Abstract
Despite the knowledge that many drugs affect men and women differently, few studies exploring the effects of marijuana use on cognition have included women. Findings from both animal and human studies suggest marijuana may have more marked effects in women. This study examined sex differences in the acute effects of marijuana on cognition in 70 (n= 35 male, 35 female) occasional users of marijuana. Tasks were chosen to tap a wide variety of cognitive domains affected by sex and/or marijuana including attention, cognitive flexibility, time estimation, and visuospatial processing. As expected, acute marijuana use impaired performance on selective and divided attention, time estimation, and cognitive flexibility. While there did not appear to be sex differences in marijuana's effects on cognition, women requested to discontinue the smoking session more often than men likely leading to an underestimation of differences. Further study of psychological differences in marijuana's effects on men and women following both acute and residual effects of marijuana is warranted.
Keywords: acute effects, marijuana, cannabis, cognition, sex, gender
Introduction
Marijuana (Cannabis sativa) has been used by 28% of persons between the ages of 18-25 in the United States (US DHHS 2007). Smoking marijuana commonly causes acute subjective effects such as euphoria, depersonalization, altered time sense, lethargy, and drowsiness (Hollister 1986). Subjective and behavioral effects of marijuana begin within minutes after smoking and last at least two hours (Barnett, Licko, & Thompson 1985; Curran, Brignell, Fletcher, Middleton, & Henry 2002).
In 1994 the National Institutes of Health (NIH) revised its inclusion policy to mandate that women be included in all NIH-supported clinical research studies (Wetherington 2007). Since that time, research has found sex differences in the effects of several drugs such as alcohol, cocaine, and nicotine (Daurignac, Perez-Diaz, Grillon, & Jouvent 2001; Fallon, Keator, Mbogori, Taylor, & Potkin 2005; Mann, et al. 2005; Shiffman & Paton 1999; Wiren, et al. 2006). Little work has been performed exploring sex differences in the effects of marijuana. Despite strong theoretical evidence of differences, a meta-analysis of driving and marijuana found fewer than 25% of studies included women in their analyses (Berghaus, Scheer, & Schmidt 1995).
Studies at both the molecular and behavioral levels of neuroscience suggest there may be sex differences in the acute effects of marijuana, although results often conflict between animal and human work (Narimatsu, et al. 1993; Narimatsu, Watanabe, Yamamoto, & Yoshimura 1991; Tang, Tran, & Wagner 2005; Tseng & Craft 2001; Wagner, Abesser, Karcher, Laser, & Kunos 2005; Watanabe, Matsunaga, Narimatsu, Yamamoto, & Yoshimura 1992). Metabolically, liver cytochrome P450 enzymes, those responsible for oxidative metabolism of cannabinoids, appear to be sex-specific in rats (Narimatsu, et al. 1993; Watanabe, et al. 1992). As a result, female rats preferentially metabolize delta-9-tetrahydrocannabinol (THC) to its highly active metabolite, while males metabolize THC to multiple compounds (Narimatsu, et al. 1991). This difference in metabolism may contribute to the more pronounced and longer acting effects of THC on nociception (Tseng & Craft 2001) and motor behavior (Cohn, Barnes, Barratt, & Pirch 1972) in female rats. Males have greater CB1 receptor density in the striatum and limbic forebrain (Gonzalez, et al. 2000; Rodriguez de Fonseca, Cebeira, Ramos, Martin, & Fernandez-Ruiz 1994) and this density appears to fluctuate across the estrous cycle in female rats (Gonzalez, et al. 2000; Rodriguez de Fonseca, et al. 1994). Further, cannabinoids appear to modulate the voltage dependence of A-type potassium currents in a sex-specific way (Tang, et al. 2005; Wagner, et al. 2005).
In contrast to rodents, however, Wall and colleagues found no sex differences in the pharmacokinetics of THC in humans (Wall, Sadler, Brine, Taylor, & Perez-Reyes 1983). Even if the pharmacokinetics of THC are similar in men and women, the distribution and excretion may still differ. Since THC is stored in fatty tissue, which tends to be more prevalent in women than men [a ratio of 27% fat in women versus 15% in men is typical (Sloan 1967; Sloan, Burt, & Blyth 1962)], the distribution and excretion of THC may differ between the sexes (Huestis 1999). While studies have not yet examined this, the greater amount of fat tissue in women may result in greater amounts of THC being stored. Once stored in the body, THC remains for several weeks, due to its long biological half-life of 4-12 days, being gradually metabolized in the liver and excreted in the urine (Grotenhermen 2003).
