Abstract
Previous immunological studies indicated that the Lyme disease spirochete, Borrelia burgdorferi, expresses Erp outer surface proteins during mammalian infection. We conducted analyses of Erp expression throughout the entire tick-mammal infectious cycle, which revealed that the bacteria regulate Erp production in vivo. Bacteria within unfed nymphal ticks expressed little to no Erp proteins. However, as infected ticks fed on mice, B. burgdorferi increased production of Erp proteins, with essentially all transmitted bacteria expressing these proteins. Mice infected with B. burgdorferi mounted rapid IgM responses to all tested Erp proteins, followed by strong immunoglobulin G responses that generally increased in intensity throughout 11 months of infection, suggesting continued exposure of Erp proteins to the host immune system throughout chronic infection. As naive tick larvae acquired B. burgdorferi by feeding on infected mice, essentially all transmitted bacteria produced Erp proteins, also suggestive of continual Erp expression during mammalian infection. Shortly after the larvae acquired bacteria, Erp production was drastically downregulated. The expression of Erp proteins on B. burgdorferi throughout mammalian infection is consistent with their hypothesized function as factor H-binding proteins that protect the bacteria from host innate immune responses.
The causative agent of Lyme disease, Borrelia burgdorferi, is transmitted to humans and other warm-blooded hosts via the bites of infected ixodid ticks. There are three postembryonic stages of these ticks: larva, nymph, and adult, each of which takes only one blood meal. There is essentially no transovarial transmission of B. burgdorferi, so emergent larvae are uninfected and acquire the bacteria by feeding on infected hosts. Fully engorged larvae molt to nymphs, which can transmit B. burgdorferi to the hosts on which they feed. Fed nymphs molt to adults, the females of which feed, lay eggs, and then die. In most areas where Lyme disease is endemic, both the larval and nymphal stages feed on the same host species, and B. burgdorferi perpetuates through a cycle between these tick stages and their warm-blooded hosts. To complete this infectious cycle, the bacteria must interact with many different host and vector tissues, as well as evade clearance by the host's immune system. To do so, B. burgdorferi apparently senses its environment and coordinates the synthesis of numerous proteins.
Among the bacterial proteins known to be expressed during mammalian infection are the Erp lipoproteins. These outer surface proteins are encoded by allelic genes located on the cp32 plasmids of B. burgdorferi (57). A potential function for these proteins is suggested by observations that they bind the complement inhibitory factor H proteins of numerous different vertebrates (5, 6, 26, 29-31, 54). It is hypothesized that a bacterium needs to express a wide repertoire of Erp proteins so that it can bind the factor H molecules from a wide variety of mammalian hosts, allowing the bacteria to establish infections of a diverse range of hosts (54).
It is well known that B. burgdorferi differentially expresses Erp proteins in vitro in response to temperature and chemical signals (1, 3, 7, 23, 25, 38, 53). Such regulation suggests that expression of these proteins is also regulated by B. burgdorferi during the natural infectious cycle. Bacteria cultured at 23°C produce very little Erp proteins, while those shifted from 23 to 34°C express significantly greater Erp protein levels (25, 53, 56). These temperatures mimic those experienced by B. burgdorferi within the midguts of unfed ticks (ambient temperature) and in ticks during feeding on warm-blooded animals (warming from ambient to blood temperature) (48, 56). B. burgdorferi increases expression levels of the unrelated OspC protein when cultured bacteria are shifted from 23 to 34°C and also upregulates OspC production during tick feeding (20, 34, 39, 47, 48). The similarities in Erp and OspC expression patterns in vitro led to the suggestion that Erp protein levels may also increase during transmission from ticks to warm-blooded hosts (56), a hypothesis which we have addressed with the present study. While several immunological studies demonstrated that Erp proteins are produced during mammalian infection (3, 33, 36, 38, 53, 59), the timing of that expression has not yet been addressed. For that reason, we examined the production of Erp proteins by B. burgdorferi as it enters mammalian hosts during tick feeding, as well as immune responses to Erp proteins throughout persistent murine infection. Finally, Erp protein expression as bacteria were transmitted from infected mice to naive, feeding tick larvae was also examined.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. burgdorferi was grown in either Barbour-Stoenner-Kelly II (BSK-II) (8) or BSK-H (Sigma, St. Louis, Mo.) medium supplemented with 6% rabbit serum. A clone derived from the subculture B31-MI (10, 21) was utilized in the tick-mouse infection studies described below. Subculture B31-MI contains all B. burgdorferi B31 plasmids except cp32-2, cp32-5, and cp9-2 but is not clonal (10, 21, 37). Clones of this culture were obtained by plating in semisolid BSK media, as previously described (32). Twenty clones were selected aseptically and grown in liquid media, and 103 bacteria of each clone were injected subcutaneously into 4- to 6-week-old female BALB/c mice. Sera were obtained 4 weeks postinjection, and infectivity was assessed by Western blotting utilizing a B31-MI whole-cell lysate. All clones were found to be infectious in the mice, and clone B31-MI-16 was randomly selected for further study. A plasmid content analysis of B31-MI-16 was undertaken to ensure that no plasmids were lost during the cloning process, and the profile obtained was identical to that of the parent B31-MI strain.
Tick rearing and infection.
Adult Ixodes scapularis ticks were obtained from Jerry Bowman (Oklahoma State University, Stillwater) and then fed and mated on New Zealand White rabbits. Completely engorged females were held in a humidified chamber until eggs were laid. After hatching, 200 larvae each were fed on female BALB/c mice previously infected with B. burgdorferi strain B31-MI-16 (see above). Larvae fed to repletion and were then allowed to molt to nymphs in the humidified chamber. The infectivity rate of the engorged larval and flat nymphal ticks was assessed by indirect immunofluorescence analysis (IFA) (see below) utilizing a B. burgdorferi B31 rabbit polyclonal anti-membrane protein antibody (K. Babb, unpublished results) and Alexa Fluor 594-labeled goat anti-rabbit immunoglobulin G (IgG; Molecular Probes, Eugene, Oreg.). The infectivity rate was found to be 90%.
Temporal analysis of B. burgdorferi in ticks and tick bite sites.
