Abstract
3-Deoxy-3-fluoro-d-glucopyranose crystallizes from acetone to give a unit cell containing two crystallographically independent molecules. One of these molecules (at site A) is structurally homogeneous and corresponds to 3-deoxy-3-fluoro-β-d-glucopyranose, C6H11FO5, (I). The second molecule (at site B) is structurally heterogeneous and corresponds to a mixture of (I) and 3-deoxy-3-fluoro-α-d-glucopyranose, (II); treatment of the diffraction data using partial-occupancy oxygen at the anomeric center gave a high-quality packing model with an occupancy ratio of 0.84:0.16 for (II):(I) at site B. The mixture of α- and β-anomers at site B appears to be accommodated in the lattice because hydrogen-bonding partners are present to hydrogen bond to the anomeric OH group in either an axial or equatorial orientation. Cremer–Pople analysis of (I) and (II) shows the pyranosyl ring of (II) to be slightly more distorted than that of (I) [θ(I) = 3.85 (15)° and θ(II) = 6.35 (16)°], but the general direction of distortion is similar in both structures [ϕ(I) = 67 (2)° (B C1,C4) and ϕ(II) = 26.0 (15)° (C3 TB C1); B = boat conformation and TB = twist-boat conformation]. The exocyclic hydroxymethyl (–CH2OH) conformation is gg (gauche–gauche) (H5 anti to O6) in both (I) and (II). Structural comparisons of (I) and (II) to related unsubstituted, deoxy and fluorine-substituted monosaccharides show that the gluco ring can assume a wide range of distorted chair structures in the crystalline state depending on ring substitution patterns.
Comment
Fluorosugars (Taylor, 1988 ▶) are saccharide derivatives in which one or more of the hydroxy groups or H atoms in the saccharide are substituted by fluorine. For example, substitution at the anomeric OH group, giving glycosyl fluorides, activates the anomeric center in chemical glycosylation reactions (Yokoyama, 2000 ▶). The altered internal electronic structure in fluorosugars relative to the parent (unsubstituted) saccharide renders them useful for the investigation of enzyme reaction mechanisms, since such substitution can be exploited for the stabilization of putative intermediates along a reaction trajectory (White et al., 1996 ▶). In addition, substitution of fluorine for hydroxy groups or H atoms alters the hydrogen-bonding character of saccharides, thus affecting their binding properties with various receptors (Buchini et al., 2008 ▶).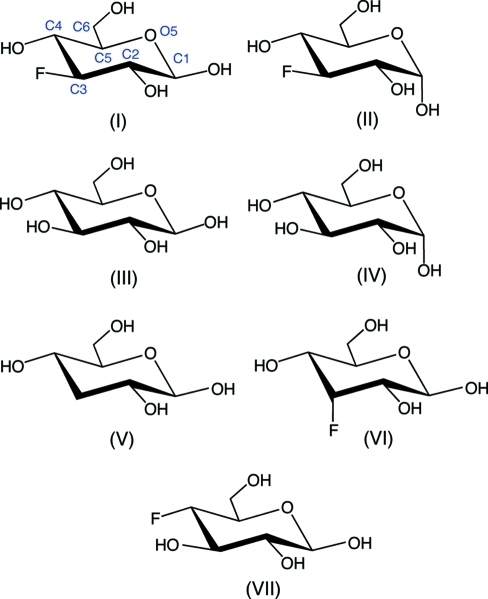
We have been preparing fluorosugars as tools to investigate the mechanisms of protein-bound saccharide rearrangements that accompany non-enzyme-catalyzed protein glycation (Chetyrkin et al., 2008 ▶). We reported recently the X-ray crystal structure of 4-deoxy-4-fluoro-β-d-glucopyranose (4DFG) and showed that its packing and that of the parent β-d-glucopyranose are very similar (Zhang et al., 2010 ▶). Furthermore, we showed that the anomeric configuration of 4DFG molecules in the crystal lattice is not completely homogeneous; careful fitting of the electron density at O1 showed that a small percentage (<5%) of the α-anomer appears to be accommodated in the lattice.