While no acute differences in subjective “highness” ratings or objective measures of plasma THC levels have been found after smoking marijuana (Cocchetto, Owens, Perez-Reyes, DiGuiseppi, & Miller 1981; Mathew, Wilson, & Davis 2003; Miller & Branconnier 1983; Wall, et al. 1983), women report a greater increase in subjective measures of dizziness and a greater drop in mean arterial blood pressure than men (Mathew, et al. 2003). Tolerance may also develop faster in women as they show less tachycardia than males while smoking a second marijuana cigarette (Cocchetto, et al. 1981). Functionally, females exhibit decreased spectral power on steady state evoked potentials following acute administration, yet no change is found in men (Skosnik, Krishnan, Vohs, & O'Donnell 2006). Acute Marinol (synthetic THC) administration showed no sex differences on behavioral measures of impulsivity (McDonald, Schleifer, Richards, & de Wit 2003), while acute marijuana enhanced spatial working memory in females but not males (Makela, et al. 2006). The authors do not address it in their paper, however, it is interesting to note that following the placebo marijuana administration, women performed considerably worse than normative data provided by the Cambridge Automated Neuropsychological Testing Battery (personal correspondence) suggests is typical. Women ages 16-23 average only 9.23 between-search errors on the eight-box task, while Makela's participants made 17 between-search errors after placebo and 7 between-search errors after acute marijuana administration. Not only are sex differences found in the acute effects of marijuana, there also appear to be sex differences in the residual effects of chronic marijuana smoking, e.g. on a visuospatial memory task females who frequently smoke marijuana had more impairment than men (Pope, Jacobs, Mialet, Yurgelun-Todd, & Gruber 1997).
The current study aimed to examine sex differences in effects of acute marijuana administration on cognition in a between-subjects, double-blinded design. This was part of a larger pilot study examining sex differences in the effects of marijuana on simulated driving performance. Cognitive tasks were chosen to tap a wide variety of cognitive domains used in driving and affected by sex and/or marijuana including attention, perception, and executive function (Solowij 1998). It was hypothesized that women would be more vulnerable than men to the acute effects of marijuana.
Methods
Subjects
A total of seventy (70) age-matched occasional marijuana smokers [n = 35 males, 35 females; 18 to 31 years of age; mean age = 20.9 years (SD 2.9)] were recruited from the Iowa City, Iowa area through word of mouth, fliers, and local newspaper advertisements from 2004-2006. Participants underwent a brief phone screening to determine if they were current, occasional users of marijuana, defined as those who reported their current use of marijuana as at least once per month but no more frequently than ten times per month. Drug naïve participants were not recruited due to ethical concerns that it may be a “gateway drug” and individuals are more likely to have adverse reactions during the first several uses. Specific inclusion/exclusion criteria are presented in Table 1.
Table 1.
Inclusion/Exclusion criteria
| Inclusion criteria: |
| ■ Current use of marijuana 1-10 times per month |
| ■ Normal or corrected to normal vision |
| ■ Age 18-40 years |
| ■ Negative pregnancy test (females) |
| ■ Negative drug screen for Phencyclidine, Benzodiazepines, Cocaine, Amphetamines, Opiates, and Barbiturates |
| Exclusion criteria: |
| ■ Naive to use of marijuana |
| ■ Use of methamphetamine, cocaine, opiates, ecstasy, mushrooms, sleeping pills or any injected of inhaled drug of abuse more than 5 times in life or within the prior three months |
| ■ History of significant medical problems such as diabetes, heart disease, or respiratory disorders |
| ■ Use of medications other than birth control or acne medication |
This study was approved by the University of Iowa Institutional Review Board, informed consent obtained prior to participation in the study, and participants were financially compensated. Participants were studied in an age-matched, between-subject, double-blinded design as described elsewhere (Anderson, Rizzo, Block, Pearlson, & O'Leary in press). Briefly, qualifying participants were asked to abstain from marijuana use for one week prior to the visit to minimize the impact of residual drug effects on performance. All testing was completed with participant's wearing their corrective lenses if needed. A brief screening was performed in which visual acuity and contrast sensitivity were assessed. Heart rate and blood pressure were measured to obtain baseline measures for comparison of active marijuana and placebo. No participants were excluded as part of the brief in person screening. A urine drug screen (TRIAGE Drugs of Abuse Panel Kit) was administered to participants to test for THC and six other drugs of abuse. Positive THC tests were found in 25.7% of the participants. No participants were excluded due to pregnancy.
Baseline cognitive assessment included estimated current and premorbid intelligence using the Matrix Reasoning from the Wechsler Adult Intelligence Scale – III (Wechsler 1997) and the American National Adult Reading Test (ANART) (Grober & Sliwinski 1991), respectively. An additional assessment was completed to establish criteria which participants must meet prior to discharge from the study. This assessment checked participants’ orientation to time, date, and location. It evaluated the presence of paranoia, delusions, and hallucinations. Measures of subjective “high” were also obtained. For this, participants were asked to evaluate how “high” they felt on a zero to ten scale with zero being no effect and ten being the “highest” they have ever felt after smoking marijuana. Participants completed this “high” scale at baseline (time = -10 minutes), immediately after smoking (time = 0 minutes), before beginning neuropsychological testing (time = ~30 minutes), immediately after neuropsychological testing (time = ~90 minutes) and prior to study discharge (time = ~120 minutes). To address potential confounds of fatigue or sleepiness, all participants completed the Stanford Sleepiness Scale (Hoddes, Zarcone, Smythe, Phillips, & Dement 1973) at similar time points to the “high” scale.
