Pierce, Rockford, Ill.).
Mice were immunized with soluble antigens through the use of the following protocol: 2% aluminum hydroxide low-viscosity Rehydragel (alum) (Reheis, Inc., Berkeley Heights, N.J.) was diluted 1:10 with PBS containing 25 μg of DOC Ag. Mice were injected with 200 μl of the solution in the nape of the neck on days 0 and 14 which was followed on day 28 by a challenge infection consisting of L3 contained within a diffusion chamber (which was recovered 4 days later).
Serum transfers and antibody purification.
Serum used in transfers was obtained from mice which were immunized and challenged as described above. The serum was collected at either 3 or 5 weeks after a booster immunization from mice immunized with live L3 and 18 days after a booster immunization from mice immunized with the DOC Ag. Serum (recovered from blood after exsanguination of mice anesthetized with isoflurane) was filter sterilized with a 0.2-μm-pore-size syringe filter and stored at −80°C.
Serum was passed over a protein G-Sepharose column (Pharmacia, Peapack, N.J.) to purify the IgG fraction. The flowthrough volume containing IgA, IgE, and IgM was then passed over IgA and IgE affinity columns, leaving only IgM in the flowthrough solution. Antibody fractions were eluted from the column with 0.5 M acetic acid (pH 3.0), neutralized with saturated Tris-HCl (pH 9.8), dialyzed overnight in PBS, and concentrated with an Ultrafree-15 centrifugal filter device (Millipore). Total IgM and IgG concentrations in the serum were determined by enzyme-linked immunosorbent assay (as described below) before and after purification.
Passive transfer of the purified antibody and whole immune sera was done by injection either directly into the diffusion chamber or into the subcutaneous pocket around the implanted diffusion chamber at the time of challenge. No differences were seen between the results obtained with each method. The amount of purified antibody transferred into the mice was equal to the amount of antibody found in 100 μl of either unfractionated naive or immune serum. The antibody was administered in 200 μl of PBS. All serum transfer experiments were terminated at 24 h postchallenge.
Cell and complement depletion.
Depletion of granulocytes was accomplished using the monoclonal antibody (MAb) RB6-8C5 (18). Mice were given an intraperitoneal injection of 0.5 mg of antibody 3 days before the challenge as well as at the time of challenge. An isotype control antibody was not included in these studies, because multiple previous experiments have shown that these antibodies have no effect on parasite viability (31), (32). Granulocyte depletion was verified with blood smears taken at the time of recovery and from analysis of the fluid in the diffusion chambers.
Cobra venom factor (CVF) (Sigma) (used for depletion of complement) was injected (400 μg per kg of body weight) intraperitoneally into mice at 24 h prior to and at the time of challenge (14).
Enzyme-linked immunosorbent assay.
Maxisorp 96-well plates (Nunc Inc., Naperville, Il.) were coated with 2 μg of rat anti-mouse IgG (Sigma)/ml or 5 μg of rat anti-mouse IgM (Pharmingen, San Diego, Calif.)/ml overnight at 4°C. Plates were then blocked with borate buffer solution (0.17 M boric acid, 0.12 M NaCl, 0.05% Tween 20, 0.025% bovine serum albumin, 1 mM EDTA, pH 8.2) at room temperature (RT) for 30 min. The plates were washed with distilled water, and the samples and appropriate standards were serially diluted in borate buffer solution and then incubated for 2 h at 37°C. The plates were washed with distilled water and then incubated with biotinylated goat anti-mouse IgG or IgM (Pharmingen) for 1 h at RT. The plates were washed, and ExtrAvidin peroxidase (Sigma) was added for 30 min at RT. After the final washing, the peroxidase substrate ABTS [2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate)] (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was added. ABTS color reactions were measured at 410 nm on an MR5000 microplate reader (Dynatech Laboratories, Chantilly, Va.).
Western blotting.
DOC-soluble L3 antigens (100 μg) were separated in a sodium dodecyl sulfate-8 to 16% polyacrylamide electrophoresis slab gel on a Mini-PROTEAN 3 apparatus (Bio-Rad Corporation, Hercules, Calif.). Electrophoresis was run at 30 mA for 2 h, after which proteins were transferred to a nitrocellulose membrane (Bio-Rad) through the use of a Trans-Blot apparatus (Bio-Rad) at 100 V for 1 h at 4°C. The nitrocellulose membranes were then blocked with 5% Carnation brand nonfat dry instant milk (Nestlé Food, Glendale, Calif.). The membranes were washed in PBS-0.05% Tween 20, cut into strips, and then incubated in a 1:1000 dilution of whole serum for 1 h at RT. The membrane strips were washed in PBS-0.05% Tween 20 and then treated with anti-mouse IgG horseradish peroxidase-conjugated antibody (Pharmingen) or anti-mouse IgM F(ab′)2 horseradish peroxidase-conjugated antibody (ICN/Cappell, Aurora, Ohio) for 45 min at RT and washed. Western blots were processed using enhanced chemiluminescence (NEN Life Sciences Products Inc., Boston, Mass.) and then developed with Biomax ML film (Eastman Kodak, Rochester, N.Y.).