We report herein the X-ray crystal structure of 3-deoxy-3-fluoro-d-glucopyranose (3DFG) crystallized from acetone (Fig. 1 ▶). The crystalline lattice contains two independent molecules at two distinct sites, sites A and B. Site A is occupied by a single structurally homogeneous form of 3DFG, namely 3-deoxy-3-fluoro-β-d-glucopyranose, (I). Site B, however, is structurally heterogeneous, containing an 84:16 ratio of 3-deoxy-3-fluoro-α-d-glucopyranose, (II), and (I) based on a partial-occupancy fitting of the model to the diffraction data. In comparison, 1H and 13C NMR analyses of 3DFG in 2H2O solvent show that the α- and β-pyranoses are present in an approximate 47:53 (α:β) ratio (Zhang & Serianni, private communication).
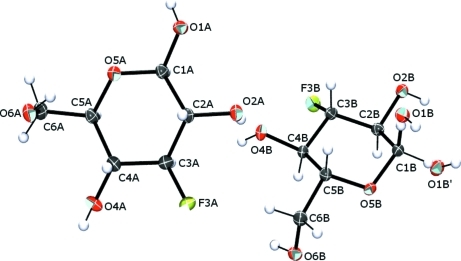
Figure 1.
Labeling scheme for molecules A and B in the title compound. Displacement ellipsoids are depicted at the 50% probability level. Atoms O1B and O1B′ are the disordered hydroxy groups corresponding with compounds (II) and (I), respectively, at site B.
In this paper, the X-ray crystal structures of (I) and (II) are compared to those of related unsubstituted, deoxy and fluorine-substituted saccharides: β-d-glucopyranose, (III) (Kouwijzer et al., 1995 ▶); α-d-glucopyranose, (IV) (Mostad, 1994 ▶); 3-deoxy-β-d-ribo-hexopyranose, (V) (Zhang et al., 2007 ▶); 3-deoxy-3-fluoro-β-d-allopyranose, (VI) (Myers et al., 1997 ▶); 4-deoxy-4-fluoro-β-d-glucopyranose, (VII) (Zhang et al., 2010 ▶). While the model for (II) represent a weighted average of contributions made by both the α- and β-pyranose forms of 3DFG, the contribution from the α-anomer is highly dominant, thus justifying a comparison to α-d-glucopyranose, (IV). Throughout the following discussion we refer to structure (II) with the knowledge that this structure is not homogeneous, thus comparisons involving (II) are made with this limitation in mind.
A comparison of several structural parameters obtained from X-ray crystal structure analyses of (I)–(VII) is shown in Table 1 ▶. Corresponding C—C and C—O bond lengths in (I) and (II) are comparable, except for r C1,O5, which is substantially longer in (II) [1.4383 (19) Å] than in (I) [1.4176 (19) Å]. Interestingly, r C1,O1 in (I) and (II) are very similar. In contrast, both r C1,O5 and r C1,O1 are minimally affected by the anomeric configuration in unsubstituted d-glucopyranoses (III) and (IV). The C—F bond length averages 1.400 (4) Å in (I), (II) and (VII), whereas r C3,F is 1.414 (2) Å in (VI). Thus, axial C—F bonds appear elongated by 0.01 Å relative to the same bond in an equatorial orientation.