For B. burgdorferi transmission studies, 10 to 20 infected flat nymphal ticks each were placed on 4- to 6-week-old naive female BALB/c mice. Feeding nymphal ticks were forcibly removed with fine forceps after 24, 48, or 72 h on mice. Often during removal a piece of skin remained attached to the hypostome of the feeding tick. These attached skin samples were carefully dissected away from the tick for analysis. All ticks and skin pieces were examined immediately after tick removal. Ticks that had just completed feeding at 96 h and postfed ticks at 120, 144, 168, 192, and 264 h were also examined. For B. burgdorferi acquisition studies, 200 naive larvae were placed on each B. burgdorferi-infected mouse (see above) and feeding larval ticks were forcibly removed with fine forceps after 24, 48, or 72 h on mice. In addition, unattached larvae that had just completed feeding after 96 h, as well as larvae 120, 144, 168, 192, and 264 h postattachment, were analyzed. An average of two different ticks were examined at each time point and for each examined protein.
Ticks or tissues were dissected into 10 μl of phosphate-buffered saline (PBS) on glass slides and allowed to air dry overnight. Slides were then fixed and permeabilized in acetone for 15 min. Slides were allowed to air dry and then blocked for 1 h at room temperature in PBS containing 0.2% bovine serum albumin (BSA) and 10% goat serum. After being washed in PBS-0.2% BSA, slides were incubated overnight at 4°C in monospecific rabbit polyclonal antisera that recognized either ErpA/I/N, ErpL, or ErpQ (16) diluted 1:100 or mouse monoclonal antibody B5, which is specific for OspC (35), diluted 1:10 in PBS-0.2% BSA. Slides were then washed and incubated for 1 h at room temperature in a 1:10 dilution of murine anti-FlaB monoclonal antibody H9724 (9) (provided by Tom Schwan, Rocky Mountain Laboratories, Hamilton, Mont.) or B. burgdorferi B31 rabbit polyclonal anti-membrane protein antibody. After being washed, slides were incubated in 1:1,000 dilutions of both Alexa Fluor 488-labeled goat anti-mouse IgG and Alexa Fluor 594-labeled goat anti-rabbit IgG (Molecular Probes) for 45 min at room temperature. Slides were then washed, dried, and mounted in glycerol for viewing. Slides were viewed and images were captured with an Axiophot epifluorescence microscope at ×400 and a Spot digital camera (Zeiss, Hallbergmoos, Germany). Labeled bacteria within 25 random fields were counted to determine the proportions of Erp- and OspC-positive bacteria relative to the FlaB- or B. burgdorferi membrane protein antibody-positive bacteria, as either of the last two antibodies labels all bacteria present in a given field. On average, 20 to 30 bacteria were observed in each skin sample, with at least two skin samples examined for each time point and protein.
Measurement of antibody responses directed against Erp proteins during mammalian infection.
To assess the timing and type of antibody response directed against Erp proteins by B. burgdorferi-infected animal hosts, enzyme-linked immunosorbent analyses (ELISA) were conducted on serum samples collected from individual B. burgdorferi-infected mice over an 11-month period. B. burgdorferi B31-MI-16-infected nymphs (see above) were allowed to feed to repletion on 19 4- to 6-week-old female BALB/c mice. Serum samples were then obtained from each mouse at the following time points after the conclusion of tick feeding over the course of 11 months: weekly for the first month, biweekly from months 2 through 6, and monthly from months 7 through 11. Sera were then utilized in ELISA to determine whether these mice produced antibodies that recognized recombinant ErpA/I/N, ErpL, and ErpQ proteins (15). In addition, antibody responses to the infection-associated OspC protein were analyzed by the same means. However, for some mice at certain time points, insufficient serum was obtained to perform ELISA on all the proteins. An average of 10 mice were analyzed at each time for each protein.
To carry out ELISA, 96-well Maxisorp Nunc-Immuno plates (Nalge Nunc International, Naperville, Ill.) were coated overnight at 4°C with 1 μg of either recombinant ErpA/I/N, ErpL, ErpQ, or OspC/well in 50 μl of PBS. Plates were washed three times in PBS-0.5% Tween 20 (PBS-T) and blocked for 1 h at room temperature in PBS-T containing 10% fetal bovine serum. After three washes, triplicate wells of plates were incubated for 1 h at room temperature with 200 μl of either 1:100-diluted B. burgdorferi-infected mouse sera or 1:100-diluted sera obtained from uninfected female BALB/c mice. Following three PBS-T washes, triplicate wells were incubated with a 1:30,000 dilution of either alkaline phosphatase-conjugated goat anti-mouse IgM or goat anti-mouse IgG (Sigma) for 1 h at room temperature. Two hundred microliters of para-nitrophenyl phosphate (Sigma)/well was added after the plates were washed, and color was allowed to develop for 30 min at room temperature. The absorbance at 405 nm was then read with a VersaMax tunable microplate reader and Softmax Pro software (Molecular Devices, Sunnyvale, Calif.), and the values obtained for the uninfected mouse sera were subtracted from those obtained for the B. burgdorferi-infected mouse sera. Values greater than 2 standard deviations from the mean obtained from uninfected mouse sera were considered positive. The averages and standard deviations of samples in triplicate wells were also calculated with this software. All values were then put into the MySQL database server (www.MySQL.com.; MySQL Incorporated, Seattle, Wash.) for grouping and further analysis.
Effects of culture temperature on Erp expression.
To assess the effect of continuous growth at 34°C on Erp expression, B31-MI bacteria were grown in BSK-H at 23°C to mid-exponential phase (1 × 107 to 5 × 107 bacteria/ml), diluted 1:100 into fresh media, and shifted to 34°C. Cultures were then maintained at 34°C for three more passages. Cell lysates were prepared from bacteria grown at each passage, and 5 μg of each was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes, and Western blot analysis was conducted utilizing monospecific rabbit polyclonal ErpA/I/N antisera (16) or mouse monoclonal antibody B5, specific for OspC (35). Bound antibodies were detected as mentioned above. Blots were then stripped and incubated with murine anti-FlaB monoclonal antibody H9724 to ensure that equal quantities of protein were loaded in all lanes. Bound antibodies were detected by enhanced chemiluminescence (Amersham).
