Abstract
Salmonella enterica serovar Enteritidis is a major cause of food-borne diseases in industrialized countries. The incidence of S. enterica serovar Enteritidis infections has increased substantially in recent decades, and S. enterica serovar Enteritidis is now one of the leading serovars of Salmonella in the United States. A unique epidemiological characteristic of S. enterica serovar Enteritidis is its association with chicken shell eggs, since approximately 80% of all human gastrointestinal diseases can be traced to contaminated egg products. Eggs are contaminated when bacteria from reproductive tissues of infected hens are packaged into the eggs and persist inside the hostile egg albumen environment. Therefore, resistance to egg albumen is an important aspect in the transmission of S. enterica serovar Enteritidis. We identified a gene, yafD from S. enterica serovar Enteritidis, whose overexpression conferred upon S. enterica serovar Typhimurium enhanced resistance to egg albumen, while disruption of this gene in S. enterica serovar Enteritidis rendered the organism more susceptible to egg albumen. YafD is homologous to members of an exonuclease-endonuclease-phosphatase family, including some enzymes involved in DNA repair. Furthermore, we discovered that egg albumen has nuclease activities and uses both circular and linear DNA as substrates. We propose that YafD provides a survival advantage to S. enterica serovar Enteritidis in eggs by repairing DNA damage caused by egg albumen and that it may be one of the biologic determinants that contribute to the epidemiological association of S. enterica serovar Enteritidis with egg products.
In the last three decades, Salmonella enterica serovar Enteritidis emerged from being a minor serovar of Salmonella to become one of the most common serovars associated with food-borne illnesses in the United States (3, 5, 6, 17, 20, 32). Between 1993 and 1997, S. enterica serovar Enteritidis was responsible for more food-borne gastroenteritis outbreaks and deaths than any other bacterium in the United States (35). From 1990 to 2001, state and territorial health departments reported 677 S. enterica serovar Enteritidis outbreaks, which accounted for 23,366 illnesses, 1,988 hospitalizations, and 33 deaths (5), and the costs associated with human salmonellosis due to S. enterica serovar Enteritidis have been estimated to range from $150 million to $870 million annually (11). Most of the cases are associated with eating contaminated eggs (5, 6, 35). The United States table egg industry produced 67.3 billion eggs in 1998(34), and it is estimated that 1 in 20,000 eggs produced in the United States is contaminated with S. enterica serovar Enteritidis (10, 11). Thus, every year, more than 3 million eggs in the United States are potentially contaminated.
Unsolved questions regarding the epidemiology of S. enterica serovar Enteritidis include why it has emerged in the last 30 years as a major Salmonella serovar in industrialized countries and why it is associated with egg products (3, 17). Other Salmonella serovars, including S. enterica serovar Typhimurium, infect chickens and may cause human infection when cracked eggs are consumed. In contrast, S. enterica serovar Enteritidis contaminates the contents of intact eggs and is the major egg-associated human pathogen (3, 27, 32, 38). It is postulated that S. enterica serovar Enteritidis colonizes ovaries and oviducts of chickens and subsequently contaminates eggs as they form (12, 13, 21, 22, 39). Keller et al. showed previously that clinical strains of both S. enterica serovar Enteritidis and S. enterica serovar Typhimurium colonized the tissues of hen reproductive tracts and forming eggs. However, all of the S. enterica serovar Typhimurium and the majority of the S. enterica serovar Enteritidis were killed by the time eggs were laid (26, 27). This study suggested that the ability of S. enterica serovar Enteritidis to survive in eggs is crucial for the transmission of this bacterium and may have contributed to the emergence of S. enterica serovar Enteritidis as a major cause of human salmonellosis in industrialized countries. We wished to investigate S. enterica serovar Enteritidis determinants for survival inside eggs. Since S. enterica serovar Enteritidis is often found in the albumen of contaminated eggs, especially in naturally contaminated eggs (14, 15, 22, 39), we studied the resistance of S. enterica serovar Enteritidis to egg albumen.
We report here the identification of yafD as a gene essential for the resistance of S. enterica serovar Enteritidis to egg albumen. We provide evidence that YafD may play a role in the repair of DNA damage caused by egg albumen and hence may facilitate the survival of S. enterica serovar Enteritidis in chicken eggs.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. The clinical isolate of S. enterica serovar Typhimurium used, ST3744, was kindly provided by Sharon Abbott of the Department of Health Services, State of California. The clinical isolate of S. enterica serovar Enteritidis, SE2472, has been described previously (29, 30). The latter organism is a clinical isolate of phage type 4 and is virulent in mouse infections. Escherichia coli DH5α (Gibco/BRL, Gaithersburg, Md.) was used as the host for all recombinant DNA manipulations. Plasmid vector pRB3-273C (4) was used to construct a Sau3A I genomic DNA library from isolate SE2472 (29). Plasmids pRB3yafD and pRB3xthA were constructed by inserting PCR-amplified S. enterica serovar Enteritidis genomic DNA, corresponding to nucleotides 295457 to 296841 and 1730923 to 1732500 of the S. enterica serovar Typhi genome, respectively, into pRB3-273C with appropriate linkers. Plasmids pKD4 and pKD46 used for mutagenesis of S. enterica serovar Enteritidis were generously provided by Barry Wanner (Purdue University, West Lafayette, Ind.) (8). Bacteriophage P22 was used for generalized transduction (31). All bacterial strains were grown in Luria-Bertani (LB) broth (Difco, Sparks, Md.) at 37°C with shaking. Antibiotics were added as appropriate.
TABLE 1.