Table 1. Comparison of structural parameters in (I)–(VII).
| β-3DFGlcp, (I) | α-3DFGlcp, (II) | β-Glcp, (III) | α-Glcp, (IV) | β-3DGlcp, (V) | β-3DFAllp, (VI) | β-4DFGlcp, (VII) | |
|---|---|---|---|---|---|---|---|
| Bond lengths (Å) | |||||||
| C1—C2 | 1.529 (2) | 1.529 (2) | 1.511 (4) | 1.535 (2) | 1.5250 (18) | 1.520 (3) | 1.5208 (18) |
| C2—C3 | 1.521 (2) | 1.519 (2) | 1.513 (4) | 1.521 (2) | 1.5216 (19) | 1.513 (2) | 1.5286 (19) |
| C3—C4 | 1.519 (2) | 1.517 (2) | 1.531 (4) | 1.523 (2) | 1.5295 (18) | 1.515 (2) | 1.5158 (19) |
| C4—C5 | 1.534 (2) | 1.530 (2) | 1.519 (4) | 1.526 (2) | 1.5329 (18) | 1.528 (3) | 1.5348 (18) |
| C5—C6 | 1.511 (2) | 1.519 (2) | 1.513 (4) | 1.510 (2) | 1.5088 (18) | 1.523 (3) | 1.515 (2) |
| C1—O1 | 1.388 (2) | 1.382 (2) | 1.394 (4) | 1.390 (3) | 1.4005 (17) | 1.390 (2) | 1.3682 (17) |
| C1—O5 | 1.4175 (19) | 1.4383 (19) | 1.431 (3) | 1.435 (2) | 1.4153 (16) | 1.432 (2) | 1.4415 (16) |
| C2—O2 | 1.4218 (18) | 1.4210 (19) | 1.429 (3) | 1.424 (2) | 1.4227 (15) | 1.415 (2) | 1.4228 (16) |
| C3—O3 | 1.427 (3) | 1.421 (2) | 1.4272 (16) | ||||
| C3—F | 1.3960 (18) | 1.4029 (18) | 1.414 (2) | ||||
| C4—O4 | 1.422 (2) | 1.4265 (19) | 1.422 (3) | 1.431 (2) | 1.4325 (16) | 1.421 (2) | |
| C4—F | 1.4019 (16) | ||||||
| C5—O5 | 1.436 (2) | 1.4358 (19) | 1.439 (3) | 1.434 (2) | 1.4379 (16) | 1.446 (2) | 1.4285 (17) |
| C6—O6 | 1.435 (2) | 1.434 (2) | 1.424 (4) | 1.426 (2) | 1.4289 (16) | 1.424 (2) | 1.4286 (19) |
| Bond angles (°) | |||||||
| C1—C2—C3 | 108.27 (13) | 109.86 (13) | 113.1 (2) | 111.3 (1) | 108.96 (10) | 109.76 (12) | 111.63 (11) |
| C2—C3—C4 | 110.73 (14) | 111.90 (12) | 109.8 (2) | 109.7 (1) | 110.76 (11) | 111.61 (12) | 110.13 (11) |
| C3—C4—C5 | 109.24 (13) | 111.19 (12) | 109.5 (2) | 111.3 (1) | 110.53 (11) | 110.69 (12) | 111.98 (12) |
| C4—C5—O5 | 110.25 (13) | 109.13 (12) | 108.3 (2) | 108.5 (1) | 110.62 (10) | 108.15 (13) | 108.32 (11) |
| C5—O5—C1 | 112.67 (13) | 113.19 (11) | 112.0 (2) | 113.4 (1) | 112.78 (10) | 111.89 (12) | 112.60 (10) |
| O5—C1—C2 | 108.80 (12) | 108.38 (13) | 109.3 (2) | 110.0 (1) | 109.76 (11) | 109.62 (12) | 107.84 (11) |
| C4—C5—C6 | 113.52 (13) | 112.94 (13) | 115.0 (2) | 111.9 (1) | 111.06 (11) | 113.57 (12) | 113.09 (12) |
| Torsion angles (°) | |||||||
| C1—C2—C3—C4 | −56.72 (17) | −52.09 (18) | −49.7 (3) | −51.25 (11) | −54.39 (14) | −51.65 (16) | −50.81 (14) |
| C1—O5—C5—C4 | 61.57 (16) | 62.60 (16) | 66.5 (3) | 62.34 (12) | 60.09 (13) | 63.98 (14) | 64.47 (14) |
| C2—C3—C4—C5 | 53.87 (18) | 50.21 (18) | 52.6 (3) | 53.56 (11) | 51.35 (14) | 52.17 (16) | 49.68 (14) |
| C2—C1—O5—C5 | −64.37 (16) | −64.90 (17) | −61.9 (3) | −60.91 (12) | −63.83 (13) | −64.88 (15) | −65.54 (13) |
| C3—C4—C5—O5 | −54.54 (17) | −53.46 (17) | −60.5 (3) | −57.89 (11) | −52.64 (14) | −56.43 (16) | −55.55 (14) |