Smoking Session
Marijuana cigarettes were stored in locked refrigerator prior to use to retain their potency. Approximately twenty-four hours before use, the marijuana cigarette was removed from storage and humidified with a saline solution. The marijuana cigarettes were administered via a paced smoking paradigm, according to standard laboratory procedures (Block, Farinpour, & Braverman 1992). Briefly, participants were instructed to inhale for three seconds, hold their breath for five seconds, and given twenty-seven seconds between inhalations to rest. The experimenter timed the protocol and instructed the participants for each puff. Participants were asked to continue this inhalation procedure until the marijuana cigarette was consumed or they reached an “uncomfortable” level of highness (Gorelick & Heishman 2006). The number of puffs and the time taken to finish the marijuana cigarette were recorded. The smoking session was conducted in the General Clinical Research Center (GCRC) “Environmental Chamber”, a stainless steel cage with controlled airflow venting to the outside of the hospital. The experimenter sat outside the chamber and communicated with the participant through a glass partition and a two-way intercom system. Participants were informed they would be receiving “one of two possible doses of marijuana.” The marijuana cigarettes, provided by the National Institute of Drug Abuse, were administered in a between-subjects, within-sex, randomized, double-blinded design and contained either approximately 0% delta-9-tetrahydrocannabinol (THC) (placebo) or 2.9% delta-9-THC (active) doses of marijuana. Both active and placebo cigarettes were 85 mm length and 25 mm in circumference. The average weight was 0.790 grams equating to approximately 0 mg THC in the placebo condition (because it was marijuana with the THC removed, trace amounts may still be present) and 22.9 mg THC in the active cigarette. This dose was similar to amounts previously utilized in our laboratory (O'Leary, et al. 2000; O'Leary, et al. 2002).
Cognitive Assessment
Approximately thirty minutes after smoking, participants took part in a battery of neuropsychological tasks. The order of the tests was pseudo-randomly varied between participants to minimize possible effects of sequence or fatigue. The following tests were included in the protocol.
Useful Field of View
The Useful Field of View (UFOV) task assesses speed of processing, divided attention and selective attention (Ball, Owsley, Sloane, Roenker, & Bruni 1993; Edwards, et al. 2005). Participants are required to make a forced choice identification of a central target and/or the location of a peripheral target. The duration of stimulus presentation is varied to determine the fastest threshold at which subject's can correctly detect the target(s). The fastest possible time the program allowed to correctly identify stimuli was 16 msec, thus providing a ceiling to performance levels.
Time Estimation
This test measured the subject's ability to estimate time duration. Participants were asked at the completion of the Useful Field of View task, how long they spent completing that entire task. The average task duration was approximately ten minutes. Accuracy was assessed as a ratio of (actual time minus estimated time) divided by actual time. The resulting score is positive if subjects underestimate the amount of time that has passed and negative when participants over estimate the passage of time.
Trail Making Test—Parts A and B
The Trail Making Test is a widely used and reliable measure of executive functions (Reitan & Davison 1974). This is a paper-and-pencil task in which participants must connect items in an increasing sequence as quickly and as accurately as possible. The Trail Making Test Part A requires tracking of only numbers, thereby providing a baseline estimate of motor function. The Trail Making Test Part B requires cognitive flexibility and planning as participants must shift from connecting numbers to letters and back to numbers again (e.g., 1 to A, A to 2, 2 to B, B to 3, etc). A cognitive flexibility score is created by taking the ratio of Trails B divided by Trails A time to complete. This ratio score has been found to be less susceptible to demographic influences than the more standard subtraction of Trails B minus Trails A times to completion, however, both measures are provided (Horton & Roberts 2002).
3-D Structure from Motion
For this task, we used a two-answer forced choice shape identification paradigm in which the observer is asked to report the shape of a rotating object (either a random-dot sphere or a random-dot cube) (Rizzo, Nawrot, & Zihl 1995). Varying amounts of random dot noise, resulting in signals of 5%, 10%, 15%, 20%, 25%, or 30% were added to a square background region (2 inches × 2 inches) surrounding the target to prevent shape identification from non-motion cues such as edges or dot density, and to index the difficulty of the task. From percent correct performance at each signal level, threshold (defined as 75% correct) was determined using probit analysis.
Task Switching
For this task, participants had to identify odd versus even numbers or consonant versus vowels by making a force-choice button press response (Vecera & Rizzo 2003). Tasks varied from independently presented letters and numbers, to a “cued” condition in which participants were instructed before each trial whether a letter or a number would appear, and finally “random” trials in which no warning was provided.
Following the cognitive assessment, sobriety tests were performed and participants returned to the General Clinical Research Center for further monitoring. Baseline orientation measures (date, time, and location) and presence of psychosis were again assessed. Vital signs were recorded as were sleepiness and “highness”. Participants were provided a meal and asked to remain at the research center for further monitoring. Participants were not told the criteria for study discharge: a subjective “high” rating of 3 or less. Heart rate was utilized as a secondary, objective measure of “high” and was required to be within ten beats of baseline. This discharge criterion was chosen based on standards previously utilized in our laboratory (O'Leary, et al. 2000; O'Leary, et al. 2002). Upon meeting criteria for discharge, participants were provided with a voucher for cab fare and returned home. For their safety, they were asked to remain at home and specifically not to drive for the remainder of the day.