RESULTS
B. burgdorferi within transmitting nymphal ticks.
Two previous studies demonstrated that B. burgdorferi within engorged nymphal ticks produces detectable levels of erp transcripts or Erp proteins (23, 25). However, in those two studies, the ticks were examined for Erp expression either after completion of feeding or at only one time point during feeding. The present study represents the first analysis to accurately and quantitatively pinpoint the time frame of Erp expression during nymphal tick feeding and B. burgdorferi transmission.
All erp loci are preceded by essentially identical promoter regions, suggesting that all are coregulated (57). Consistent with this expectation, several studies demonstrated that all the strain B31 erp loci are regulated in similar manners and that all that strain's Erp proteins are produced simultaneously (our unpublished results; 7, 16, 53). For this reason, we selected three antigenically distinct, genetically unlinked Erp proteins as representatives for detailed characterization. As it has been suggested that the Erp family may be divided into three subgroups (2), we also made sure that the selected proteins, ErpA/I/N, ErpL, and ErpQ, represent each of those subgroups.
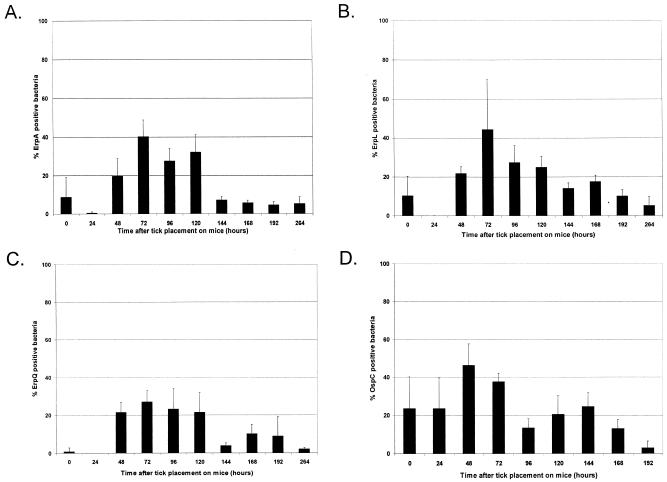
IFA indicated that spirochetes within unfed nymphs produced barely detectable amounts of all Erp proteins analyzed (Fig. 1A to C). Twenty-four hours after the initiation of feeding, bacteria within nymph midguts did not produce detectable levels of Erp proteins. However, the percentage of Erp-positive bacteria increased as feeding progressed, reaching a peak of approximately 40% after 72 h of feeding. Ninety-six hours after the initiation of feeding, the ticks had completed feeding and dropped off their murine hosts. Erp expression levels by bacteria within fully engorged ticks then declined, until, by 144 h after the initiation of feeding, Erp protein production had dropped to levels approximating those seen in unfed nymphs.
FIG. 1.
Temporal analysis of B. burgdorferi B31 Erp and OspC expression in feeding nymphal ticks. Naive BALB/c mice were infested with B31-MI-16-infected nymphal ticks. Ticks were removed at various time intervals during feeding, examined at drop-off (96 h), or analyzed at various points after the conclusion of blood feeding by double-labeling immunofluorescence for B. burgdorferi expressing Erp (A to C) or OspC (D) proteins. Bars, mean percentages of Erp- or OspC-positive bacteria counted in 25 random fields; error bars, 1 standard deviation of the means. (A) ErpA/I/N; (B) ErpL; (C) ErpQ; (D) OspC.
Since OspC levels have been previously demonstrated to increase during tick feeding (19, 20, 34, 39, 47, 48), we also examined expression of this protein as a positive control. A small percentage of bacteria within unfed ticks expressed detectable levels of OspC (Fig. 1D). As ticks fed, the numbers of bacteria producing OspC increased dramatically, peaking at 48 h and then declining. As was seen for the Erp proteins, OspC expression levels decreased after the conclusion of blood feeding.
Ixodid ticks take in the largest volumes of blood toward the end of the feeding process, a phenomenon that is known as the “big sip” (50). Noting that relative expression levels of Erp and OspC proteins peaked during the last 2 days of nymphal feeding, we examined the engorging nymphs to determine whether the peaks of protein synthesis corresponded with the nymphal big sip period. The relative engorgement of ixodid ticks is best examined by comparisons of abdomen size relative to that of the scutum (18, 51). As ticks feed, new cuticle epidermal cells are synthesized to accommodate the large volume of blood being imbibed. As a result, the cuticle of the abdomen increases in size. However, the sclerotized scutum does not grow (12, 28). During the first 48 h of nymphal feeding, the cuticle/scutum ratio remained constant but increased dramatically between 48 and 96 h (Table 1). We conclude that the maximal expression of Erp and OspC proteins by bacteria within the tick midgut coincides with the enhanced blood feeding and bacterial transmission levels of the big sip period of feeding.
TABLE 1.
Measurement of tick cuticle and scutal lengths
| Time after tick placement on mice (h) | Length (mm)a of:
|
Cuticle/scutum ratio | |
|---|---|---|---|
| Cuticle | Scutum | ||
| 0 | 1.14 ± 0.0633 | 0.63 ± 0.050 | 1.8 |
| 24 | 1.13 ± 0.0450 | 0.55 ± 0.043 | 2.1 |
| 48 | 1.35 ± 0.130 | 0.60 ± 0.052 | 2.2 |
| 72 | 2.07 ± 0.193 | 0.62 ± 0.075 | 3.3 |
| 96 | 2.39 ± 0.247 | 0.53 ± 0.040 | 4.5 |
Represents average ± 1 standard deviation of measurements obtained from 12 nymphs at each time point.