| C3—C2—C1—O5 | 60.44 (16) | 57.50 (17) | 53.2 (3) | 54.20 (11) | 59.82 (13) | 56.87 (16) | 57.15 (14) |
| C3—C4—C5—C6 | −173.13 (15) | −173.09 (14) | −179.8 (3) | −177.00 (9) | −171.12 (13) | −176.81 (12) | −174.82 (12) |
| O5—C5—C6—O6 | −64.46 (17) (gg) | −63.23 (17) (gg) | −60.4 (3) (gg) | 70.51 (12) (gt) | 74.22 (13) (gt) | −72.64 (15) (gg) | −59.56 (15) (gg) |
Note: gg is gauche–gauche and gt is gauche–trans.
The largest endocyclic bond angle in (I) and (II) is the C5—O5—C1 angle [112.66 (12) and 113.19 (11)°, respectively], thus mimicking behavior in (III) and (IV), where this angle is 112.0 (2) and 113.4 (1)°, respectively. A similar trend is observed in (V)–(VII), where the same angle averages 112.4 (5)°. The exocyclic C4—C5—C6 bond angles in (I)–(VII) are relatively large compared to endocyclic C—C—C bond angles; in (I)–(VII), the former angle averages 113.0 (12)° compared to the three endocyclic C—C—C bond angles, which average 110.6 (12)°.
The endocyclic torsion angles in (I)–(II) vary from 50 to 65° (absolute values), indicating the presence of pyranosyl rings distorted from idealized chair conformations. Similar deviations are observed in (III)–(VII). In addition, the C3—C4—C5—C6 torsion angle varies from 171 to 180° (absolute values), also indicating different degrees and/or types of ring distortion in (I)–(VII). Further insight into these distortions was obtained by calculating Cremer–Pople parameters in (I)–(VII) (Table 2 ▶; Cremer & Pople, 1975 ▶). The extent of ring distortion, embodied in the value of θ, is slightly greater for (II) [θ = 6.35 (16)°] than for (I) [θ = 3.85 (15)°]. Within (I)–(VII), the most significant ring distortion is observed in (III) [θ = 8.0 (3)°], followed by (VII) [θ = 7.16 (13)°]. The direction of the ring distortion, embodied in the value of ϕ, varies widely in (I)–(VII). Average values of ϕ increase as follows: (II) and (VII), 18 (12)°; (I) and (V), 63 (6)°; (III) and (IV), 321 (3)°; (VI), 358°. Thus, the pyranosyl rings of (II) and (VII) are distorted towards C3 TB C1, those of (I) and (V) towards B C1,C4, those of (III) and (IV) towards O5 TB C2, and that of (VI) towards C3,O5 B (B = boat conformation and TB = twist-boat conformation). The effect of the anomeric configuration on 3DFG pyranose ring shape is small, mimicking the behavior of unsubstituted d-glucopyranose anomers (III) and (IV). Interestingly, both (I) and (V) show similar ring distortions, suggesting that C3 deoxygenation and C3 fluorine substitution exert similar effects on the overall pyranose ring shape, at least in the crystalline state. Likewise, inversion of configuration at C3 of (I), giving (VI), induces a measurable but not radical change in ring distortion in that the ϕ values for the two structures are within ~70° of one another; an ~60° shift in ϕ is also observed upon moving the F atom in (I) from C3 to C4, giving (VII).