Bacterial strains and plasmids
| Bacterial strain or plasmid | Characteristic(s) | Source or reference |
|---|---|---|
| E. coli DH5α | F−φ80d lacZΔM15Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ thi-1 gyrA96 relA1 | Gibco/BRL |
| S. enterica serovar Enteritidis strains | ||
| SE2472 | Clinical isolate, mouse virulent and egg resistant | 30 |
| SE2472ΔyafD | yafD::kan derivative of SE2472 | This study |
| SE2472ΔyafE | yafE::kan derivative of SE2472 | This study |
| SE2472ΔxthA | xthA::kan derivative of SE2472 | This study |
| S. enterica serovar Typhimurium strains | ||
| ST3744 | Clinical isolate, egg albumen susceptible | This study |
| ST3744ΔyafD | yafD::kan derivative of ST3744 | This study |
| Plasmids | ||
| pBluescript II KS | Apr, high-copy-number plasmid | Stratagene |
| pKD4 | Apr, Kanr oriRγ | 8 |
| pKD46 | Apr, containing the Red recombinase of λ phage | 8 |
| pRB3-273C | Apr, low- to medium-copy-number plasmid for Salmonella | 4 |
| pK3 | Derivative of pRB3-273C containing yafD and yafE | This study |
| pRB3yafD | Derivative of pRB3-273C containing yafD | This study |
| pRB3xthA | Derivative of pRB3-273C containing xthA | This study |
Screening of SE2472 genomic DNA library for plasmid conferring resistance to egg albumen.
The SE2472 genomic DNA library in pRB3-273C (29) was transformed into ST3744 by electroporation and plated on LB agar plates supplemented with 100 μg of ampicillin per ml. After overnight incubation at 37°C, colonies were scraped off the plates and resuspended thoroughly in LB broth. Organic, antibiotic-free chicken eggs from a local farm were disinfected by immersion in 70% ethanol and then dried and cracked into a sterile container. Egg albumen from six to eight eggs was pooled and beaten with an electric mixer for 3 min at the lowest speed. Approximately 1 × 105 organisms transformed with the genomic library were mixed with 10 ml of egg white. After 24 h of incubation at 37°C, 3 ml of the bacterium-egg mixture was diluted in phosphate-buffered saline (PBS), centrifuged, and plated on LB agar supplemented with 100 μg of ampicillin per ml. The surviving bacteria were scraped from the plates after overnight incubation and subjected to two more rounds of screening in egg albumen, each for 72 h at 37°C. Plasmid DNA from surviving colonies after the three rounds of screening was purified and transformed into fresh ST3744, and the resistance of the transformants was compared to that of ST3744 transformed with vector pRB3-273C.
Assay of survival of Salmonella in egg albumen.
Egg albumen was prepared as described above. An overnight culture of bacteria was added to 2 ml of albumen in an Eppendorf tube to a concentration of 1 × 103 to 2 × 103 CFU/ml and thoroughly mixed. The tube was incubated at 37°C, and 30-μl aliquots of the mixture were plated on LB agar plates at the beginning and after different periods of incubation. The surviving bacteria were enumerated after overnight incubation. The bacterial concentration (in CFU per milliliter) was calculated and plotted against time of incubation.
Construction of targeted mutants of SE2472.
yafD, yafE, and xthA deletion mutants were constructed by homologous recombination by using the RED recombinase system (8, 29). Since the efficiency of homologous recombination with short homologous sequences is much lower in S. enterica serovar Enteritidis than in E. coli, flanking DNA fragments longer than those previously reported were used (8, 29). The coding sequence of the gene to be mutated and surrounding intergenic regions were cloned into a pGEM-derived vector, and subsequently the coding region was replaced by a kanamycin resistance cassette (an XbaI fragment from pKD4). These manipulations generated a plasmid containing the kanamycin resistance cassette flanked by DNA homologous to the DNA that surrounded the gene to be mutated. More specifically, the plasmid pK3 insert (corresponding to nucleotides 295457 to 297792 of the S. enterica serovar Typhi genome), which contained the yafD and yafE genes, was subcloned into a pGEM-derived vector (Promega, Madison, Wis.). The xthA sequence (corresponding to nucleotides 1731061 to 1732478 of the S. enterica serovar Typhi genome) was amplified from the genomic DNA of SE2472 by PCR and cloned into the pGEM-derived vector. The sequence encoding YafD (except for the last four amino acids), YafE (except for the first four amino acids), or XthA (except for the first and last two amino acids) was replaced by the kanamycin resistance cassette. The homologous sequences in these constructs ranged from 100 bp to 1 kb long. The inserts containing the interrupted alleles of yafD, yafE, or xthA were amplified by PCR and used to construct the mutants. Prospective mutants were characterized by using primers outside the homologous regions and within the kanamycin resistance cassette, as described previously (8, 29). Once homologous recombination was confirmed, the deletion mutations were transduced into fresh cultures of SE2472 by using phage P22, and phage-free colonies were selected for further analysis. The ΔyafD mutation was also transduced into ST3744 with P22 for egg resistance assays.
Assays of survival of bacteria after exposure to hydrogen peroxide and UV irradiation.
Freshly transformed bacteria were cultured in 2 ml of LB broth at 37°C overnight with shaking. Antibiotics were added as appropriate. Twenty microliters of an overnight culture was added to 2 ml of LB broth containing 1 mM hydrogen peroxide. The cultures were grown at 37°C with shaking at 225 rpm. After exposure to hydrogen peroxide, aliquots of cultures were diluted in PBS and plated in triplicate. Bacterial colonies were enumerated by determining the number of CFU after overnight incubation.
The susceptibility of bacteria to UV irradiation was assayed by plating serial dilutions of bacteria on LB agar plates and exposing the plates to a 30-W germicidal lamp at a distance of 33 cm. A set of control plates was prepared without UV irradiation. All plates were then incubated at 37°C overnight, and the surviving bacteria were counted. The numbers of colonies on the UV-irradiated plates were compared to the numbers on unirradiated plates.
Nuclease activity of egg albumen.
Plasmid pBluescript II KS (Stratagene, La Jolla, Calif.) DNA was purified by the alkaline lysis method (1) and used to assay the nuclease activity of egg albumen. Egg albumen was prepared as described above for the bacterial survival assay. One microliter of pBluescript plasmid DNA (3 μg/μl) was mixed with 100 μl of egg albumen in PBS (various dilutions were used) and incubated at 37°C for 10 min. The reaction mixture was then extracted with phenol-chloroform, and 10 μl was removed from the aqueous phase for analysis by gel electrophoresis.