B. burgdorferi in nymphal tick bite sites.
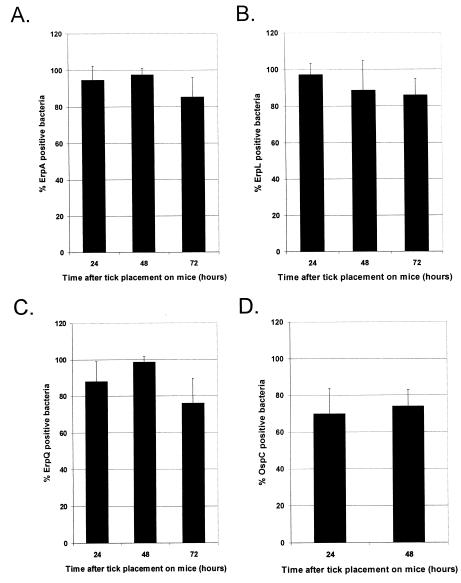
A caveat to studies such as those described above is that bacteria examined within the midgut of a feeding tick are those that did not make the trip from the midgut to the salivary glands and then into the skin of the host. Thus, they are not necessarily representative of the population of successfully transmitted bacteria. Taking this into account, we utilized double-labeling immunofluorescence techniques to examine skin taken from the nymphal tick bite sites at various time intervals during spirochete transmission. After 24 and 48 h of feeding, almost 100% of the spirochete population in each tick bite site skin sample was Erp positive. After 72 h of tick feeding, detectable Erp expression by spirochetes within the skin samples appeared to drop slightly, with over 80% of bacteria producing detectable levels of Erp proteins (Fig. 2A to C).
FIG. 2.
Temporal analysis of B. burgdorferi B31 Erp and OspC expression in tick bite site skin. Naive BALB/c mice were infested with B31-MI-16-infected nymphal ticks. Ticks were removed at various time intervals during feeding, and the skin attached to the tick hypostome was carefully dissected away. The tick bite site skin pieces were then examined by double-labeling immunofluorescence for B. burgdorferi expressing Erp (A to C) or OspC (D) proteins. Bars, mean percentages of Erp- or OspC-positive bacteria counted in 25 random fields; error bars, 1 standard deviation of the means. (A) ErpA/I/N; (B) ErpL; (C) ErpQ; (D) OspC.
Previous studies have shown that transmitted spirochetes producing OspC may be detected in the skin of the host during tick feeding (34, 39). We were also able to detect OspC-positive spirochetes in the tick bite site skin samples, with a significant majority of spirochetes producing detectable levels of OspC (Fig. 2D).
Antibody responses to Erp proteins during mammalian infection.
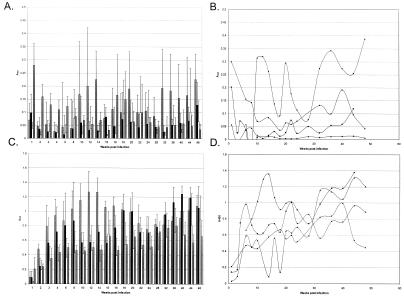
The above-described studies indicate that B. burgdorferi expresses Erp proteins as it enters mammalian hosts. These data are consistent with previous observations that humans and other vertebrates infected with B. burgdorferi produce antibodies directed against Erp proteins (3, 24, 25, 33, 36, 38, 53, 58, 59). We next examined how long Erp expression persists during mammalian infection. B. burgdorferi is exceedingly difficult to find in vertebrate tissues and assess directly. However, Erp expression during mammalian infection can be assessed indirectly through examination of antibody responses directed against these proteins throughout the infection period. In many previous studies, murine antibody responses to a particular antigen have been monitored by obtaining serum samples from different groups of mice at each time point. As a result, data obtained from such studies must be interpreted carefully because of possible variations in immune responses among different mice. To avoid this potential problem, we instead examined the IgM and IgG titers of individual mice. In total, serum samples were obtained from 19 different infected mice over an 11-month period, and IgM and IgG antibody responses to Erp proteins were assessed by ELISA. The mice all mounted rapid IgM responses, followed by robust IgG responses that persisted at high levels throughout the entire 11 months of infection (Fig. 3). Intriguingly, IgM antibodies directed against ErpA/I/N were stronger and of longer duration than those produced in response to ErpL or ErpQ, peaking at 3.5 months of infection, declining, and periodically increasing in the later stages of infection (Fig. 3A). IgG titers produced in response to all examined Erp proteins also varied over time, and often increased, suggestive of continued stimulation of the immune systems throughout duration of the infection (Fig. 3C). These fluctuations are even more dramatic when ELISA results for each mouse were considered individually (Fig. 3B and D and data not shown).
FIG. 3.
IgM (A and B) and IgG (C and D) antibody responses to Erp and OspC proteins mounted by mice infected with B. burgdorferi for 11 months. All examined mice yielded similar results. (A and C) Averaged responses for all mice. Bars (from left to right), ErpA, ErpL, ErpQ, and OspC, respectively. Error bars, 1 standard deviation of the means. (B and D) Analysis of a single, representative mouse (mean A405s obtained from triplicate wells). Diamonds, ErpA; squares, ErpL; triangles, ErpQ; circles, OspC.
The production of antibodies directed against OspC during mammalian infection has been well documented. One of the diagnostic antigens against which human Lyme disease patients produce antibodies is OspC (11, 14, 17, 22). Mice infected with B. burgdorferi also produce antibodies directed against OspC (46, 61). For these reasons, sera from B. burgdorferi-infected mice were also assessed for the presence of antibodies directed against OspC. Strong IgM responses directed against OspC were detected within the first few weeks of infection; these responses converted to IgG responses that persisted throughout infection (Fig. 3).
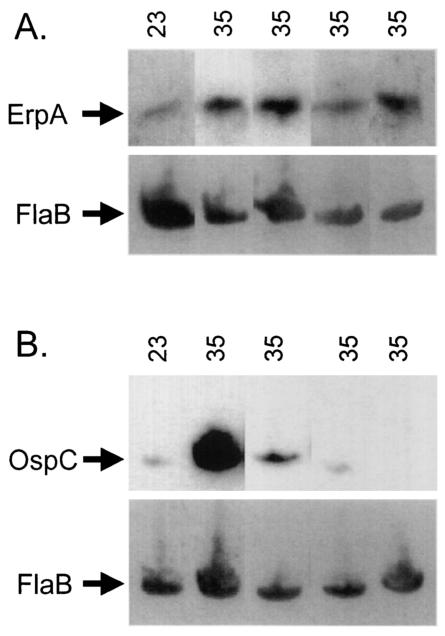
As noted above, B. burgdorferi increases synthesis of Erp proteins in vitro when cultures are shifted from 23 to 34°C, mimicking conditions in the feeding tick. However, it was not known what the effect on Erp expression would be when the spirochetes were cultured for prolonged times at warmer temperatures, a condition similar to that experienced during prolonged mammalian infection. For example, in vitro expression of OspC declines during continuous cultivation at 37°C (47). For this reason, we assessed Erp expression levels of bacteria grown at 23°C, shifted to 34°C, and then maintained at 34°C for an additional three passages (Fig. 4). As expected, both ErpA/I/N and OspC concentrations increased in cultures after shifts from 23 to 34°C. However, in contrast to the waning of OspC expression observed when B. burgdorferi was maintained at 34°C, Erp protein production remained high, with no decrease in expression level detected after four consecutive passages at 34°C. These data indicate that sustained exposure of B. burgdorferi to temperatures approximating those experienced during mammalian infection results in continual production of Erp proteins by those bacteria.