Statistical analyses
An age-matched grouping of males and females were chosen from a larger sample of eighty-five participants. Two groups (male and female) × two drug doses (placebo and active THC) analysis of variance were utilized to examine group differences in general intellectual function. First, group differences in the following baseline demographic measures were evaluated: age; education; weight; body mass index (BMI); frequency of marijuana use; duration of marijuana use and weekly alcohol intake. Next, the following baseline cognitive testing variables were examined: WAIS-III Matrix Reasoning Scaled Score and NART WAIS-R estimate of intelligence. Repeated measures analyses of variance were used to evaluate the effects of marijuana smoking on subjective “high,” heart rate, and Stanford Sleepiness Scale scores.
The post-smoking cognitive assessment included eight variables in addition to those in the task switching which was analyzed separately. The variables are as follows: 1-4) a reaction time threshold from each section of the Useful Field of View task (speed of processing, divided attention, and two selective attention tasks); 5-6) Trail Making Test cognitive flexibility scores including both ratio and subtraction of time to complete for the Trail Making Test forms A and B; 7) time estimation accuracy ratio; and 8) threshold of signal detection for the 3-D Structure from Motion task.
Because of the repeated nature of the task, task switching was analyzed separately. Mean reaction time was computed separately for trials requiring a switch in task or key and those requiring no switch. This resulted in five additional mean reaction time variables: the individual task, the cued condition requiring a switch in task or response, the cued condition in which no switch in task or response was needed, the random condition requiring a switch in task or response, and the random condition in which no switch in task or response was needed. Because the individual task did not have a switch component to it, its mean reaction time was analyzed separately in a two group (male, female) × two drug doses (placebo, active) ANOVA. A two groups (male, female) × two drug doses (placebo, active) × two tasks (cued, random) × 2 conditions (switch, no switch) ANOVA was conducted for task switching mean reaction time.
Results
Demographic characteristics of the groups are presented in Table 2. Due to matching of age, no group differences were found for this variable or for education. No differences in estimated premorbid intelligence were found with the Adult North American Reading Test or Matrix Reasoning Section of the Wechsler Adult Intelligence Scale Version III. Men weighed more than women, although no significant difference in body mass index was observed. No group differences were observed in the frequency or duration of marijuana use; however, men consumed more alcohol per week than women.
Table 2.
Demographic and Baseline Testing
| Task | Placebo THC | Active THC | Overall F | Sex F | THC F | Sex × THC F | ||
|---|---|---|---|---|---|---|---|---|
| Males (n= 17) | Females (n=17) | Males (n=18) | Females (n=18) | |||||
| Age | 20.4 (2.4) | 21.0 (2.6) | 20.8 (2.9) | 21.4 (3.9) | 0.35 | 0.69 | 0.36 | 0.01 |
| Education | 13.2 (1.4) | 14.1 (2.0) | 13.4 (1.3) | 13.8 (1.5) | 1.06 | 2.78 | 0.01 | 0.45 |
| Weight (kg) | 78.6 (10.7) | 71.1 (14.0) | 79.5 (10.6) | 68.7 (17.2) | 2.86* | 8.15** | 0.06 | 0.27 |
| Body Mass Index (BMI) | 24.3 (2.2) | 25.1 (5.0) | 24.7 (3.1) | 25.7 (6.1) | 0.34 | 0.77 | 0.25 | 0.01 |
| Handedness (% right handed) | 76.5% | 82.4% | 88.9% | 83.3% | ||||
| Frequency of marijuana use (times per month) | 4.5 (2.9) | 4.5(2.9) | 4.4 (2.7) | 4.1 (3.0) | 0.07 | 0.03 | 0.11 | 0.07 |
| Duration of marijuana use (# of months) | 29.4 (21.8) | 34.3(27.7) | 36.8 (20.8) | 38.2 (25.9) | 0.38 | 0.26 | 0.85 | 0.08 |
| Alcohol use (drinks per week) | 9.7 (7.1) | 7.4 (8.7) | 12.4 (7.9) | 9.1 (7.4) | 1.19 | 2.03 | 1.27 | 0.06 |
| ANART estimated IQ | 115.4 (4.9) | 115.3 (5.6) | 114.7 (4.6) | 113.9 (4.0) | 0.31 | 0.12 | 0.74 | 0.07 |
| Matrix Reasoning Scaled Score | 12.8 (2.2) | 12.4 (2.7) | 12.1 (1.9) | 12.6 (2.2) | 0.36 | 0.01 | 0.28 | 0.75 |
NART = North American Reading Test
p < 0.05
p < 0.01
Women requested to discontinue the smoking session more often than men (0% of men requested to discontinue the active cigarette, while 44.4% of the women did so). The number of puffs to complete the marijuana cigarette and the corresponding amount of time taken did not differ between sexes or doses [F(3, 69) = 0.91, p = 0.44]. The number of puffs taken ranged between 7 and 16 and the time to complete the smoking paradigm ranged from approximately 4 to 9 minutes. No significant differences were found between the women who did and did not complete the marijuana cigarette for body mass index, weight, previous use in years or joints per month, alcohol intake, age at which the participant began using marijuana or the frequency of other drug use. All participants who requested to discontinue the active cigarette had a negative drug screen suggesting they may have underestimated their history of prior marijuana use. No significant differences in “high,” heart rate, or cognitive test performance were found for those that completed the marijuana cigarette versus those that did not; thus all participants are included in these analyses. It should be noted, the large dropout rate may cause an underestimation in cognitive differences between men and women after acute marijuana use.