Electron microscopy.
L3 were fixed for 30 min in 0.25% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) containing 1% sucrose, embedded, and then processed for immunoelectron microscopy. Thin sections of embedded worms were incubated with purified IgM (diluted 1:5) or IgG (diluted 1:100) from control and immunized mice followed by incubation with a suspension of 15-nm-diameter gold particles coated with protein A, as previously described (19, 21, 22).
Statistics.
All experiments consisted of at least five mice per group; experiments were performed at least twice, and consistent results were obtained from the repeated experiments. Data presented are from all of the performed experiments. Statistical analysis was determined by multifactorial analysis of variance; probability values of less than 0.05 were considered significant.
RESULTS
Passive transfer of immunity to mice with serum and antibody.
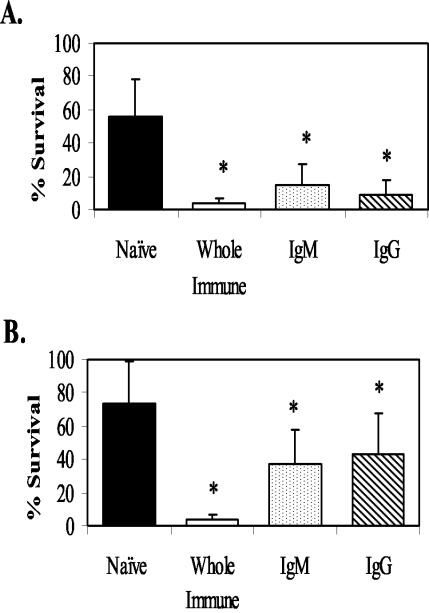
Serum and antibody transfers were performed to determine when after immunization with live L3, IgG became protective against larval S. stercoralis in mice. IgG and IgM antibody fractions were purified from whole serum at 3 and 5 weeks after the booster immunization. Transfer of IgG, IgM, and whole serum was by subcutaneous injection of antibody around the implanted diffusion chamber. Serum collected at 3 weeks after the booster immunization caused a more than 80% reduction in the number of live larvae recovered. Larval killing at that time was mediated by whole immune serum, purified IgM, and IgG (Fig. 1A). Transfer of whole serum collected at 5 weeks after the booster immunization caused a significant reduction in the survival of the challenge infection, as did transfer of purified IgG and IgM (Fig. 1B). As the level of protection attributed to IgG was higher at 3 weeks after the booster immunization than at 5 weeks, IgG collected 3 weeks after the booster immunization was used in the subsequent studies.
FIG. 1.
Mean percentage of live larval recovery from mice that received the transfer of whole immune sera, purified IgM, and purified IgG recovered 3 weeks (A) and 5 weeks (B) after booster immunization with live L3. The data represent the means and standard deviations of the results for 10 animals per group. The asterisks indicate statistical differences (P ≤ 0.05) from control values.
The mechanism by which IgG from mice immunized with live L3 elicits larval killing.
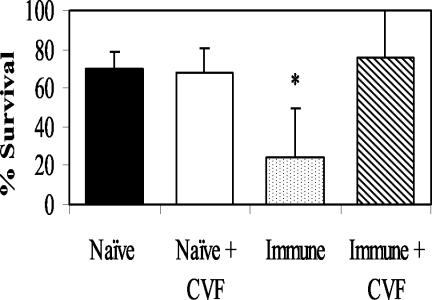
To test whether IgG requires complement activation to kill the larvae, CVF was used to block the complement cascade by the depletion of the C3 component. The ability of immune IgG to kill the parasites was significantly diminished when complement activation was blocked (Fig. 2). Cell recruitment into diffusion chambers did not alter either in the number of cells or in the types of cells after CVF treatment.
FIG. 2.
Effect of CVF treatment of mice that have received transfer of naïve and immune IgG on the survival of challenge larvae. The data represent the means and standard deviations of the results for 10 animals per group. The asterisks indicate statistical differences (P ≤ 0.05) from control values.