Table 2. Cremer–Pople puckering parameters in (I)–(VII).
| Compound | θ (°) | ϕ (°) | Q (Å) | q2 (Å) | q3 (Å) |
|---|---|---|---|---|---|
| (I) | 3.87 (15) | 67 (2) | 0.5916 (16) | 0.0399 (16) | 0.5902 (16) |
| (II) | 6.35 (16) | 26.0 (15) | 0.5723 (16) | 0.0638 (16) | 0.5688 (16) |
| (III) | 8.0 (3) | 319 (2) | 0.580 (3) | 0.080 (3) | 0.575 (3) |
| (IV) | 3.83 (13) | 323.2 (19) | 0.5696 (13) | 0.0386 (13) | 0.5684 (13) |
| (V) | 4.80 (14) | 59.0 (16) | 0.5734 (14) | 0.0484 (14) | 0.5714 (14) |
| (VI) | 5.93 (15) | 358.4 (16) | 0.5815 (16) | 0.0602 (15) | 0.5784 (16) |
| (VII) | 7.16 (13) | 9.5 (11) | 0.5775 (14) | 0.0726 (13) | 0.5730 (14) |
The exocyclic hydroxymethyl conformation in (I)–(VII) is either gg (H5 anti to O6) or gt (C4 anti to O6) (gg = gauche–gauche and gt = gauche–trans). In gluco isomers (I)–(V) and (VII), this distribution is consistent with NMR findings in which both gg and gt conformers are detected in comparable proportions in solution, with tg (tg = trans–gauche) present in very minor abundance (Thibaudeau et al., 2004 ▶).
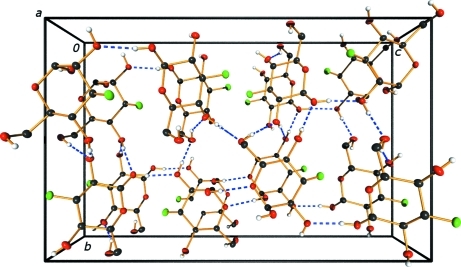
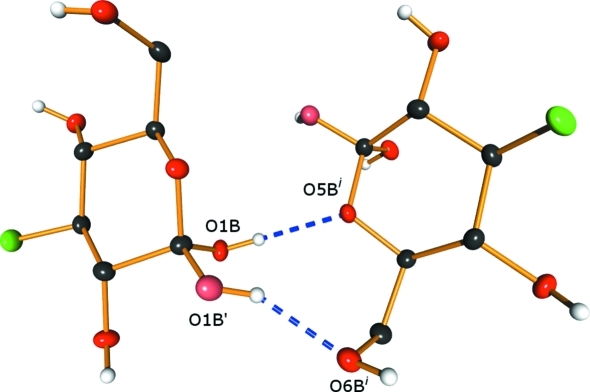
Extensive hydrogen-bonding interactions are observed within the crystalline lattice containing (I) and (II) (Fig. 2 ▶). In (I), all of the hydroxy groups serve as donors and all but O2A are mono-acceptors of hydrogen bonds. Atom O2A accepts one ‘normal’ hydrogen bond from O6B [2.7348 (16) Å] and a longer one from O4B [3.1418 (18) Å]. The ring O atom in (I), O5A, and the F atom are not involved in hydrogen bonding. In (II), the ordered hydroxy groups (O2B, O4B and O6B) serve as hydrogen-bond donors and mono-acceptors. Atoms O1B and O1B′, the α- and β-anomer positions of this hydroxy group, respectively, are both accommodated by hydrogen bonding to nearby hydroxy groups. Atom O1B forms a contact to O5B of a neighboring molecule (related by the screw axis along the a axis), and O1B′ forms a contact to O6B of the same neighboring molecule (Fig. 3 ▶). Thus, both the α- and β-anomers in site B are accommodated in a ‘pocket’ within the lattice in which they can reside with minimal distortion of the local environment.
Figure 2.
Hydrogen-bonding scheme for the title structure, viewed along the a axis. Dashed lines represent hydrogen bonds.