For analysis of nuclease activity in the active components of egg albumen, egg albumen was digested with 1.25 mg of protease K per ml for 2 h at 37°C and heated at 70°C for 20 min. In some samples, EDTA was added at a concentration of 10 mM to the egg albumen before plasmid DNA was added. Nuclease assays were carried out with egg albumen treated with protease K, with heat, with EDTA, or with a combination of these treatments. Nuclease assays with treated egg albumen were performed as described above for the nuclease assays performed with untreated egg albumen.
To test the effect of egg albumen on intracellular plasmid DNA, we exposed SE2472 or the ΔyafD mutant transformed with the pBluescript plasmid to egg albumen. SE2472(pBluescript II) or ΔyafD(pBluescript II) was inoculated into 150 ml of LB broth supplemented with 100 μg of ampicillin per ml and cultured overnight at 37°C with shaking. Fifty milliliters of each culture was used as an untreated sample. The remaining 100 ml of the overnight culture of SE2472(pBluescript II) or ΔyafD(pBluescript II) was spun down and washed with PBS. The bacteria were resuspended in 30 ml of egg albumen and incubated at 37°C for 24 h. After incubation, the bacteria were spun down and washed once with PBS. Plasmid DNA from all samples was purified by the alkaline lysis method (1) and analyzed by agarose gel electrophoresis. The amounts of nicked and supercoiled plasmid DNA were quantified by using the Quantity One gel imaging system (Bio-Rad, Richmond, Calif.).
RESULTS
Plasmid pK3 that contains yafD and yafE confers resistance to egg albumen on S. enterica serovar Typhimurium ST3744.
We developed an in vitro assay to determine the resistance of S. enterica serovar Enteritidis to egg albumen. In this assay, bacteria were mixed with egg albumen, and the survival of the bacteria was determined by plating. To identify genes that enhance the survival of S. enterica serovar Enteritidis in egg albumen, we employed a gain-of-function screening approach to identify DNA fragments that increased survival of an egg-susceptible isolate of bacteria in egg albumen. We transformed an egg-susceptible S. enterica serovar Typhimurium isolate, ST3744, with a Sau3A I genomic DNA library of egg-resistant S. enterica serovar Enteritidis isolate SE2472 constructed with plasmid vector pRB3-273C (4, 29). The transformants were subjected to three rounds of selection in egg albumen, and plasmid DNA from surviving colonies was isolated and transformed into a fresh culture of ST3744. The survival of ST3744 transformed with the plasmids was compared to the survival of the organisms transformed with the vector pRB3-273C.
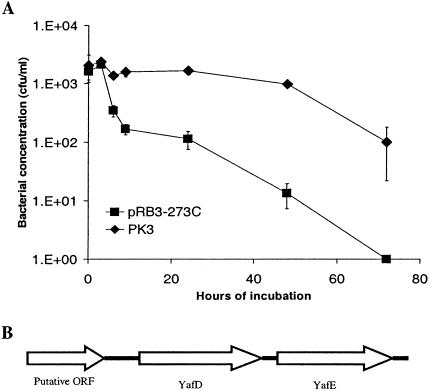
One plasmid, pK3, was found to consistently result in increased survival of ST3744 in egg albumen (Fig. 1A). This plasmid contained a 2.3-kb DNA insert. Sequence analysis indicated that the insert was comprised of two open reading frames homologous to yafD and yafE of E. coli and a partial open reading frame encoding a putative transmembrance efflux protein (http://www.ncbi.nih.gov) (Fig. 1B). Since the partial open reading frame encoded only 150 amino acids of the predicted 399 amino acids encoded by the open reading frame and since the insert lacked any promoter or regulatory sequence for the open reading frame, yafD or yafE or both were deemed likely to be responsible for the phenotype observed.
FIG. 1.
Plasmid pK3 enhanced the survival of S. enterica serovar Typhimurium ST3744 in egg albumen. (A) Survival of ST3744 transformed with plasmid pK3 or the vector pRB3-273C in egg albumen. ST3744 transformed with either plasmid pK3 or the vector pRB3-273C was incubated with egg albumen at 37°C. The survival of bacteria was determined and plotted against incubation time. At least three experiments were performed, and the results of a representative experiment performed in triplicate are shown. The error bars indicate standard deviations. (B) Open reading frames identified in the insert of plasmid pK3.
yafD gene is necessary for SE2472 resistance to egg albumen.
Since yafD or yafE enhanced the survival of ST3744 in egg albumen, we next determined if yafD or yafE or both are necessary for the survival of S. enterica serovar Enteritidis in egg albumen. yafD and yafE deletion mutants were constructed by homologous recombination by using the RED recombinase system (8, 29), in which the yafD or yafE coding sequence was replaced by a kanamycin resistance cassette (Kanr). Successful disruption of these genes by homologous recombination was confirmed by PCR performed with primers external to the homologous regions and within the kanamycin resistance cassette and by sequencing of the junction regions. Mutations were then transduced into fresh SE2472 with bacterial phage P22, and phage-free colonies were selected for further analysis.
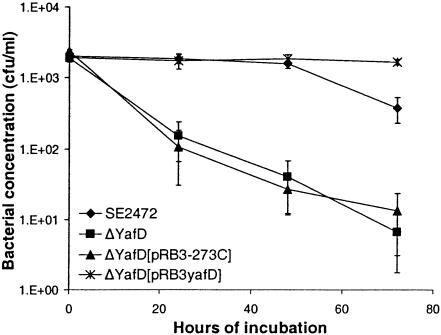
The ΔyafD and ΔyafE mutants of SE2472 had the same morphology on LB agar plates. Both of the mutants had the same growth kinetics as wild-type strain SE2472 in both LB broth and M9 minimal medium, indicating that they did not have general growth defects (data not shown). The susceptibility of the ΔyafD and ΔyafE mutants of SE2472 to egg albumen was compared to that of wild-type strain SE2472. The ΔyafE mutant of SE2472 had a survival pattern indistinguishable from that of wild-type strain SE2472 (data not shown), while the ΔyafD mutant was significantly more susceptible to egg albumen (Fig. 2). The susceptibility was detectable after 24 h of incubation and was even more evident after 48 h. After 72 h of incubation, the concentration of the ΔyafD mutant or the pRB3-273C vector-transformed mutant had decreased by approximately 100-fold, while the concentration of wild-type strain SE2472 had decreased by less than 10-fold compared with the concentration in the initial inoculum. Therefore, there was a more-than-10-fold difference in survival between the wild type and the ΔyafD mutant. The pRB3yafD plasmid (which contained the upstream and coding regions of yafD cloned in the pRB3-273C vector) fully restored resistance to egg albumen to the ΔyafD mutant, while the vector pRB3-273C did not change the susceptibility of the ΔyafD mutant. Interestingly, the plasmid pRB3yafD-transformed ΔyafD mutant survived better than the wild-type strain SE2472 survived after prolonged incubation (72 h) (Fig. 2). This was likely due to a gene dosage effect of the multicopy plasmid, providing further evidence that YafD plays a role in resistance to egg albumen. This is also consistent with our initial observation that plasmid pK3 enhanced the survival of ST3744 in egg albumen.