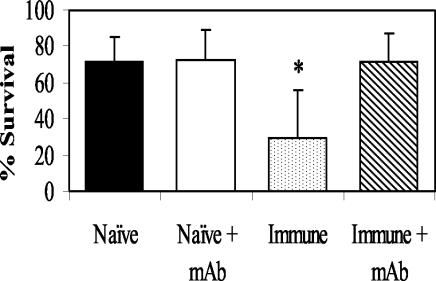
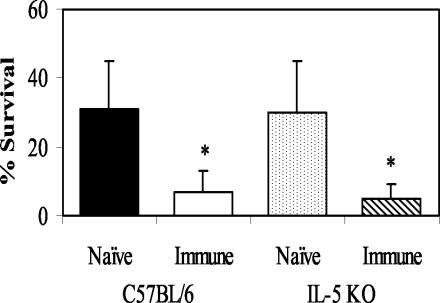
The role of cells in the IgG killing process was initially studied by using the MAb RB6-8C5 to deplete both eosinophils and neutrophils. Treatment of mice with RB6-8C5 inhibited larval killing after passive transfer of immune IgG (Fig. 3). Differential analysis of the cells in the diffusion chambers in the RB6-8C5-treated mice showed significantly decreased levels of neutrophils. Equally low levels of eosinophils were found in both the MAb-treated mice and nontreated controls (data not shown). Immune IgG was then transferred into IL-5 KO mice, which are deficient in eosinophils. Purified immune IgG transferred protective immunity to the IL-5 KO mice (Fig. 4), thus suggesting that eosinophils are not required for larval killing by IgG but rather that neutrophils are the required cells.
FIG. 3.
Effect of granulocyte depletion with MAb RB6-8C5 on immunity transferred with purified immune IgG. The data represent the means and standard deviations of the results for 10 animals per group. The asterisks indicate statistical differences (P ≤ 0.05) from control values.
FIG. 4.
Transfer of naïve and immune IgG into wild-type C57BL/6J mice and IL-5 KO mice to determine whether eosinophils are required for protective immunity. The data represent the means and standard deviations of the results for 10 animals per group. The asterisks indicate statistical differences (P ≤ 0.05) from control values.
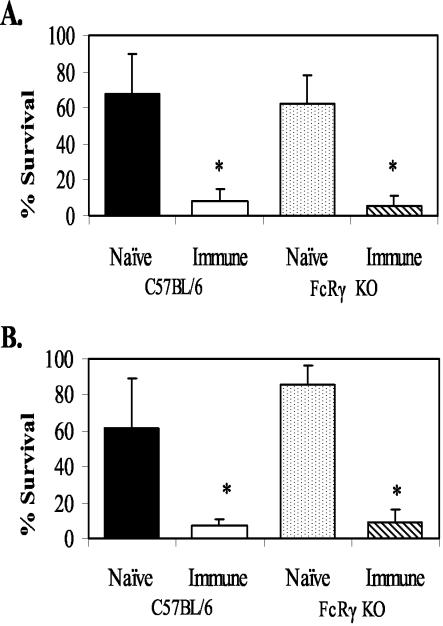
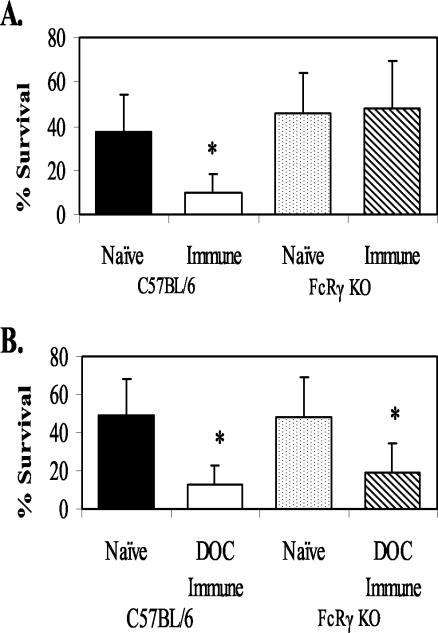
FcRγ KO mice are missing the γ-chain required for FcγRI, FcγRIII, and FcɛR receptor surface expression (37). Cells in these mice are therefore unable to bind to IgG and IgE. FcRγ KO mice were immunized with L3 to determine whether protective immunity would be different from that seen with wild-type mice. Immunization of FcRγ KO mice with challenge either 1 week after the booster immunization (when IgM was the functioning isotype) (4) or 3 weeks after the booster immunization (when IgM and IgG were the functioning isotypes) resulted in protective immunity (Fig. 5). However, transfer of purified IgG (recovered 3 weeks after booster immunization with live L3) into FcRγ KO mice failed to transfer immunity (Fig. 6A). This was in marked contrast to the results seen with IgG recovered from mice immunized with DOC Ag, which could transfer immunity to the FcRγ KO mice (Fig. 6B). Diffusion chambers implanted in wild-type and FcRγ KO mice that received either no IgG, control IgG, or IgG from animals immunized with live or solubilized L3 all contained equal numbers of infiltrating neutrophils. The numbers of infiltrating neutrophils ranged from 4 × 106 to 8 × 106, with no statistical differences between groups.
FIG. 5.
Effect of immunization of C57BL/6J and FcRγ KO mice on the survival of challenge larvae at 1 week (A) and 3 weeks (B) after booster immunization. The data represent the means and standard deviations of the results for 12 animals per group for the experiment at 1 week after booster immunization and 5 animals per group for the experiment at 3 weeks after booster immunization. The asterisks indicate statistical differences (P ≤ 0.05) from control values.
FIG. 6.