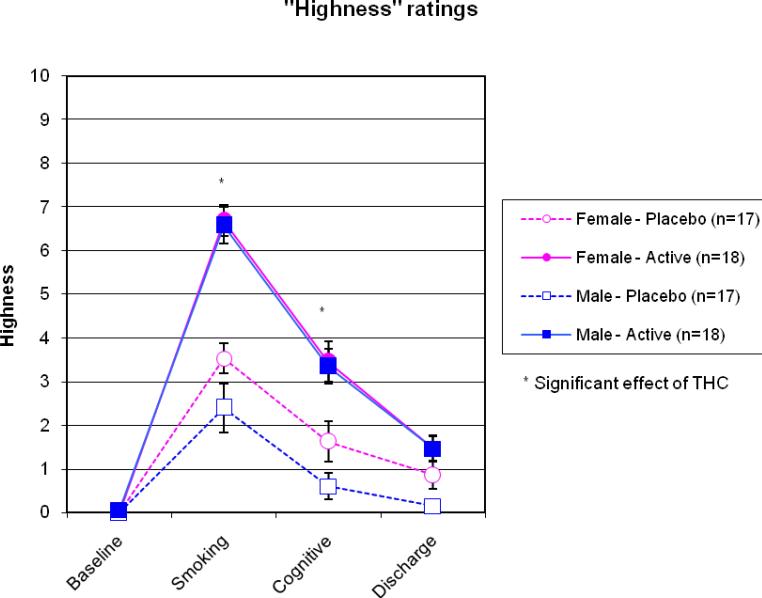
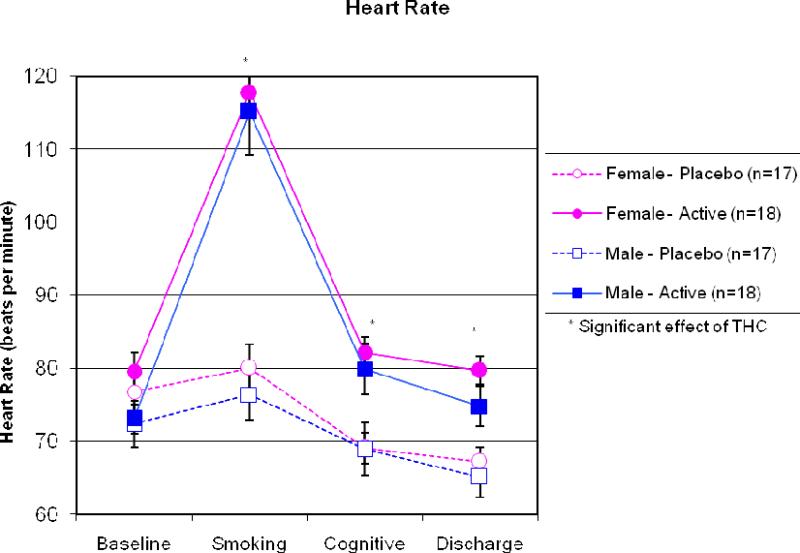
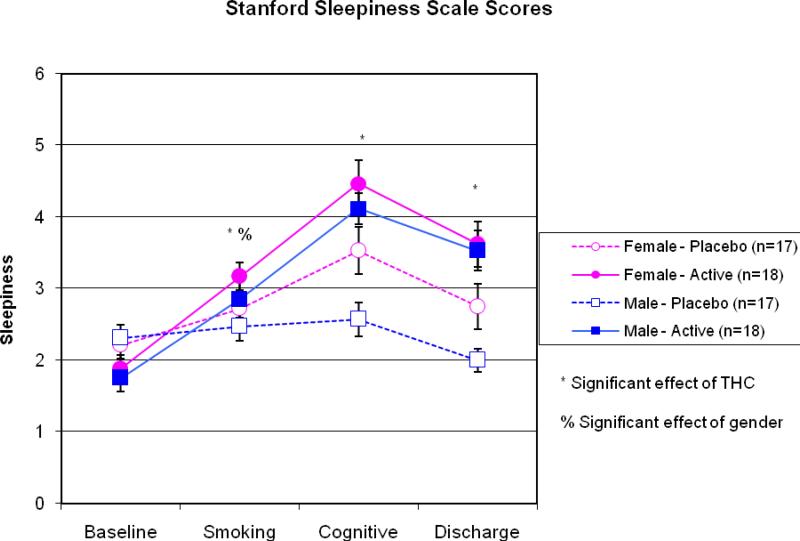
As expected, heart rate and ratings of “high” increased immediately after smoking the active cigarette and declined throughout the test session. No sex differences in “high” scores or heart rate were found at any time point. See Figure 1 for ratings of “high” and Figure 2 for heart rate. Subjective ratings of sleepiness based on the Stanford Sleepiness Scale increased significantly following administration of the active marijuana dose. Women reported significantly more sleepiness than men immediately prior to cognitive testing, although sex differences were not found at any other time points. See Figure 3 for ratings of somnolence.
Figure 1.