FIG. 2.
Resistance of the ΔyafD mutant of SE2472 to egg albumen. Wild-type strain SE2472, the ΔyafD mutant, and vector pRB3-273C- and plasmid pRB3yafD-transformed ΔyafD mutants were incubated with egg albumen at 37°C. The survival of bacteria was determined by plating, and the results were plotted against incubation time. At least three experiments were performed, and the results of a representative experiment performed in triplicate are shown. The error bars indicate standard deviations.
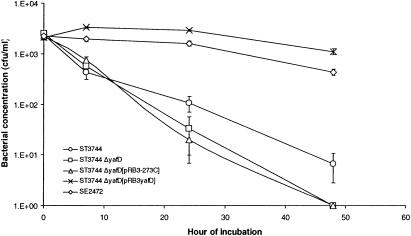
We also tested whether yafD is necessary for survival of the egg-susceptible S. enterica serovar Typhimurium isolate ST3744 in egg albumen. The ΔyafD mutation was transduced into ST3744, and the mutant was analyzed to determine its resistance to egg albumen. As shown in Fig. 3, deletion of yafD from ST3744 further decreased the survival of the bacterial strain in egg albumen only slightly. Not only did complementation of the mutation with plasmid pRB3yafD increase the survival of the ΔyafD mutant of ST3744 in egg albumen, but the complemented mutant also exhibited much better survival in egg albumen than the wild-type parental strain exhibited. This again confirmed that overexpression of YafD increased survival of Salmonella in egg albumen.
FIG. 3.
Resistance of the ΔyafD mutant of ST3744 to egg albumen. Wild-type strain SE2472, wild-type strain ST3744, the ΔyafD mutant of strain ST3744, and vector pRB3-273C- and plasmid pRB3yafD-transformed ΔyafD mutants were incubated with egg albumen at 37°C. The survival of bacteria was determined by plating, and the results were plotted against incubation time. At least three experiments were performed, and the results of a representative experiment performed in triplicate are shown. The error bars indicate standard deviations.
YafD is homologous to members of an endonuclease-exonuclease-phosphatase family.
Homologs of YafD are present in E. coli, S. enterica serovar Typhimurium, and S. enterica serovar Typhi. The function of yafD has not been studied genetically and therefore is unknown (http://www.ncbi.nih.gov). In S. enterica serovar Typhimurium, the yafD locus is located near 6 min on the genome in the region of tRNA and rRNA genes, and it is not located within the known pathogenicity islands (16). The chromosomal location of yafD in S. enterica serovar Enteritidis has not been reported. However, given the similarity in genome organization between S. enterica serovar Typhimurium and S. enterica serovar Enteritidis, yafD is expected to localize to the corresponding region in S. enterica serovar Enteritidis.
On the basis of the amino acid sequence encoded by yafD, the protein is predicted to be a possible cytoplasmic protein, and this protein exhibits sequence homology to members of anendonuclease-exonuclease-phosphatase family (gnl|CDD|8588,pfam03372). The overall levels of homology to the consensus sequence of the family are 22% identity and 36% similarity for 216 amino acids of the 259-amino-acid reading frame (Fig. 4). The endonuclease-exonuclease-phosphatase family includes magnesium-dependent endonucleases, apurinidic-apirimidinic endonucelases (AP endonucleases), and phosphatases. Endonucleases control the restriction systems (37), and phosphatases are involved in intracellular signaling. AP endonculeases are DNA repair enzymes that remove debased nucleotides in DNA and allow repair of damaged DNA (28).
FIG. 4.
Comparison of the amino acid sequence of S. enterica serovar Enteritidis YafD and the consensus sequence of an endonuclease-exonuclease-phosphatase family (EEP) (gnl|CDD|8588, pfam03372). The residues with a black background are identical in the two sequences, and the residues with a gray background are similar.
ΔyafD mutant of S. enterica serovar Enteritidis is more susceptible to DNA-damaging conditions.
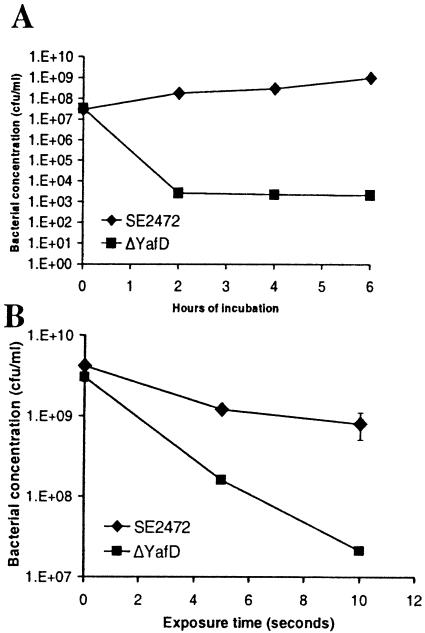
Since YafD has sequence homology to members of the endonuclease-exonuclease-phosphatase family, some of which are DNA repair enzymes, we explored the possibility that YafD is involved in DNA repair. If this is true, YafD may be involved in resistance to other conditions that induce DNA damage, such as exposure to hydrogen peroxide and UV irradiation. To test this hypothesis, we exposed the ΔyafD mutant of S. enterica serovar Enteritidis to hydrogen peroxide and UV irradiation and compared its survival to that of wild-type parental strain SE2472. As expected, the ΔyafD mutant was much more susceptible than wild-type strain SE2472 to both hydrogen peroxide and UV irradiation (Fig. 5). Therefore, these results demonstrate that yafD is necessary for S. enterica serovar Enteritidis to resist hydrogen peroxide and UV irradiation. In contrast to the egg albumen survival assay, in which yafD expressed from plasmid pRB3yafD fully restored the resistance of the ΔyafD mutant, the same plasmid increased the survival of the ΔyafD mutant in hydrogen peroxide and UV irradiation assays but did not restore the survival to the wild-type level (data not shown). This suggests that although YafD is necessary for S. enterica serovar Enteritidis to resist stresses caused by egg albumen, hydrogen peroxide, and UV irradiation, the exact lesions of the damaged DNA, the repair of the lesions, and the regulation of yafD under these stress conditions are not identical.