FIG. 4.
Analysis of Erp and OspC protein expression by B. burgdorferi grown at a constant 34°C. B31-MI bacteria were grown at 23°C to mid-late logarithmic phase, diluted 1:100 into fresh media, and shifted to 34°C. Cultures were then maintained at 34°C for three more passages. Cell lysates were prepared from bacteria grown at each passage, and Western blot analysis utilizing specific antibodies directed against ErpA/I/N (A) and OspC (B) was conducted. Each blot was then stripped and reprobed with murine anti-FlaB monoclonal antibody to ensure that equal quantities of protein were loaded in all lanes.
Assessment of larval ticks acquiring B. burgdorferi.
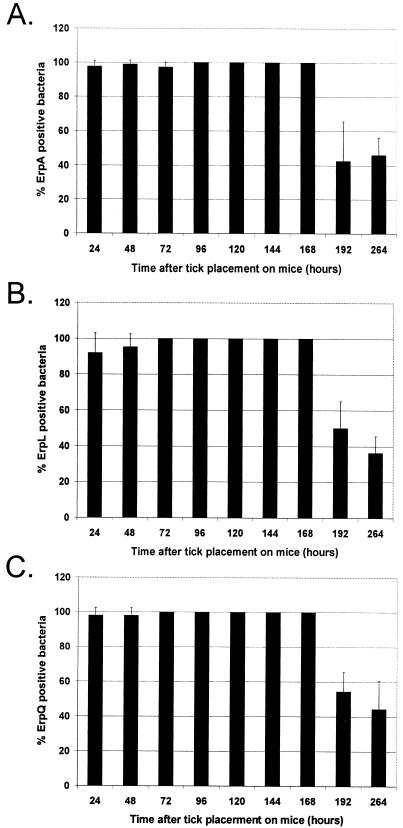
Until the present study, very little was known about Erp expression by bacteria during transmission from infected mammals to feeding larvae. While nonquantitative reverse transcription-PCR analysis indicated that erp genes are transcribed by spirochetes within fully engorged larvae (23), that study examined larvae only after completion of feeding. To complete our analysis of Erp expression during the mammal-tick infectious cycle, double-labeling IFA was conducted on larvae removed at intervals during feeding or following the conclusion of blood feeding. Erp proteins were detected in approximately 100% of the spirochetes within ticks at all stages of feeding and for several days after drop-off (96 h; Fig. 5). The number of Erp-expressing bacteria then began to decrease 3 to 4 days after completion of tick feeding.
FIG. 5.
Temporal analysis of B. burgdorferi B31 Erp expression in feeding larval ticks. B31-MI-16-infected BALB/c mice were infested with naive larval ticks. Ticks were removed at various time intervals during feeding and examined at drop-off (96 h) or at various points after the conclusion of blood feeding by double-labeling immunofluorescence for B. burgdorferi expressing Erp (A to C). Bars, mean percentages of Erp-positive bacteria counted in 25 random fields; error bars, 1 standard deviation of the means. (A) ErpA/I/N; (B) ErpL; (C) ErpQ.
DISCUSSION
These studies represent the first analysis of Erp expression patterns throughout the entire tick-mammal infectious cycle. Erp production by B. burgdorferi in unfed nymphs and in both larvae and nymphs examined several days after completion of blood feeding was found to be low. However, bacteria increased the synthesis of Erp proteins during transmission from infected nymphs to mice. Bacteria appeared to continue expressing these proteins during chronic mammalian infection, apparently in response to the sustained warm temperature of the mammalian body, and essentially all bacteria transmitted from infected mice to feeding larvae expressed every examined Erp protein.
While earlier studies demonstrated that Erp proteins are produced by spirochetes within fed nymphal ticks (23, 25), neither the level of expression nor the timing of synthesis of these proteins during the transmission process had been undertaken. We observed that the proportion of Erp-expressing bacteria within the tick midgut increased dramatically during tick feeding and then declined following detachment from the host. These data extend the results of another study, which detected expression of Erp proteins by bacteria in the salivary glands of ticks after 55 h of feeding (25). It is presumably of great importance to the establishment of infection that those spirochetes successfully transmitted into the mammalian host overwhelmingly express Erp proteins.
Many Erp proteins have been demonstrated to bind host factor H, thus preventing killing by the host alternative pathway of complement-mediated killing. It may seem paradoxical that the factor H-binding Erp proteins are not expressed at higher levels within the midguts of transmitting nymphs, since host blood is present within this environment. However, tick saliva contains components that inhibit the alternative pathway of complement (45, 60). In addition, a recent report suggested that bacteria within feeding nymphs are not affected by complement (43). Thus, it may not be necessary for B. burgdorferi within the midguts of feeding ticks to express Erp proteins at high levels, since the need to inactivate complement within the vector is nullified. However, B. burgdorferi transmitted from the vector to the mammalian host is exposed to the full onslaught of the host alternative pathway during the establishment of infection. It is likely that this necessity is responsible for our observations that essentially all transmitted B. burgdorferi bacteria expressed every tested Erp protein.
Our studies demonstrated that successfully transmitted B. burgdorferi can be phenotypically very distinct from bacteria remaining in the tick midgut. While at most 40 to 50% of bacteria in tick midguts expressed Erp proteins, essentially all bacteria in skin surrounding the tick bite site produced every examined Erp protein. This demonstrates that the protein content of bacteria within tick midguts is not necessarily representative of those bacteria that are transmitted to the vertebrate host. Thus it is possible that erroneous conclusions may be drawn from studies that analyze only the midgut contents of fed ticks when exploring expression of borrelial proteins thought to be involved in spirochete transmission.