Subjective ratings of “highness” by sex at each time point. Participants receiving the active marijuana dose rating themselves significantly “higher” than those receiving placebo at each time point after smoking. Women rated themselves as significantly “higher” than men immediately after smoking and prior to cognitive testing.
Figure 2.
Heart rate for each sex and dose at each time point. After smoking, active marijuana increased heart rate significantly more than placebo. No sex differences were observed.
Figure 3.
Stanford Sleepiness Scale scores for each sex and dose at each time point. Active marijuana increased sleepiness compared to placebo in participants prior to and post-cognitive testing, and at discharge. Women exhibited more sleepiness than men prior to cognitive testing, but at no other time points.
As shown in Table 3, marijuana significantly increased the time needed to successfully detect stimuli in the selective and divided attention components of the Useful Field of View task. Under the influence of marijuana, participants overestimated the ten minute duration; this same duration was underestimated in participants who received the placebo cigarette. No significant effects of marijuana or sex were found for visuospatial processing as assessed with the 3-D Structure from Motion task or cognitive flexibility as assessed with the Trail Making Test.
Table 3.
Cognitive testing post smoking
| Task | Placebo | Active | Overall F | Sex | THC | Sex by THC | ||
|---|---|---|---|---|---|---|---|---|
| Males (n=17) | Females (n=17) | Males (n=18) | Females (n=18) | |||||
| UFOV 1 (msec) | 16 (0) | 16 (0) | 16.4(1.6) | 16 (0) | 0.96 | 0.94 | 0.94 | 0.94 |
| UFOV 2 (msec) | 16.0 (0) | 16.8 (3.4) | 23.6 (17.1) | 28.9 (32.7) | 1.84 | 0.47 | 4.76* | 0.25 |
| UFOV 3 (msec) | 68.3(39.1) | 63.2 (19.3) | 83.3 (56.4) | 73.8 (21.2) | 0.94 | 0.67 | 2.07 | 0.06 |
| UFOV 4 (msec) | 154.0 (64.7) | 169.1 (50.1) | 214.3 (77.8) | 206.9 (62.6) | 3.54* | 0.06 | 10.05** | 0.53 |
| Trails B-A | 29.1 (9.4) | 23.7 (10.6) | 42.5 (23.6) | 39.9 (15.0) | 5.17* | 1.05 | 14.75*** | 0.13 |
| Trails B/A*100 | 2.4 (0.6) | 1.9 (0.5) | 2.7 (1.0) | 2.6 (0.7) | 2.94* | 2.25 | 6.20* | 0.88 |
| Time Estimation | 0.03 (0.46) | 0.07 (0.46) | -0.44 (0.82) | -0.13 (0.58) | 2.42 | 1.42 | 5.05* | 0.85 |
| Structure from Motion | 9.7 (2.9) | 9.8 (1.9) | 10.0 (2.6) | 10.9 (2.0) | 0.91 | 0.80 | 1.29 | 0.52 |
UFOV = Useful Field of View
p < 0.05
p < 0.01
p < 0.001
Task switching showed no gender or drug differences when letters and numbers were presented separately. When participants were cued as to whether a letter or a number would be appearing next and the response required a shift in either category or response type (i.e., from a letter to a number, from odd to even, from consonant to vowel, etc.), significant effects of sex and drug were found, however there was no sex by drug interaction. Similarly, when participants were cued as to the upcoming condition and no switch in response type was needed, there was a significant effect of marijuana and sex, but no sex by drug interaction. With randomly presented letters and numbers no significant sex, drug, or interactions were identified for either trials that required or did not require a switch.
Discussion
Despite efforts to legalize marijuana for medicinal and/or recreational use, few studies have investigated sex differences in marijuana's effects on cognition. This study investigated sex differences in the effects of acute marijuana administration on cognition. Seventy age-matched participants (N = 35 men; N = 35 women) were randomly assigned to smoke either an active or placebo marijuana cigarette in a double-blind, between-subjects design after which they completed a standardized neuropsychological test battery. Active marijuana impaired selective and divided attention and time estimation (Murray 1986; O'Leary, et al. 2003; Wilson, Ellinwood, Mathew, & Johnson 1994).
Findings from the present study support previous literature showing an effect of acute marijuana administration on cognition in both sexes. Marijuana was found to affect estimation of a ten minute interval duration, selective and divided attention tasks, and cognitive flexibility. Participants receiving the placebo cigarettes underestimated the duration of time to complete the Useful Field of View Task whereas subjects who received the active marijuana overestimated this duration (i.e., they thought more time had passed than actually had). Previous research suggests subjects under the influence of acute marijuana use experience time as passing more quickly relative to real time (O'Leary, et al. 2003). Similar to the results of this study, such an effect leads to an overestimation of a duration.