FIG. 5.
Resistance of the wild type and the ΔyafD mutant of SE2472 to hydrogen peroxide (A) and UV irradiation (B). (A) The wild-type and ΔyafD mutant of strain SE2472 were incubated in LB broth containing 1 mM hydrogen peroxide at 37°C. The survival of bacteria was determined by plating, and the results were plotted against incubation time. (B) Serial dilutions of the wild-type and ΔyafD mutant of SE2472 were plated on LB agar plates and exposed to UV irradiation. The concentrations of surviving bacteria were plotted against exposure time. At least three experiments were performed, and the results of a representative experiment performed in triplicate are shown. The error bars indicate standard deviations and are within the symbols in some instances.
Egg albumen has nuclease activity.
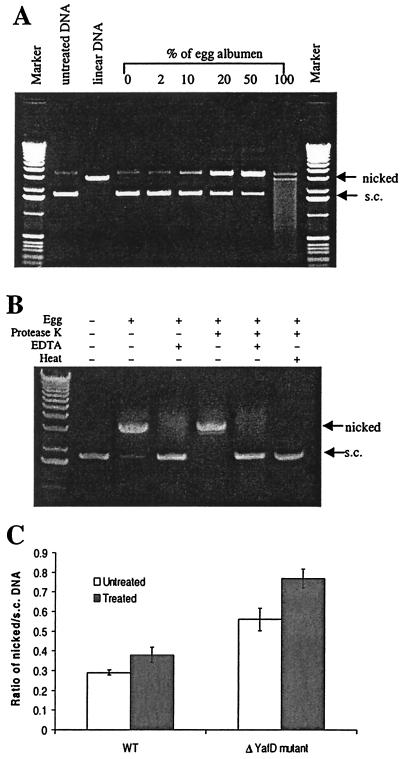
We showed that yafD of S. enterica serovar Enteritidis is necessary for resistance of this organism to egg albumen and to DNA-damaging conditions, such as exposure to hydrogen peroxide and UV irradiation. However, no DNA-damaging activity has been reported for egg albumen, and there has been no report of a role of DNA repair in bacterial resistance to egg albumen. To investigate the possibility that egg albumen causes DNA damage, we performed a nuclease assay with egg albumen. Supercoiled pBluescript plasmid DNA was incubated with different concentrations of egg albumen, the endonuclease activity was detected by examining the conversion of supercoiled DNA to nicked or linear DNA, and the exonuclease activity was detected by examining the degradation of linear DNA. As shown in Fig. 6A, conversion of supercoiled DNA to nicked DNA increased as the concentration of egg albumen used to treat the plasmid DNA increased. In the sample treated with undiluted egg albumen, all supercoiled DNA was converted to nicked and linear DNA, and there was degradation of linear DNA as well. Thus, egg albumen has both endonuclease and exonuclease activities.
FIG. 6.
Nuclease activities of egg albumen. (A) Electrophoresis analysis of the effects of egg albumen on naked plasmid DNA. Supercoiled pBluescript II KS DNA (s.c.) was incubated with different concentrations of egg albumen for 10 min at 37°C. (B) Effect of egg albumen on DNA in conjunction with protease K, EDTA, and heat treatments. Plasmid pBluescript II was incubated with untreated egg albumen or egg albumen treated as indicated at the top of the gel. The treatments included one or more of the following: protease K, EDTA, and heat. The DNA-nicking activity was measured by the conversion of supercoiled DNA to nicked DNA. (C) Effect of egg albumen on intracellular pBluescript II DNA. Plasmid DNA was purified from wild-type strain SE2472 (WT) or the ΔyafD mutant before treatment with egg albumen (untreated) or after treatment with egg albumen (treated). Purified DNA was electrophoresed on agarose gels in triplicate, and the ratio of nicked DNA to supercoiled DNA was used to determine the nicking of plasmid DNA. At least three experiments were performed, and the results of a representative experiment performed in triplicate are shown. The error bars indicate standard deviations.
We next probed the chemical nature of the nuclease activities. To determine if the nuclease activities are from proteins, we performed the nuclease assay with heat-inactivated egg albumen and in the presence of EDTA, which is a chelator of divalent ions that are necessary for most nucleases. Since heating egg albumen causes coagulation and renders it unsuitable for further analysis, we first treated the egg albumen with protease K to prevent coagulation caused by heating (75°C for 20 min). The protease K digestion alone did not eliminate the nuclease activity of egg albumen (Fig. 6B) or affect the plasmid DNA (data not shown). However, either heat treatment or addition of EDTA eliminated the nuclease activities, indicating that the nuclease activities in egg albumen are likely due to a protein(s) (Fig. 6B). The nuclease activities were resistant to protease K, probably due to the fact that a large amount of small proteins remained detectable by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis after protease digestion (data not shown). Therefore, egg albumen contains small, protease K-resistant proteins that have nuclease activities, which is a novel antimicrobial mechanism of egg albumen.