We consistently detected B. burgdorferi within the skin of mice at the tick bite sites within 24 h of tick attachment. Several other researchers have observed bacteria in the tick bite wound after a similarly short time of attachment (4, 27, 39, 40, 49). At first glance, these data seem to contradict results of other studies indicating that ticks must feed for at least 48 h before transmitting Lyme disease (13, 41, 51). However, a review of those reports indicates that, while efficiency of transmission greatly increases after 2 days of tick feeding, occasional infections were observed even when ticks fed for less time. Thus, it appears that during the first day or two of feeding, either very few bacteria are transmitted and are readily eliminated by the immune systems of most hosts or most of those bacteria transmitted are physiologically unable to establish disseminated infection.
It is intriguing that B. burgdorferi expressed only low levels of Erp proteins during infection of unfed ticks. Furthermore, Erp expression levels decreased following completion of feeding by both larvae and nymphs. The reasons for these phenomena are unknown, although it is possible that expression of Erp proteins by B. burgdorferi is inhibitory to their survival during the tick molting process, necessitating clearance of Erp proteins from the bacterial outer surface in order to adhere to the tick midgut and persist through the molt. Ongoing studies in our laboratory are addressing this possibility.
Our studies of OspC expression by bacteria during nymphal tick infection paralleled those of other researchers that showed that the proportion of OspC-positive bacteria increases during tick feeding and then diminishes after completion of engorgement (19, 20, 34, 39, 44, 47, 48). Similar to some of those earlier studies but in contrast with others, we detected expression of OspC in unfed nymphs. The reasons for these differences are unknown but may be due to the titers of antibodies used, accessibilities of different antibody preparations for their epitopes, or variations in conditions under which ticks were maintained.
B. burgdorferi-infected mice mounted rapid IgM responses to Erp and OspC proteins, followed by robust IgG responses that persisted at high levels throughout 11 months of infection. Additionally, while the IgG responses to Erp and OspC proteins remained high, titers exhibited wave-like patterns where the antibody levels would be extremely high for a time, wane a bit, and then skyrocket back up. This pattern is suggestive of periodic restimulation of the immune system, possibly due to episodes of bacterial multiplication and dissemination within the host. Descriptions of human Lyme disease include episodic occurrences of symptoms such as arthritis and fever (42, 52), which may also be attributable to periodic reemergence of B. burgdorferi from immune-privileged sites in the host.
Higher titers of antibodies were detected as recognizing ErpA/I/N than ErpL, with levels of antibodies recognizing ErpQ being lowest. It was previously shown that stronger antibody responses are produced in response to recombinant ErpA/I/N than to most other recombinant Erp proteins (53). This is perhaps not surprising, as ErpA/I/N is encoded by three identical genes (erpA, erpI, and erpN), which conceivably results in production of far greater levels of this protein than of Erps encoded by a single gene. Analyses using transcriptional fusions to reporter genes have demonstrated that the erpA transcriptional promoter is relatively strong, which may also result in larger quantities of this protein being synthesized (our unpublished results). Also, ErpA/I/N is highly similar to ErpP (10, 55), so IgG antibodies recognizing ErpA/I/N could initially have been directed against the other protein. Similarly, ErpL is very similar to ErpY (10, 55), which might enhance the production of antibodies directed against the two proteins. On the other hand, ErpQ is not antigenically similar to any other B31 Erp proteins (10, 55), so antibodies recognizing this protein are likely specific for only that protein, resulting in measurably lower levels of antibodies than those recorded for ErpA/I/N and ErpL. It is also possible that less ErpQ protein is produced during infection or that antibodies directed against ErpQ are weaker than those directed against ErpA/I/N and ErpL.
Several studies have demonstrated that all the Erp proteins of B. burgdorferi strain B31 are regulated in similar manners and are produced simultaneously (our unpublished results; 7, 16, 53). Combined with results from the present study, these data strongly suggest that transmitted bacteria express their entire repertoire of Erp proteins during transmission from tick to mammal and from mammal to tick. However, a recent report suggested that some strain B31 Erp proteins might instead be sequentially expressed during mammalian infection (36). That conclusion was based on differences in the immune responses of infected mice to needle injection of cultured bacteria, with the assumption that failure to detect antibodies that recognized a particular Erp protein corresponded with lack of expression of that protein by the infecting bacteria. Three lines of evidence argue against that conclusion. First, as discussed in the previous paragraph, the strength of the host immune response is not necessarily reflective of whether or not bacteria produced a certain protein during infection. Levels of antibodies directed against apparently nonexpressed proteins may have been below thresholds of detection. Second, all erp loci are preceded by nearly identical promoter regions, so it is difficult to envision conditions in which some promoters will be active while others are inactivated. For example, studies of the B. burgdorferi proteins that specifically bind erp promoters indicate that those DNA binding proteins interact with every tested erp locus (our unpublished results; 7). Third, studies of erp transcriptional promoters indicate that they vary in strength, primarily due to differences in the −10 sequences and that this variation results in the bacteria producingdifferent quantities of each Erp protein (our unpublished results). For these reasons, we consider it far more likely that the apparently conflicting results are actually due to differences in antigenicity and relative expression levels of different erp genes and their proteins, rather than to some proteins being produced while other erp genes are completely silenced.
In summary, we demonstrated that B. burgdorferi regulates Erp protein production during the natural vertebrate-tick infectious cycle. While bacteria within unfed nymphal ticks produced extremely low levels of Erp proteins, expression of these proteins increased during tick feeding, with essentially all B. burgdorferi bacteria transmitted to mammalian hosts producing every examined Erp protein. Infected mice maintained antibody responses suggestive of sustained exposure to Erp proteins throughout long-term infection. In vitro experiments suggest that B. burgdorferi expresses Erp proteins in response to the sustained warm temperature of the mammalian host. Also consistent with that hypothesis, detectable levels of Erp proteins were produced by essentially all B. burgdorferi bacteria transmitted from infected mice to naive larvae. Erp production was then drastically downregulated after the ticks acquired the bacteria. Continued characterization of the factor H-binding Erp proteins will undoubtedly enhance knowledge of the mechanisms utilized by B. burgdorferi to evade the host innate immune response and cause persistent infection. Furthermore, characterization of mechanisms used by these bacteria to sense their environment and control synthesis of proteins required for mammalian infection will provide novel targets for therapies to prevent and treat Lyme disease.