Figure 3.
Local hydrogen-bonding scheme for the α- and β-anomers present in the lattice. The light-colored O atoms (O1B′) (pink in the electronic version of the paper) represent the minor component. Dashed lines represent hydrogen bonds. [Symmetry code: (i) x −  , −y +
, −y +  , −z.]
, −z.]
Experimental
3-Deoxy-3-fluoro-d-glucose was synthesized by a chemical route described in detail in the Supplementary material (Cruz-Gregorio et al., 2005 ▶; Podlasek & Serianni, 1994 ▶; Angyal et al., 1979 ▶; Tropper et al., 1992 ▶). The final purified product was dissolved in a minimal volume of hot acetone, and the solution was left at room temperature until clear colorless crystals formed overnight. After recrystallization from acetone, the crystals were harvested for structure determination.
Crystal data
C6H11FO5
M r = 182.15
Orthorhombic,
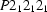
a = 5.1249 (2) Å
b = 13.7748 (4) Å
c = 21.9297 (6) Å
V = 1548.12 (9) Å3
Z = 8
Cu Kα radiation
μ = 1.33 mm−1
T = 100 K
0.27 × 0.13 × 0.08 mm
Data collection
Bruker APEXII diffractometer
Absorption correction: numerical (SADABS; Sheldrick, 2003 ▶) T min = 0.718, T max = 0.900
15073 measured reflections
2847 independent reflections
2755 reflections with I > 2σ(I)
R int = 0.026
Refinement
R[F 2 > 2σ(F 2)] = 0.027
wR(F 2) = 0.070
S = 1.06
2847 reflections
236 parameters
H-atom parameters constrained
Δρmax = 0.24 e Å−3
Δρmin = −0.18 e Å−3
Absolute structure: Flack (1983 ▶), 1117 Friedel pairs
Flack parameter: 0.05 (12)
Of the two crystallographically independent molecules located, one was found to be ordered and is the β-anomer of the 3-deoxy-3-fluoro-d-glucose. The second molecule was found to contain a mixture of both the α- and β-anomers. The site occupancies were refined and summed to unity giving a ratio of 0.836 (4):0.164 (4). The major component was refined with anisotropic displacement parameters and the minor component with isotropic displacement parameters.
Hydroxy H atoms were initially located from a difference Fourier map but were subsequently included in geometrically constrained positions, with O—H distances of 0.84 Å. All other H atoms were included in calculated positions and constrained to ride on their parent atoms, with C—H = 0.99 (methylene) or 1.00 Å (methine). For all H atoms, U iso(H) values were constrained to 1.2U eq(parent atom).
The absolute configuration was determined by the retention of the known configuration throughout the synthesis and comparison of intensities of Friedel pairs of reflections [Flack parameter = 0.05 (12); Flack, 1983 ▶]. Further, an analysis of the Hooft y parameter [0.05 (5); Hooft et al., 2008 ▶] also agrees; the P2(true) and P3(true) values are both 1.000 (measures of enantiopurity of the crystal).
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP in SHELXTL (Sheldrick, 2008 ▶) and POV-Ray (Cason, 2003 ▶); software used to prepare material for publication: XCIF (Sheldrick, 2008 ▶) and publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S0108270110040096/dn3151sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S0108270110040096/dn3151Isup2.hkl
Table 3. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1A—H1′⋯O2Bi | 0.84 | 1.87 | 2.7089 (16) | 174 |
| O2A—H2′⋯O4B | 0.84 | 1.87 | 2.6969 (17) | 169 |
| O4A—H4′⋯O6Bii | 0.84 | 1.88 | 2.7187 (16) | 173 |
| O6A—H6′⋯O4Aiii | 0.84 | 1.96 | 2.7138 (17) | 148 |
| O1B—H1′′⋯O5Biv | 0.84 | 1.94 | 2.7663 (17) | 168 |
| O1B′—H1B′′⋯O6Biv | 0.84 | 1.89 | 2.642 (8) | 148 |
| O2B—H2′′⋯O6Av | 0.84 | 1.84 | 2.6745 (16) | 177 |
| O4B—H4′′⋯O1Avi | 0.84 | 1.94 | 2.7155 (16) | 153 |
| O4B—H4′′⋯O2Avi | 0.84 | 2.58 | 3.1418 (18) | 125 |
| O6B—H6′′⋯O2Avi | 0.84 | 1.90 | 2.7348 (16) | 174 |
Symmetry codes: (i) 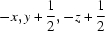 ; (ii)
; (ii) 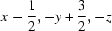 ; (iii)
; (iii) 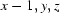 ; (iv)
; (iv) 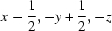 ; (v)
; (v) 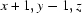 ; (vi)
; (vi) 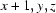 .