To test if egg albumen can affect intracellular DNA and if yafD protects bacteria from the nuclease activity of the egg albumen, we exposed either the wild type or the ΔyafD mutant transformed with the pBluescript plasmid to egg albumen for 24 h and analyzed the plasmid DNA after exposure. The nicking of plasmid DNA was measured by determining the ratio of nicked plasmid DNA to supercoiled plasmid DNA. The DNA of plasmid pBluescript purified from the untreated ΔyafD mutant bacteria showed more nicking than the DNA of plasmid pBluescript purified from wild-type S. enterica serovar Enteritidis showed (ratios of nicked DNA to supercoiled DNA, 0.560 ± 0.038 and 0.289 ± 0.012, respectively). The plasmid from the egg albumen-treated ΔyafD mutant also had a much higher ratio of nicked DNA to supercoiled DNA than the plasmid purified from the wild-type bacteria treated with egg albumen had (0.768 ± 0.047 and 0.379 ± 0.038, respectively). Since the plasmids from both the wild-type and the ΔyafD mutant bacteria displayed increased nicking after exposure to egg albumen (the ratios of nicked DNA to supercoiled DNA after treatment and before treatment were 0.379 ± 0.038 and 0.289 ± 0.012, respectively, for the wild type and 0.768 ± 0.047 and 0.560 ± 0.038, respectively, for the ΔyafD mutant), these results indicate that exposure to egg albumen caused nicking of the intracellular plasmid. The plasmid from the ΔyafD mutant showed a higher degree of nicking than the plasmid from the wild-type bacteria (Fig. 6C), suggesting that YafD may be necessary for repairing the nicked DNA.
ΔxthA mutant of S. enterica serovar Enteritidis is more susceptible to egg albumen.
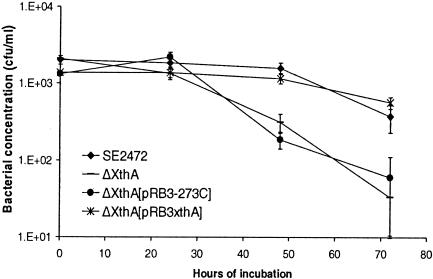
To further demonstrate that DNA repair is necessary for S. enterica serovar Enteritidis to resist egg albumen, we generated a targeted mutation at the xthA locus, encoding the exonuclease III, which is the main AP endonuclease of bacteria (28). The coding sequence of xthA was replaced by a kanamycin resistance gene by using the Red recombinase system (8, 29). The resistance of the Δxth mutant of S. enterica serovar Enteritidis to egg albumen was tested in survival assays and compared to that of wild-type parental strain SE2472. As shown in Fig. 7, the ΔxthA mutant was significantly more susceptible than strain SE2472 to egg albumen. To complement the ΔxthA mutant, we cloned a copy of the xthA gene into plasmid pRB3-273C and transformed the resulting plasmid, pBR3xthA, into the ΔxthA mutant. Plasmid pRB3xthA restored the resistance of the ΔxthA mutant to egg albumen, while the vector pRB3-273C-transformed mutant remained as susceptible as the untransformed ΔxthA mutant. This indicates that the DNA repair enzyme exonuclease III is necessary for S. enterica serovar Enteritidis to resist egg albumen and supports the notion that one of the antimicrobial mechanisms of egg albumen involves damage of DNA.
FIG. 7.
Resistance of the ΔxthA mutant of SE2472 to egg albumen. Wild-type strain SE2472, the ΔxthA mutant, and vector pRB3-273C- and plasmid pRB3xthA-transformed ΔxthA mutants were incubated with egg albumen at 37°C. The survival of bacteria was determined by plating, and the bacterial concentration was plotted against incubation time. At least three experiments were performed, and the results of a representative experiment performed in triplicate are shown. The error bars indicate standard deviations and are within the symbols in some instances.
DISCUSSION
S. enterica serovar Enteritidis is unique among Salmonella serovars because it frequently contaminates the contents of eggs. Most cases of human infection result from the consumption of contaminated raw eggs (18, 19, 32, 35). Other serovars of Salmonella, including S. enterica serovar Typhimurium, may infect chickens, but they do not persist inside the eggs and eggs are not commonly implicated as vehicles in human infections caused by S. enterica serovar Typhimurium (27, 32, 38). Therefore, survival in eggs is very important for the transmission of S. enterica serovar Enteritidis. To our knowledge, the molecular mechanisms of the resistance to eggs and the genetic factors involved have not been reported previously. Here we describe an analysis of the molecular basis of the resistance of S. enterica serovar Enteritidis to eggs and identification of the yafD gene as a bacterial determinant required for this resistance.
Egg albumen restricts the growth and survival of bacteria. The restrictive effects of egg albumen on Salmonella growth are primarily due to iron limitation generated by ovotransferrin (2, 7). Ovotransferrin is a potent iron chelator, and it inhibits bacterial growth by binding free iron and keeping it inaccessible to bacteria (2, 7). Another major antimicrobial protein in egg albumen is believed to be lysozyme. This protein is a muramidase and affects some gram-positive bacteria (33, 40), although it is considered unable to penetrate the outer membrane of gram-negative bacteria (9, 33, 40). In recent years, there have been reports of pore-forming activities that are novel antimicrobial properties of ovotransferrin and lysozyme. Ovotransferrin has been shown to have bactericidal activity independent of its iron chelation properties (23, 24). A cationic peptide in the N lobe of ovotransferrin was found to cross the outer membrane of gram-negative bacteria and damage the cytoplasmic membrane (23, 24). Lysozyme has also been shown to form pores in gram-negative bacteria through cationic and hydrophobic properties. This activity is independent of the muramidase activity (25, 36).