Effect on larval survival of transfer of immune IgG from mice immunized with live L3 (A) or with soluble DOC antigen (B) into C57BL/6J and FcRγ KO mice. The data represent the means and standard deviations of results for 10 animals per group. The asterisks indicate statistical differences (P ≤ 0.05) from control values.
Morphological and molecular targets of protective IgM and IgG.
Western blotting was performed to determine what antigens the protective IgM and IgG recognized in DOC-solubilized S. stercoralis larvae. Analysis of the Western blots showed that both the IgM and the IgG recognized approximately 20 antigens. Of these 20 antigens, only 8 were recognized by both IgM and IgG (Fig. 7). IgM and IgG from naïve mice did not bind to any of the solubilized S. stercoralis larval antigens (data not shown).
FIG. 7.

Antigen recognition in Western blot analysis of protective IgM and IgG (recovered from mice 3 weeks after immunization with live L3) from solubilized S. stercoralis larval antigens. The bars represent bands recognized by both IgM and IgG.
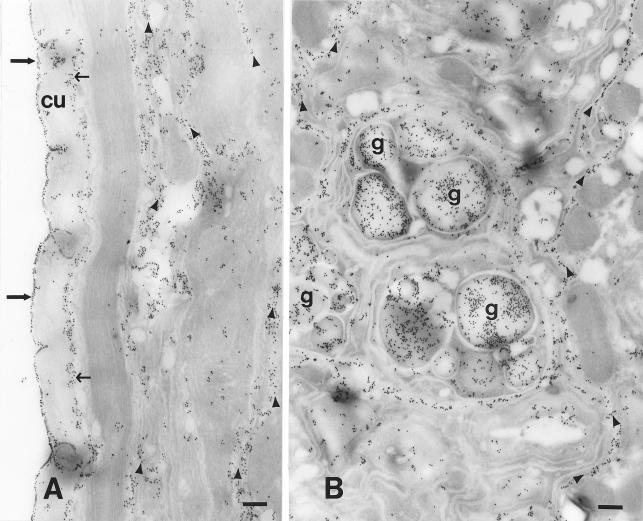
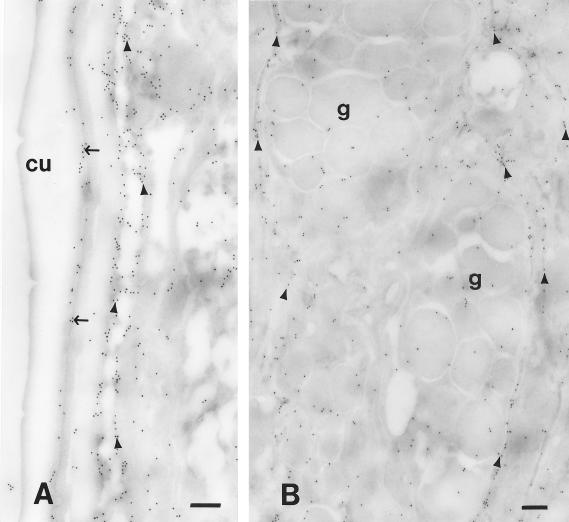
Immunoelectron microscopy was performed to determine where protective IgM (recovered 1 week after booster immunization) and IgG (recovered 3 weeks after booster immunization) bound to the L3. It was determined that IgM bound to the surface of the cuticle, basal cuticle-hypodermis, coelomic cavity, and glandular esophagus (Fig. 8), whereas the IgG bound only to the basal cuticle-hypodermis and the coelomic cavity (Fig. 9).
FIG. 8.
Immunoelectron microscopy performed to determine where protective IgM recovered 1 week after booster immunization bound to the larvae of S. stercoralis. Cu, cuticle; g, glandular esophagus granules. The thin arrows indicate labeling in the basal cuticle-hypodermis area, the thick arrows indicate labeling on the surface of the cuticle, and the arrowheads indicate labeling in the coelomic cavity. Bar, 250 nm.
FIG. 9.
Immunoelectron microscopy performed to determine where protective IgG, recovered at 3 weeks after booster immunization, bound to the larvae of S. stercoralis. Cu, cuticle; g, glandular esophagus granules. The thin arrows indicate labeling in the basal cuticle-hypodermis area; the arrowheads indicate labeling in the coelomic cavity. Bar, 250 nm.
DISCUSSION
The first objective of this study was to determine whether IgG from mice immunized with live L3 had the ability to transfer protection to challenge infections in naïve mice. Previous studies have shown that IgM was the only protective antibody isotype in sera obtained 1 week after booster immunization (4). IgG was purified from the sera of mice immunized with live worms at 3 or 5 weeks after booster immunization to determine whether allowing the immune response to mature would result in a protective IgG response. It was determined that both IgM and IgG were protective at 3 and 5 weeks after booster immunization and that the levels of protection produced by the two antibody isotypes were equal at both time points. Purified IgM and IgG from 3 weeks after booster immunization could individually transfer immunity at the same high level as the whole serum. However, this result was not seen at 5 weeks after the booster immunization, whole serum being more protective than IgM or IgG alone. It is possible that at 5 weeks after booster immunization, effective protective immunity requires both IgM and IgG; alternatively, another isotype or serum component may be required. IgG recovered from mice 3 weeks after booster immunization with live L3 was capable of contributing to an effective protective immune response against larval S. stercoralis and was studied in the ensuing experiments.