.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Disease (research grant No. DK065138).
Footnotes
Supplementary data for this paper are available from the IUCr electronic archives (Reference: DN3151). Services for accessing these data are described at the back of the journal.
References
- Angyal, S., Bethell, G. S. & Beveridge, R. (1979). Carbohydr. Res. 73, 9–18.
- Bruker (2009). APEX2 (Version 2009-9) and SAINT (Version 7.66A). Bruker AXS Inc., Madison, Wisconsin, USA.
- Buchini, S., Buschiazzo, A. & Withers, S. G. (2008). Angew. Chem. Int. Ed. 47, 2700–2703. [DOI] [PubMed]
- Cason, C. J. (2003). POV-Ray Version 3.6.2. Persistence of Vision Raytracer Pty. Ltd, Victoria, Australia.
- Chetyrkin, S. V., Zhang, W., Hudson, B. G., Serianni, A. S. & Voziyan, P. A. (2008). Biochemistry, 47, 997–1006. [DOI] [PubMed]
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Cruz-Gregorio, S., Hernández, L., Vargas, M., Quintero, L. & Sartillo-Piscil, F. (2005). J. Mex. Chem. Soc. 49, 20–23.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Hooft, R. W. W., Straver, L. H. & Spek, A. L. (2008). J. Appl. Cryst. 41, 96–103. [DOI] [PMC free article] [PubMed]
- Kouwijzer, M. L. C. E., van Eijck, B. P., Kooijman, H. & Kroon, J. (1995). Acta Cryst. B51, 209–220.
- Mostad, A. (1994). Acta Chem. Scand. 48, 276–278.
- Myers, C. B., Ramanjulu, M. M., Carrell, H. L. & Glusker, J. P. (1997). Struct. Chem. 8, 65–71.
- Podlasek, C. A. & Serianni, A. S. (1994). J. Biol. Chem. 269, 2521–2528 [PubMed]
- Sheldrick, G. M. (2003). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Taylor, N. F. (1988). Fluorinated Carbohydrates: Chemical and Biochemical Aspects, ACS Symposium Series, No. 274. Washington, DC: American Chemical Society.
- Thibaudeau, C., Stenutz, R., Hertz, B., Klepach, T., Zhao, S., Wu, Q., Carmichael, I. & Serianni, A. S. (2004). J. Am. Chem. Soc. 126, 15668–15685. [DOI] [PubMed]
- Tropper, F. D., Andersson, F. O., Grand-Maitre, C. & Roy, R. (1992). Carbohydr. Res. 229, 149–154. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- White, A., Tull, D., Johns, K., Withers, S. G. & Rose, D. R. (1996). Nat. Struct. Biol. 3, 149–154. [DOI] [PubMed]
- Yokoyama, M. (2000). Carbohydr. Res. 327, 5–14. [DOI] [PubMed]
- Zhang, W., Noll, B. C. & Serianni, A. S. (2007). Acta Cryst. C63, o578–o581. [DOI] [PubMed]
- Zhang, W., Oliver, A. G. & Serianni, A. S. (2010). Acta Cryst. C66, o496–o498. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S0108270110040096/dn3151sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S0108270110040096/dn3151Isup2.hkl