In this study, we identified DNA damage as a novel mechanism of the bactericidal activity of egg albumen. Both endonuclease and exonuclease activities were detected by incubating egg albumen with circular supercoiled plasmid DNA. In vitro assays indicated that egg albumen converts supercoiled plasmid DNA to nicked and linear DNA and eventually degrades DNA (Fig. 6A and B). In addition, intracellular plasmid DNA showed increased nicking after exposure to egg albumen (Fig. 6C), suggesting that it is possible that genomic DNA of bacteria is susceptible to the nuclease activities of egg albumen as well. Consistent with the nuclease activities of egg albumen, YafD, which exhibits sequence homology with nucleases, including AP endonucleases, is necessary for S. enterica serovar Enteritidis to survive in egg albumen. To further examine the notion that DNA repair is necessary for the resistance to egg albumen, we generated a targeted mutation of xthA, which encodes the main AP endonuclease of bacteria, and found that the ΔxthA mutant was also more susceptible to egg albumen (Fig. 7). This indicates that damaging bacterial DNA is one of the mechanisms that egg albumen uses to control bacteria and that DNA repair enzymes are involved in repairing lesions generated by egg albumen. The nuclease activities of egg albumen are likely to be mediated by a protein(s), since heat inactivation and addition of EDTA eliminated the nuclease activities. The chemical identities of the nuclease activities, the exact types of DNA lesions caused by egg albumen, and how the nucleases of egg albumen gain entry into bacteria are not yet understood. One possible explanation for the effect that the egg albumen nucleases have on bacterial DNA is that they gain entry into bacterial cells through the pores formed by lysozyme and ovotransferrin in the cell wall and subsequently damage the chromosomal DNA.
We demonstrated that yafD is essential for survival of S. enterica serovar Enteritidis in egg albumen. To our knowledge, yafD is the first gene shown to be essential for bacterial survival in egg albumen. Overexpression of YafD from a plasmid (pK3) conferred resistance upon the egg-susceptible S. enterica serovar Typhimurium isolate ST3744, and the ΔyafD mutant of S. enterica serovar Enteritidis SE2472 was more susceptible to egg albumen. The ΔyafD mutant exhibited normal growth kinetics in both LB broth and M9 minimal medium, indicating that its susceptibility to egg albumen is not due to general growth defects. YafD exhibits sequence homology with members of an exonuclease-endonuclease-phosphatase family, some of which are involved in DNA repair. Although this homology does not reveal the function of YafD because of the large number of proteins in the family and their diverse functions, we hypothesize that YafD may function in DNA repair and facilitate the repair of lesions generated by egg albumen. Consistent with the hypothesized role in DNA repair, the ΔyafD mutant is more susceptible to DNA-damaging conditions, such as exposure to hydrogen peroxide and UV irradiation. Although hydrogen peroxide, UV irradiation, and egg albumen are expected to cause different lesions in DNA, YafD may be involved in common aspects of the repair of these lesions.
The yafD gene is found in many species of bacteria, including E. coli and other serovars of Salmonella, such as S. enterica serovar Typhi and S. enterica serovar Typhimurium. Although S. enterica serovar Enteritidis is a human-pathogenic serovar that is uniquely associated with egg albumen, yafD coding sequences from several isolates of S. enterica serovar Typhimurium and S. enterica serovar Enteritidis are identical except at the nucleotides that encode amino acid 33, which is serine in S. enterica serovar Enteritidis and asparagine in S. enterica serovar Typhimurium. It has not been determined if the differences in sequence and possibly regulation of yafD in S. enterica serovar Enteritidis and S. enterica serovar Typhimurium contribute to the increased resistance of S. enterica serovar Enteritidis to egg albumen. It is also highly likely that the resistance of S. enterica serovar Enteritidis to egg albumen is mediated by multiple factors in addition to yafD. Nevertheless, we have identified a new genetic determinant of S. enterica serovar Enteritidis that may be essential for the organism's widespread epidemiologic association with egg products.
Acknowledgments
We thank Stuart Linn and Hiroshi Nikaido of the University of California at Berkeley for their invaluable suggestions and advice. We also thank Barry Wanner of Purdue University for the reagents of the Red recombinase system and Sharon Abbott of the State of California Department of Health Services for the Salmonella isolates.
This study was supported by grants AI43032 and USDA 2002-35201-11543 to L.W.R.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Ausubel, F., R. Brent, R. Kingston, D. Moore, J. Smith, and K. Struhl. 1997. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 2.Baron, F., M. Gautier, and G. Brule. 1997. Factors involved in the inhibition of growth of Salmonella enteritidis in liquid egg white. J. Food Prot. 60:1318-1323. [DOI] [PubMed] [Google Scholar]
- 3.Baumler, A. J., B. M. Hargis, and R. M. Tsolis. 2000. Tracing the origins of Salmonella outbreaks. Science 287:50-52. [DOI] [PubMed] [Google Scholar]
- 4.Berggren, R. E., A. Wunderlich, E. Ziegler, M. Schleicher, R. C. Duke, D. Looney, and F. C. Fang. 1995. HIV gp120-specific cell-mediated immune responses in mice after oral immunization with recombinant Salmonella. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10:489-495. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2003. Outbreaks of Salmonella serotype Enteritidis infection associated with eating shell eggs—United States, 1999-2001. JAMA 289:540-541. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2000. Surveillance for foodborne disease outbreaks—United States, 1993-1997. Morb. Mortal. Wkly. Rep. 49:1-72. [PubMed] [Google Scholar]
- 7.Chart, H., and B. Rowe. 1993. Iron restriction and the growth of Salmonella enteritidis. Epidemiol. Infect. 110:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, B. D., R. Dulbecco, H. N. Eisen, and H. S. Ginsberg. 1980. Microbiology, 3rd ed. Harper & Row, Hagerstown, Md.
- 10.Ebel, E., and W. Schlosser. 2000. Estimating the annual fraction of eggs contaminated with Salmonella enteritidis in the United States. Int. J. Food Microbiol. 61:51-62. [DOI] [PubMed] [Google Scholar]
- 11.Food Safety and Inspection Service. 1998. Salmonella enteritidis risk assessment for shell eggs and egg products. Final report. U.S. Department of Agriculture, Washington, D.C.