Mice were treated with CVF to eliminate complement activation and thereby determine whether activation of the complement cascade is required for IgG to function in killing the larvae. It was determined that IgG could not transfer immunity when the recipient mice were treated to block complement activation. Both the alternative and the classical pathways of complement activation have been shown to occur on the surface S. stercoralis L3 in vitro (7), and it was previously shown that complement activation was also required for IgM to effect larval killing in mice (4). In vitro studies have also shown a requirement for complement activation in killing the helminths T. spiralis (38), Loa loa (30), Angiostrongylus cantonensis (34), Nippostrongylus brasiliensis (8), and Schistosoma mansoni (2) and in the control of S. mansoni in vivo (10, 39).
In previous studies it was shown that contact between host cells and the parasites was required for IgM to kill the larvae of S. stercoralis. Furthermore, depletion of granulocytes with the MAb RB6-8C5 also eliminated IgM-dependent immunity (4). Granulocytes have also been shown to be important in controlling S. ratti infections (40). Evidence that neutrophils were the effector cells against larval S. stercoralis was derived from experiments in which IgM was transferred into IL-5 KO mice. These mice had virtually no eosinophil recruitment into the parasite's microenvironment and yet the larvae were still effectively killed, thus suggesting that neutrophils can function as the effector cells in IgM-dependent immunity (15). Results from the present study regarding the mechanism of IgG-dependent immunity mirrored the results obtained for IgM. Specifically, granulocytes were required, and judging on the basis of the results obtained in studies with IgG transferred into IL-5 KO mice, it appears that neutrophils are the effector cells in IgG-dependent immunity as well. A role for neutrophils in killing N. dubius larvae in vivo in collaboration with IgG has been previously reported (29); in vitro results with A. cantonensis also confirm the function of IgG-dependent and complement-dependent neutrophil cytotoxicity to the infective larvae (34).
FcRγ KO mice were immunized with S. stercoralis L3 to investigate whether the IgM and IgG protection functioned by an antibody-dependent cellular cytotoxicity (ADCC) mechanism. These animals lack Fc receptors; consequently, cells cannot bind to IgG, thereby impairing cell-mediated ADCC (37). Fc receptor-mediated mechanisms have been shown to be crucial in controlling primary Strongyloides venezuelensis infections (28) and resistance to the microfilariae of Brugia malayi in mice (11). Immunized FcRγ KO mice that received challenge infections either 1 or 3 weeks after a booster immunization developed protective immunity. These results can be interpreted to mean either that ADCC dependent on the presence of IgG is not required for protective immunity or that the protective IgM found in the serum at both time periods was sufficient for protective immunity in the absence of a functional IgG response.
To test the second hypothesis, IgG from mice immunized with live L3 was transferred into FcRγ KO mice; it was shown that IgG could not transfer immunity to the FcRγ KO mice. FcRγ KO mice have also been shown to have a deficit in neutrophil recruitment into sites of inflammation (6, 13, 14). In the present study, neutrophils were recruited in equal numbers in the wild-type and FcRγ KO mice receiving transferred IgG. Therefore, the deficit in protective immunity after passive transfer of IgG from mice immunized with live L3 was not caused by an absence of effector cells but rather suggests a dependency of IgG on ADCC as the mechanism of larval killing. It is of interest that IgG recovered from mice immunized with DOC Ag could transfer immunity to the FcRγ KO mice. The difference in the modes of action for the two protective IgGs may be explained by their different antigenic binding sites, with IgG from mice immunized with live L3 binding to the cuticle while IgG from mice immunized with DOC Ag bound only to internal tissues (17). These experiments therefore show that IgM from mice immunized with live L3 and IgG from mice immunized with DOC Ag kill the L3 in an ADCC-independent mechanism. This result is in contrast to that seen with IgG from mice immunized with live L3, which appears to function through a classical ADCC mechanism.
Western blot analyses performed with the protective IgM and IgG from mice immunized with live L3 showed that IgM and IgG each recognized approximately 20 antigens. DOC Ag was used in these analyses, since it was previously demonstrated that lymphocytes from mice immunized with live L3 proliferated and produced IL-5 in response to antigens found in DOC Ag and not to antigens soluble in PBS (17). Of the 20 recognized antigens, only 8 were recognized by both IgM and IgG. Immunoelectron microscopy was performed to determine where the protective IgM and IgG bound to the larvae. It was determined that IgM bound to the surface of the cuticle, the basal cuticle-hypodermis, the coelomic cavity, and the glandular esophagus, whereas IgG bound only to the basal cuticle-hypodermis and the coelomic cavity. Previous studies using fixed intact L3 in indirect fluorescent antibody assays have shown that IgM was the only antibody isotype found to bind to the surface of the larvae (4), thus corroborating the results of the present study.