The Useful Field of View has been found to be sensitive to processing speed and attention (Ball, et al. 1993; Edwards, et al. 2005). It has an established history of use in the area of driving research. Attention deficits were found for the Useful Field of View divided and selective attention tasks when active marijuana was compared to the placebo. No drug effects were found in the sustained attention task suggesting processing speed is selectively impaired by marijuana. As expected, no significant effect of marijuana or sex was found for the control section of the task switching paradigm in which letters and numbers were presented separately. This lack of difference suggests there was not an impairment of reaction time or motor performance. Marijuana impaired performance on the task switching cued condition. While this finding was surprising, it is likely due to the task construction. A 3 second cue precedes the stimulus in the cued condition. This requires participants to estimate the time duration and quickly respond when the stimulus is presented. Both random and individual conditions have only a 200 msec interstimulus interval, thus minimizing the time estimation requirements. An alternate interpretation is that following acute marijuana use more time is needed to process a cue, resulting in a bottle neck of information (i.e., an increase in the psychological refractory period). As expected, reaction time increased when stimuli were randomly presented and required a switch in task, suggesting cognitive flexibility declines under the influence of marijuana. Significant differences were found for both sex and drug, but no interaction of the two.
This study had several limitations which may result in our under-estimating the true sex differences. A between-subjects design was chosen to eliminate issues associated with practice effects. Use of a within-subjects design controlling for the effects of practice would be more powerful. As this was part of a larger study examining sex differences in the influence of marijuana on driving performance, the cognitive testing was performed 30-90 minutes after smoking, a time in which the peak effects of marijuana are starting to diminish. This study did not control for menstrual cycle phase which may have obscured potential sex difference findings (Wetherington 2007); however previous research has not identified a difference in the effects of marijuana across the menstrual cycle (Terner & de Wit 2006). Another limitation of the study was the number of participants who requested to discontinue the marijuana cigarette before completion. There was a significant sex difference in the ability of participants to complete the marijuana cigarette. Almost half of the women receiving the active cigarette requested to discontinue the smoking session, while none of the age-matched men declined. We have not previously encountered this difference which may be due to the use of the environmental chamber for smoking. The chamber has a sterile, “medical” feel while the examiner sits several doors away (yet visible through a glass window) from the participant. Previous research suggests environmental stress may sensitize the body to the psychological and physiological effects of marijuana intoxication (Suplita, Eisenstein, Neely, Moise, & Hohmann 2008). Based on these anecdotal findings, this potential sex difference in psychological effects of marijuana should be more fully examined. Our findings of an increased psychological effect corresponds with other's findings in which women report greater subjective dizziness than men (Mathew, et al. 2003). Doses administered were standard National Institute of Drug Abuse marijuana cigarettes and not adjusted by the participant's weight. While there was not a significant sex difference in body mass index, women weighed significantly less than men. Results may be further strengthened by using weight adjusted marijuana doses. This may decrease the number of female participants requesting early discontinuation of the smoking procedure and provide a more accurate estimate of sex differences in marijuana's effects on cognition. The results of this pilot study should be replicated in a fully powered study using a within-subjects design.
Summary and Conclusions
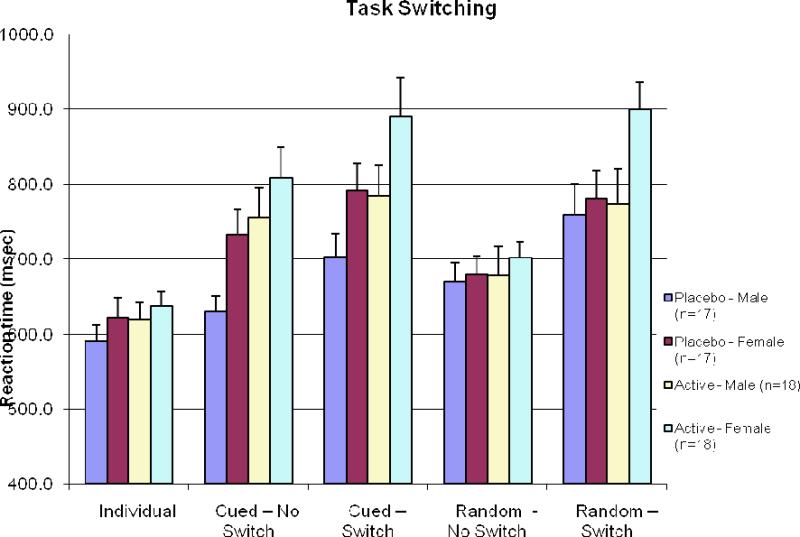
This study assessed sex differences in the effects of acute marijuana on cognition in 35 men and 35 women. As expected, marijuana did have an impact on selective and divided attention, cognitive flexibility, and time estimation. No sex by drug interactions were found for cognitive testing, however, psychological response to the smoking session was striking in that women requested to discontinue the marijuana cigarette more often than men. Despite receiving a smaller dose of marijuana, women under the influence of marijuana had a non-statistically significant slower reaction time to task switching suggesting cognitive flexibility may be a key area to focus on in future research examining sex differences in marijuana's effects.
Figure 4.
Task switching scores for each sex and dose. Active marijuana increased reaction times for the cued condition whether or not a switch in task was needed and women responded more slowly than men to the cued condition. No differences were identified in random trials and no sex by drug interactions were observed.
Footnotes
This work was supported by a National Institute of Drug Abuse grant (R01DA010551) awarded to Dr. O'Leary, a National Institute of Environmental Health Sciences grant (P30ES005605) awarded to Dr. Kline, a National Center for Research Resources General Clinical Research Centers Program grant (M01-RR-59) and the National Institute of Neurological Disorders and Stroke grant (P01NS019632) awarded to Dr. Rizzo and the Department of Neurology for use of the Simulator for Interdisciplinary Research in Ergonomics and Neuroscience.