Acknowledgments
This study was funded by U.S. National Institutes of Health grant RO1-AI44254 to Brian Stevenson. Jennifer C. Miller was supported by NIH training grant T32-AI49795.
We thank Tom Schwan for advice on the biology of ticks and their cultivation and for providing monoclonal antibodies, Robert Gilmore for the plasmid encoding recombinant OspC, Jerry Bowman for providing ticks, Robert Geraghty for assistance with ELISAs and Softmax Pro software, Christopher Miller for assistance with the MySQL database, and Natalie Mickelson and Rachel Wattier for technical assistance and helpful comments during the course of this work.
Editor: F. C. Fang
REFERENCES
- 1.Akins, D. R., K. W. Bourell, M. J. Caimano, M. V. Norgard, and J. D. Radolf. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Investig. 101:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins, D. R., M. J. Caimano, X. Yang, F. Cerna, M. V. Norgard, and J. D. Radolf. 1999. Molecular and evolutionary analysis of Borrelia burgdorferi 297 circular plasmid-encoded lipoproteins with OspE- and OspF-like leader peptides. Infect. Immun. 67:1526-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akins, D. R., S. F. Porcella, T. G. Popova, D. Shevchenko, S. I. Baker, M. Li, M. V. Norgard, and J. D. Radolf. 1995. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol. Microbiol. 18:507-520. [DOI] [PubMed] [Google Scholar]
- 4.Alekseev, A. N., L. A. Burenkova, I. S. Vasilieva, H. V. Dubinina, and S. P. Chunikhin. 1996. Preliminary studies on virus and spirochete accumulation in the cement plug of ixodid ticks. Exp. Appl. Acarol. 20:713-723. [DOI] [PubMed] [Google Scholar]
- 5.Alitalo, A., T. Meri, H. Lankinen, I. Seppälä, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. [DOI] [PubMed] [Google Scholar]
- 6.Alitalo, A., T. Meri, L. Rämö, T. S. Jokiranta, T. Heikkilä, I. J. T. Seppälä, J. Oksi, M. Viljanen, and S. Meri. 2001. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect. Immun. 69:3685-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babb, K., N. El-Hage, J. C. Miller, J. A. Carroll, and B. Stevenson. 2001. Distinct regulatory pathways control the synthesis of Borrelia burgdorferi infection-associated OspC and Erp surface proteins. Infect. Immun. 69:4146-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 9.Barbour, A. G., S. F. Hayes, R. A. Heiland, M. E. Schrumpf, and S. L. Tessier. 1986. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect. Immun. 52:549-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs of an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. Morbid. Mortal. Wkly. Rep. 44:590-591. [PubMed] [Google Scholar]
- 12.Coons, L. B., R. Rosell-Davis, and B. I. Tarnowski. 1986. Bloodmeal digestion in ticks, p. 248-279. In J. R. Sauer and J. A. Hair (ed.), Morphology, physiology, and behavioral biology of ticks. Ellis Horwood Limited, New York, N.Y.
- 13.des Vignes, F., J. Piesman, R. Heffernan, T. L. Schultze, K. C. Stafford, and D. Fish. 2001. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J. Infect. Dis. 183:773-778. [DOI] [PubMed] [Google Scholar]
- 14.Dressler, F., J. A. Whalen, B. N. Reinhardt, and A. C. Steere. 1993. Western blotting in the serodiagnosis of Lyme disease. J. Infect. Dis. 167:392-400. [DOI] [PubMed] [Google Scholar]
- 15.El-Hage, N., K. Babb, J. A. Carroll, N. Lindstrom, E. R. Fischer, J. C. Miller, R. D. Gilmore, Jr., M. L. Mbow, and B. Stevenson. 2001. Surface exposure and protease insensitivity of Borrelia burgdorferi Erp (OspEF-related) lipoproteins. Microbiology 147:821-830. [DOI] [PubMed] [Google Scholar]
- 16.El-Hage, N., and B. Stevenson. 2002. Simultaneous coexpression of Borrelia burgdorferi Erp proteins occurs through a specific, erp locus-directed regulatory mechanism. J. Bacteriol. 184:4536-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engstrom, S. M., E. Shoop, and R. C. Johnson. 1995. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 33:419-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falco, R. C., D. Fish, and J. Piesman. 1996. Duration of tick bites in a Lyme disease-endemic area. Am. J. Epidemiol. 143:187-192. [DOI] [PubMed] [Google Scholar]
- 19.Fingerle, V., U. Hauser, G. Liegl, B. Petko, V. Preac-Mursic, and B. Wilske. 1995. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus. J. Clin. Microbiol. 33:1867-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fingerle, V., G. Liegl, U. Munderloh, and B. Wilske. 1998. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus ticks removed from humans. Med. Microbiol. Immunol. 187:121-126. [DOI] [PubMed] [Google Scholar]
- 21.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidmann, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 22.Fung, B. P., G. L. McHugh, J. M. Leong, and A. C. Steere. 1994. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect. Immun. 62:3213-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilmore, R. D., Jr., M. L. Mbow, and B. Stevenson. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 24.Hefty, P. S., C. S. Brooks, A. M. Jett, G. L. White, S. K. Wikel, R. C. Kennedy, and D. R. Akins. 2002. OspE-related, OspF-related, and Elp lipoproteins are immunogenic in baboons experimentally infected with Borrelia burgdorferi and in human Lyme disease patients. J. Clin. Microbiol. 40:4256-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hefty, P. S., S. E. Jolliff, M. J. Caimano, S. K. Wikel, J. D. Radolf, and D. R. Akins. 2001. Regulation of OspE-related, OspF-related, and Elp lipoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect. Immun. 69:3618-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellwage, J., T. Meri, T. Heikkilä, A. Alitalo, J. Panelius, P. Lahdenne, I. J. T. Seppälä, and S. Meri. 2001. The complement regulatory factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 27.Hodzic, E., S. Feng, K. J. Freet, D. L. Borjesson, and S. W. Barthold. 2002. Borrelia burgdorferi population kinetics and selected gene expression at the host-vector interface. Infect. Immun. 70:3382-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemp, D. H., B. F. Stone, and K. C. Binnington. 1982. Tick attachment and feeding: role of the mouthparts, feeding apparatus, salivary gland secretions and host response, p. 119-162. In F. D. Obenchain and R. Galun (ed.), Current themes in tropical science, vol. 1. Pergamon Press, New York, N.Y.