The antigens jointly recognized by IgM and IgG may be in the basal cuticle-hypodermis and the coelomic cavity, while the antigens uniquely recognized by IgM may be on the surface of the cuticle and in the glandular esophagus. The IgM response which temporally occurs first may be directed at the antigens initially seen by the host, surface antigens, and antigens secreted by the glandular esophagus during the larval migration process. In previous studies it was shown that the protective IgG from mice immunized with DOC Ag recognized seven antigens (17), four of which were also apparently recognized (as determined on the basis of their molecular weights) by the protective IgG from mice immunized with live L3. IgG from mice immunized with DOC Ag was found to bind to the muscles and nerve cord (17). The antigens recognized by IgG from mice immunized with DOC Ag may represent proteins which are normally not seen in the immune response. These antigens may become evident only after the larvae are disrupted and solubilized. Antigens normally concealed from the host were shown to be effective immunogens against the nematodes Haemonchus contortus and Dirofilaria immitis (23, 35, 36). Therefore, it appears that IgG from mice immunized with DOC Ag and IgG from mice immunized with live L3 do not bind to the cuticle of the larvae and differ in the internal organs of the L3 to which they bind. Although they share reactivity with many antigens, the mechanisms by which they kill the larvae are different.
As the immune response to larval S. stercoralis matures in mice, a protective IgG response develops which requires complement activation, neutrophils, and FcRγ to function. It appears that killing occurs via an ADCC mechanism whereby IgG binds to the larvae and the neutrophils then bind to the Fc portion of the antibody. To kill the larvae, the neutrophils may then release one of the many toxic molecules which they contain (26). The hypothesis that IgG killing of the larvae functions through a classical ADCC mechanism is difficult to reconcile with the antibody localization studies, since IgG was found to bind to various internal organs and not to the surface of the L3. It is possible that the surface antigens to which IgG binds are not seen in the immunoelectron micrographs because they are secreted antigens that are loosely bound to the larval surface or because they represent antigens stripped during the fixation process.
It is clear from these studies that in the killing of larval S. stercoralis, IgM and IgG from mice immunized with live L3 and IgG from mice immunized with DOC Ag function by using different target antigens and different mechanisms. This finding confirms the observation made with other pathogens that the immune response is pluripotent in its ability to kill pathogens. It is therefore proposed that highly effective vaccines against S. stercoralis infections could be developed that are composed of multiple antigens inducing different types of protective immune responses. The synergy of the different types of protective immune responses would result in a vaccine that could efficiently kill the pathogen regardless of the parasite strain and host genetic background.
Acknowledgments
We acknowledge support from NIH grants AI 47189 and AI 22662.
We also thank Ofra Leon and Shalom Leon for expert technical assistance. We give special thanks to Yelena Oskov for assistance with the electron microscopy.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abraham, D., H. L. Rotman, H. F. Haberstroh, W. Yutanawiboonchai, R. A. Brigandi, O. Leon, T. J. Nolan, and G. A. Schad. 1995. Strongyloides stercoralis: protective immunity to third-stage larvae in BALB/cByJ mice. Exp. Parasitol. 80:297-307. [DOI] [PubMed] [Google Scholar]
- 2.Anwar, A. R., S. R. Smithers, and A. B. Kay. 1979. Killing of schistosomula of Schistosoma mansoni coated with antibody and/or complement by human leukocytes in vitro: requirement for complement in preferential killing by eosinophils. J. Immunol. 122:628-637. [PubMed] [Google Scholar]
- 3.Bell, R. G., J. A. Appleton, D. A. Negrao-Correa, and L. S. Adams. 1992. Rapid expulsion of Trichinella spiralis in adult rats mediated by monoclonal antibodies of distinct IgG isotypes. Immunology 75:520-527. [PMC free article] [PubMed] [Google Scholar]