- 12.Gast, R. K., and C. W. Beard. 1990. Isolation of Salmonella enteritidis from internal organs of experimentally infected hens. Avian Dis. 34:991-993. [PubMed] [Google Scholar]
- 13.Gast, R. K., and C. W. Beard. 1990. Production of Salmonella enteritidis-contaminated eggs by experimentally infected hens. Avian Dis. 34:438-446. [PubMed] [Google Scholar]
- 14.Gast, R. K., J. Guard-Petter, and P. S. Holt. 2002. Characteristics of Salmonella enteritidis contamination in eggs after oral, aerosol, and intravenous inoculation of laying hens. Avian Dis. 46:629-635. [DOI] [PubMed] [Google Scholar]
- 15.Gast, R. K., and P. S. Holt. 2001. Assessing the frequency and consequences of Salmonella enteritidis deposition on the egg yolk membrane. Poult. Sci. 80:997-1002. [DOI] [PubMed] [Google Scholar]
- 16.Groisman, E. A., and H. Ochman. 1997. How Salmonella became a pathogen. Trends Microbiol. 5:343-349. [DOI] [PubMed] [Google Scholar]
- 17.Guard-Petter, J. 2001. The chicken, the egg and Salmonella enteritidis. Environ. Microbiol. 3:421-430. [DOI] [PubMed] [Google Scholar]
- 18.Hennessy, T. W., C. W. Hedberg, L. Slutsker, K. E. White, J. M. Besser-Wiek, M. E. Moen, J. Feldman, W. W. Coleman, L. M. Edmonson, K. L. MacDonald, and M. T. Osterholm. 1996. A national outbreak of Salmonella enteritidis infections from ice cream. The Investigation Team. N. Engl. J. Med. 334:1281-1286. [DOI] [PubMed] [Google Scholar]
- 19.Henzler, D. J., E. Ebel, J. Sanders, D. Kradel, and J. Mason. 1994. Salmonella enteritidis in eggs from commercial chicken layer flocks implicated in human outbreaks. Avian Dis. 38:37-43. [PubMed] [Google Scholar]
- 20.Herikstad, H., Y. Motarjemi, and R. V. Tauxe. 2002. Salmonella surveillance: a global survey of public health serotyping. Epidemiol. Infect. 129:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humphrey, T. J., H. Chart, A. Baskerville, and B. Rowe. 1991. The influence of age on the response of SPF hens to infection with Salmonella enteritidis PT4. Epidemiol. Infect. 106:33-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphrey, T. J., A. Whitehead, A. H. Gawler, A. Henley, and B. Rowe. 1991. Numbers of Salmonella enteritidis in the contents of naturally contaminated hens' eggs. Epidemiol. Infect. 106:489-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim, H. R., E. Iwamori, Y. Sugimoto, and T. Aoki. 1998. Identification of a distinct antibacterial domain within the N-lobe of ovotransferrin. Biochim. Biophys. Acta 1401:289-303. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim, H. R., Y. Sugimoto, and T. Aoki. 2000. Ovotransferrin antimicrobial peptide (OTAP-92) kills bacteria through a membrane damage mechanism. Biochim. Biophys. Acta 1523:196-205. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim, H. R., U. Thomas, and A. Pellegrini. 2001. A helix-loop-helix peptide at the upper lip of the active site cleft of lysozyme confers potent antimicrobial activity with membrane permeabilization action. J. Biol. Chem. 276:43767-43774. [DOI] [PubMed] [Google Scholar]
- 26.Keller, L. H., C. E. Benson, K. Krotec, and R. J. Eckroade. 1995. Salmonella enteritidis colonization of the reproductive tract and forming and freshly laid eggs of chickens. Infect. Immun. 63:2443-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller, L. H., D. M. Schifferli, C. E. Benson, S. Aslam, and R. J. Eckroade. 1997. Invasion of chicken reproductive tissues and forming eggs is not unique to Salmonella enteritidis. Avian Dis. 41:535-539. [PubMed] [Google Scholar]
- 28.Lloyd, R. S., and S. Linn. 1993. Nucleases involved in DNA repair, p. 263-316. In S. Linn, R. S. Lloyd, and R. J. Roberts (ed.), Nucleases, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Lu, S., P. B. Killoran, F. C. Fang, and L. W. Riley. 2002. The global regulator ArcA controls resistance to reactive nitrogen and oxygen intermediates in Salmonella enterica serovar Enteritidis. Infect. Immun. 70:451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu, S., A. R. Manges, Y. Xu, F. C. Fang, and L. W. Riley. 1999. Analysis of virulence of clinical isolates of Salmonella enteritidis in vivo and in vitro. Infect. Immun. 67:5651-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maloy, S. R., V. J. Stewart, and R. K. Taylor. 1996. Genetic analysis of pathogenic bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakae, T., and H. Nikaido. 1975. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J. Biol. Chem. 250:7359-7365. [PubMed] [Google Scholar]
- 34.National Agricultural Statistic Service, U.S. Department of Agriculture. 1999. Layers and egg production, 1998 summary. U.S. Department of Agriculture, Washington, D.C.
- 35.Olsen, S. J., L. C. MacKinnon, J. S. Goulding, N. H. Bean, and L. Slutsker. 2000. Surveillance for foodborne disease outbreaks—United States, 1993-1997. Morb. Mortal. Wkly Rep. CDC Surveill. Summ. 49:1-62. [PubMed] [Google Scholar]
- 36.Pellegrini, A., U. Thomas, P. Wild, E. Schraner, and R. von Fellenberg. 2000. Effect of lysozyme or modified lysozyme fragments on DNA and RNA synthesis and membrane permeability of Escherichia coli. Microbiol. Res. 155:69-77. [DOI] [PubMed] [Google Scholar]
- 37.Roberts, R. J., and S. E. Halford. 1993. Type II restriction endonucleases, p. 35-88. In S. Linn, R. S. Lloyd, and R. J. Roberts (ed.), Nucleases, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.SE Pilot Project. 1995. Salmonella enteritidis Pilot Project progress report. U.S. Department of Agriculture, Lancaster, Pa.
- 39.Shivaprasad, H. L., J. F. Timoney, S. Morales, B. Lucio, and R. C. Baker. 1990. Pathogenesis of Salmonella enteritidis infection in laying chickens. I. Studies on egg transmission, clinical signs, fecal shedding, and serologic responses. Avian Dis. 34:548-557. [PubMed] [Google Scholar]
- 40.Spitznagel, J. K. 1984. Nonoxidative antimicronial reactions of leukocytes, p. 283-343. In E. Snyderman (ed.), Regulation of leukocyte function. Contemp. Top. Immunol. 14: 283-343. [DOI] [PubMed] [Google Scholar]