- 29.Kraiczy, P., J. Hellwage, C. Skerka, M. Kirschfink, V. Brade, P. F. Zipfel, and R. Wallich. 2003. Immune evasion of Borrelia burgdorferi: mapping of a complement inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur. J. Immunol. 33:697-707. [DOI] [PubMed] [Google Scholar]
- 30.Kraiczy, P., C. Skerka, V. Brade, and P. F. Zipfel. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraiczy, P., C. Skerka, M. Kirschfink, P. F. Zipfel, and V. Brade. 2001. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int. Immunopharmacol. 1:393-401. [DOI] [PubMed] [Google Scholar]
- 32.Kurtti, T. J., U. G. Munderloh, R. C. Johnson, and G. G. Ahlstrand. 1987. Colony formation and morphology in Borrelia burgdorferi. J. Clin. Microbiol. 25:2054-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam, T. T., T.-P. K. Nguyen, R. R. Montgomery, F. S. Kantor, E. Fikrig, and R. A. Flavell. 1994. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect. Immun. 62:290-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leuba-Garcia, S., R. Martinez, and L. Gern. 1998. Expression of outer surface proteins A and C of Borrelia afzelii in Ixodes ricinus ticks and in the skin of mice. Zentbl. Bakteriol. 287:475-484. [DOI] [PubMed] [Google Scholar]
- 35.Mbow, M. L., R. D. Gilmore, Jr., and R. G. Titus. 1999. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect. Immun. 67:5470-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDowell, J. V., S. Y. Sung, G. Price, and R. T. Marconi. 2001. Demonstration of the genetic stability and temporal expression of select members of the Lyme disease spirochete OspF protein family during infection in mice. Infect. Immun. 69:4831-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, J. C., J. L. Bono, K. Babb, N. El-Hage, S. Casjens, and B. Stevenson. 2000. A second allele of eppA in Borrelia burgdorferi strain B31 is located on the previously undetected circular plasmid cp9-2. J. Bacteriol. 182:6254-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller, J. C., N. El-Hage, K. Babb, and B. Stevenson. 2000. Borrelia burgdorferi B31 Erp proteins that are dominant immunoblot antigens of animals infected with isolate B31 are recognized by only a subset of human Lyme disease patient sera. J. Clin. Microbiol. 38:1569-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piesman, J. 1993. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J. Infect. Dis. 167:1082-1085. [DOI] [PubMed] [Google Scholar]
- 41.Piesman, J., T. M. Mather, R. J. Sinsky, and A. Spielman. 1987. Duration of tick attachment and Borrelia burgdorferi transmission. J. Clin. Microbiol. 25:557-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahn, D. W., and J. Evans (ed.). 1998. Lyme disease. American College of Physicians, Philadelphia, Pa.
- 43.Rathinavelu, S., A. Broadwater, and A. M. de Silva. 2003. Does host complement kill Borrelia burgdorferi within ticks? Infect. Immun. 71:822-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rathinavelu, S., and A. M. de Silva. 2001. Purification and characterization of Borrelia burgdorferi from feeding nymphal ticks (Ixodes scapularis). Infect. Immun. 69:3536-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribeiro, J. M. C. 1987. Ixodes dammini: salivary anti-complement activity. Exp. Parasitol. 64:347-353. [DOI] [PubMed] [Google Scholar]
- 46.Schwan, T. G., K. K. Kime, M. E. Schrumpf, J. E. Coe, and W. J. Simpson. 1989. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi). Infect. Immun. 57:3445-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shih, C.-M., and A. Spielman. 1993. Accelerated transmission of Lyme disease spirochetes by partially fed vector ticks. J. Clin. Microbiol. 31:2878-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonenshine, D. E. 1991. Biology of ticks, vol. 1. Oxford University Press, New York, N.Y.
- 51.Sood, S. K., M. B. Salzman, B. J. B. Johnson, C. M. Happ, K. Feig, L. Carmody, L. G. Rubin, E. Hilton, and J. Piesman. 1997. Duration of tick attachment as a predictor of the risk of Lyme disease in an area in which Lyme disease is endemic. J. Infect. Dis. 175:996-999. [DOI] [PubMed] [Google Scholar]
- 52.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 53.Stevenson, B., J. L. Bono, T. G. Schwan, and P. Rosa. 1998. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect. Immun. 66:2648-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stevenson, B., and J. C. Miller. 2003. Intra- and interbacterial genetic exchange of Lyme disease spirochete erp genes generates sequence identity amidst diversity. J. Mol. Evol. 57:309-324. [DOI] [PubMed] [Google Scholar]
- 56.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevenson, B., W. R. Zückert, and D. R. Akins. 2001. Repetition, conservation, and variation: the multiple cp32 plasmids of Borrelia species, p. 87-100. In M. H. Saier and J. García-Lara (ed.), The spirochetes: molecular and cellular biology. Horizon Press, Oxford, United Kingdom. [PubMed]
- 58.Suk, K., S. Das, W. Sun, B. Jwang, S. W. Barthold, R. A. Flavell, and E. Fikrig. 1995. Borrelia burgdorferi genes selectively expressed in the infected host. Proc. Natl. Acad. Sci. USA 92:4269-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallich, R., C. Brenner, M. D. Kramer, and M. M. Simon. 1995. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect. Immun. 63:3327-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wikel, S. K. 1999. Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol. 29:851-859. [DOI] [PubMed] [Google Scholar]
- 61.Wilske, B., V. Preac-Mursic, S. Jauris, A. Hofmann, I. Pradel, E. Soutschek, E. Schwab, G. Will, and G. Wanner. 1993. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect. Immun. 61:2182-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]