- 4.Brigandi, R. A., H. L. Rotman, W. Yutanawiboonchai, O. Leon, T. J. Nolan, G. A. Schad, and D. Abraham. 1996. Strongyloides stercoralis: role of antibody and complement in immunity to the third stage of larvae in BALB/cByJ mice. Exp. Parasitol. 82:279-289. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho, E. M., T. M. Andrade, J. A. Andrade, and H. Rocha. 1983. Immunological features in different clinical forms of strongyloidiasis. Trans. R. Soc. Trop. Med. Hyg. 77:346-349. [DOI] [PubMed] [Google Scholar]
- 6.Coxon, A., X. Cullere, S. Knight, S. Sethi, M. W. Wakelin, G. Stavrakis, F. W. Luscinskas, and T. N. Mayadas. 2001. Fc gamma RIII mediates neutrophil recruitment to immune complexes. A mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity 14:693-704. [DOI] [PubMed] [Google Scholar]
- 7.de Messias, I. J., R. M. Genta, and W. D. Mohren. 1994. Adherence of monocytes and polymorphonuclear cells to infective larvae of Strongyloides stercoralis after complement activation. J. Parasitol. 80:267-274. [PubMed] [Google Scholar]
- 8.Egwang, T. G., J. Gauldie, and D. Befus. 1984. Complement-dependent killing of Nippostrongylus brasiliensis infective larvae by rat alveolar macrophages. Clin. Exp. Immunol. 55:149-156. [PMC free article] [PubMed] [Google Scholar]
- 9.Genta, R. M., J. S. Harper III, A. A. Gam, W. I. London, and F. A. Neva. 1984. Experimental disseminated strongyloidiasis in Erythrocebus patas. II. Immunology. Am. J. Trop. Med. Hyg. 33:444-450. [DOI] [PubMed] [Google Scholar]
- 10.Goes, A. M., and F. J. Ramalho-Pinto. 1991. Protective immunity to Schistosoma mansoni in mice is dependent on antibody and complement but not on radiosensitive leukocytes. Immunol. Lett. 28:57-63. [DOI] [PubMed] [Google Scholar]
- 11.Gray, C. A., and R. A. Lawrence. 2002. A role for antibody and Fc receptor in the clearance of Brugia malayi microfilariae. Eur. J. Immunol. 32:1114-1120. [DOI] [PubMed] [Google Scholar]
- 12.Grove, D. I., and C. Northern. 1982. Infection and immunity in dogs infected with a human strain of Strongyloides stercoralis. Trans. R. Soc. Trop. Med. Hyg. 76:833-838. [DOI] [PubMed] [Google Scholar]
- 13.Hall, L. R., E. Diaconu, and E. Pearlman. 2001. A dominant role for Fc gamma receptors in antibody-dependent corneal inflammation. J. Immunol. 167:919-925. [DOI] [PubMed] [Google Scholar]
- 14.Hanski, C., M. Naumann, A. Grutzkau, G. Pluschke, B. Friedrich, H. Hahn, and E. O. Riecken. 1991. Humoral and cellular defense against intestinal murine infection with Yersinia enterocolitica. Infect. Immun. 59:1106-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbert, D. R., J. J. Lee, N. A. Lee, T. J. Nolan, G. A. Schad, and D. Abraham. 2000. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J. Immunol. 165:4544-4551. [DOI] [PubMed] [Google Scholar]
- 16.Herbert, D. R., T. J. Nolan, G. A. Schad, and D. Abraham. 2002. The role of B cells in immunity against larval Strongyloides stercoralis in mice. Parasite Immunol. 24:95-101. [DOI] [PubMed] [Google Scholar]
- 17.Herbert, D. R., T. J. Nolan, G. A. Schad, S. Lustigman, and D. Abraham. 2002. Immunoaffinity-isolated antigens induce protective immunity against larval Strongyloides stercoralis in mice. Exp. Parasitol. 100:112-120. [DOI] [PubMed] [Google Scholar]
- 18.Hestdal, K., F. W. Ruscetti, J. N. Ihle, S. E. Jacobsen, C. M. Dubois, W. C. Kopp, D. L. Longo, and J. R. Keller. 1991. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J. Immunol. 147:22-28. [PubMed] [Google Scholar]
- 19.Irvine, M., T. Huima, A. M. Prince, and S. Lustigman. 1994. Identification and characterization of an Onchocerca volvulus cDNA clone encoding a highly immunogenic calponin-like protein. Mol. Biochem. Parasitol. 65:135-146. [DOI] [PubMed] [Google Scholar]
- 20.Kopf, M., F. Brombacher, P. D. Hodgkin, A. J. Ramsay, E. A. Milbourne, W. J. Dai, K. S. Ovington, C. A. Behm, G. Kohler, I. G. Young, and K. I. Matthaei. 1996. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 4:15-24. [DOI] [PubMed] [Google Scholar]
- 21.Lustigman, S., B. Brotman, T. Huima, and A. M. Prince. 1991. Characterization of an Onchocerca volvulus cDNA clone encoding a genus specific antigen present in infective larvae and adult worms. Mol. Biochem. Parasitol. 45:65-75. [DOI] [PubMed] [Google Scholar]
- 22.Lustigman, S., J. H. McKerrow, K. Shah, J. Lui, T. Huima, M. Hough, and B. Brotman. 1996. Cloning of a cysteine protease required for the molting of Onchocerca volvulus third stage larvae. J. Biol. Chem. 271:30181-30189. [DOI] [PubMed] [Google Scholar]
- 23.McGonigle, S., E. R. Yoho, and E. R. James. 2001. Immunisation of mice with fractions derived from the intestines of Dirofilaria immitis. Int. J. Parasitol. 31:1459-1466. [DOI] [PubMed] [Google Scholar]
- 24.Medeiros, F., C. I. Baldwin, and D. A. Denham. 1996. Brugia pahangi in cats: the passive transfer of anti-microfilarial immunity from immune to non-immune cats. Parasite Immunol. 18:79-86. [DOI] [PubMed] [Google Scholar]
- 25.Murrell, K. D. 1981. Protective role of immunoglobulin G in immunity to Strongyloides ratti. J. Parasitol. 67:167-173. [PubMed] [Google Scholar]
- 26.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolan, T. J., H. L. Rotman, V. M. Bhopale, G. A. Schad, and D. Abraham. 1995. Immunity to a challenge infection of Strongyloides stercoralis third-stage larvae in the jird. Parasite Immunol. 17:599-604. [DOI] [PubMed] [Google Scholar]
- 28.Onah, D. N., F. Uchiyama, Y. Nagakui, M. Ono, T. Takai, and Y. Nawa. 2000. Mucosal defense against gastrointestinal nematodes: responses of mucosal mast cells and mouse mast cell protease 1 during primary Strongyloides venezuelensis infection in FcRγ-knockout mice. Infect. Immun. 68:4968-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penttila, I. A., P. L. Ey, and C. R. Jenkin. 1984. Infection of mice with Nematospiroides dubius: demonstration of neutrophil-mediated immunity in vivo in the presence of antibodies. Immunology 53:147-154. [PMC free article] [PubMed] [Google Scholar]
- 30.Pinder, M., A. Leclerc, and S. Everaere. 1992. Antibody-dependent cell-mediated immune reactions to Loa loa microfilariae in amicrofilaraemic subjects. Parasite Immunol. 14:541-556. [DOI] [PubMed] [Google Scholar]
- 31.Rotman, H. L., S. Schnyder-Candrian, P. Scott, T. J. Nolan, G. A. Schad, and D. Abraham. 1997. IL-12 eliminates the Th-2 dependent protective immune response of mice to larval Strongyloides stercoralis. Parasite Immunol. 19:29-39. [DOI] [PubMed] [Google Scholar]
- 32.Rotman, H. L., W. Yutanawiboonchai, R. A. Brigandi, O. Leon, G. J. Gleich, T. J. Nolan, G. A. Schad, and D. Abraham. 1996. Strongyloides stercoralis: eosinophil-dependent immune-mediated killing of third stage larvae in BALB/cByJ mice. Exp. Parasitol. 82:267-278. [DOI] [PubMed] [Google Scholar]
- 33.Schad, G. A., M. E. Hellman, and D. W. Muncey. 1984. Strongyloides stercoralis: hyperinfection in immunosuppressed dogs. Exp. Parasitol. 57:287-296. [DOI] [PubMed] [Google Scholar]
- 34.Shaio, M. F., S. C. Hou, J. G. Chen, C. C. Wu, K. D. Yang, and F. Y. Chang. 1990. Immunoglobulin G-dependent classical complement pathway activation in neutrophil-mediated cytotoxicity to infective larvae of Angiostrongylus cantonensis. Ann. Trop. Med. Parasitol. 84:185-191. [DOI] [PubMed] [Google Scholar]
- 35.Smith, T. S., E. A. Munn, M. Graham, A. S. Tavernor, and C. A. Greenwood. 1993. Purification and evaluation of the integral membrane protein H11 as a protective antigen against Haemonchus contortus. Int. J. Parasitol. 23:271-280. [DOI] [PubMed] [Google Scholar]
- 36.Smith, W. D., S. K. Smith, and J. M. Murray. 1994. Protection studies with integral membrane fractions of Haemonchus contortus. Parasite Immunol. 16:231-241. [DOI] [PubMed] [Google Scholar]
- 37.Takai, T., M. Li, D. Sylvestre, R. Clynes, and J. V. Ravetch. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell 76:519-529. [DOI] [PubMed] [Google Scholar]
- 38.Venturiello, S. M., G. H. Giambartolomei, and S. N. Costantino. 1993. Immune killing of newborn Trichinella larvae by human leucocytes. Parasite Immunol. 15:559-564. [DOI] [PubMed] [Google Scholar]
- 39.Vignali, D. A., Q. D. Bickle, M. G. Taylor, G. Tennent, and M. B. Pepys. 1988. Comparison of the role of complement in immunity to Schistosoma mansoni in rats and mice. Immunology 63:55-61. [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe, K., K. Noda, S. Hamano, M. Koga, K. Kishihara, K. Nomoto, and I. Tada. 2000. The crucial role of granulocytes in the early host defense against Strongyloides ratti infection in mice. Parasitol. Res. 86:188-193. [DOI] [PubMed] [Google Scholar]
- 41.Williams, D. J., and J. M. Behnke. 1983. Host protective antibodies and serum immunoglobulin isotypes in mice chronically infected or repeatedly immunized with the nematode parasite Nematospiroides dubius. Immunology 48:37-47. [PMC free article] [PubMed] [Google Scholar]