REFERENCES
- Anderson BM, Rizzo M, Block RI, Pearlson GD, O'Leary DS. Sex differences in the effects of marijuana on simulated driving performance. Journal of Psychoactive Drugs. doi: 10.1080/02791072.2010.10399782. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993;34(11):3110–3123. [PubMed] [Google Scholar]
- Barnett G, Licko V, Thompson T. Behavioral pharmacokinetics of marijuana. Psychopharmacology (Berl) 1985;85(1):51–56. doi: 10.1007/BF00427321. [DOI] [PubMed] [Google Scholar]
- Berghaus G, Scheer N, Schmidt P. Effects of cannabis on psychomotor skills and driving performance: a meta-analysis of experimental studies.. Paper presented at the Proceedings of the 13th International Council on Alcohol; Drugs and Traffic Safety, Adelaide, Australia. 1995. [Google Scholar]
- Block RI, Farinpour R, Braverman K. Acute effects of marijuana on cognition: relationships to chronic effects and smoking techniques. Pharmacol Biochem Behav. 1992;43(3):907–917. doi: 10.1016/0091-3057(92)90424-e. [DOI] [PubMed] [Google Scholar]
- Cocchetto DM, Owens SM, Perez-Reyes M, DiGuiseppi S, Miller LL. Relationship between plasma delta-9-tetrahydrocannabinol concentration and pharmacologic effects in man. Psychopharmacology (Berl) 1981;75(2):158–164. doi: 10.1007/BF00432179. [DOI] [PubMed] [Google Scholar]
- Cohn RA, Barnes PR, Barratt E, Pirch JH. Sex differences in response to marijuana in the rat. In: Singh JM, Miller LH, Lal H, editors. Drug Addiction Experimental Pharmacology. Vol. 1. Futura Publishing; NY: 1972. pp. 227–234. [Google Scholar]
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002;164(1):61–70. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- Daurignac E, Perez-Diaz F, Grillon C, Jouvent R. Gender and activation level in smokers. Eur Psychiatry. 2001;16(5):313–316. doi: 10.1016/s0924-9338(01)00584-3. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Vance DE, Wadley VG, Cissell GM, Roenker DL, Ball KK. Reliability and validity of useful field of view test scores as administered by personal computer. J Clin Exp Neuropsychol. 2005;27(5):529–543. doi: 10.1080/13803390490515432. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Keator DB, Mbogori J, Taylor D, Potkin SG. Gender: a major determinant of brain response to nicotine. Int J Neuropsychopharmacol. 2005;8(1):17–26. doi: 10.1017/S1461145704004730. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F, et al. Sex steroid influence on cannabinoid CB(1) receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochem Biophys Res Commun. 2000;270(1):260–266. doi: 10.1006/bbrc.2000.2406. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Heishman SJ. Methods for clinical research involving cannabis administration. Methods Mol Med. 2006;123:235–253. doi: 10.1385/1-59259-999-0:235. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13(6):933–49. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10(4):431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38(1):1–20. [PubMed] [Google Scholar]
- Horton AM, Jr., Roberts C. Derived trail making test indices in a sample of marijuana abusers: demographic effects. Int J Neurosci. 2002;112(4):429–438. doi: 10.1080/00207450290025563. [DOI] [PubMed] [Google Scholar]
- Huestis M. Pharmacokinetics of THC in inhaled and oral preparations. In: Nahas GG, Sutin KM, Harvey DJ, Agurell S, editors. Marijuana and Medicine. Humana Press; Totawa NJ: 1999. pp. 105–116. [Google Scholar]
- Makela P, Wakeley J, Gijsman H, Robson PJ, Bhagwagar Z, Rogers RD. Low doses of delta-9 tetrahydrocannabinol (THC) have divergent effects on short-term spatial memory in young, healthy adults. Neuropsychopharmacology. 2006;31(2):462–470. doi: 10.1038/sj.npp.1300871. [DOI] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res. 2005;29(5):896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Davis R. Postural syncope after marijuana: a transcranial Doppler study of the hemodynamics. Pharmacol Biochem Behav. 2003;75(2):309–318. doi: 10.1016/s0091-3057(03)00086-8. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28(7):1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- Miller LL, Branconnier RJ. Cannabis: effects on memory and the cholinergic limbic system. Psychol Bull. 1983;93(3):441–456. [PubMed] [Google Scholar]
- Murray JB. Marijuana's effects on human cognitive functions, psychomotor functions, and personality. Journal of General Psychology. 1986;113(1):23–55. doi: 10.1080/00221309.1986.9710540. [DOI] [PubMed] [Google Scholar]
- Narimatsu S, Watanabe K, Matsunaga T, Yamamoto I, Imaoka S, Funae Y, et al. Suppression of liver microsomal drug-metabolizing enzyme activities in adult female rats pretreated with cannabidiol. Biol Pharm Bull. 1993;16(4):428–430. doi: 10.1248/bpb.16.428. [DOI] [PubMed] [Google Scholar]
- Narimatsu S, Watanabe K, Yamamoto I, Yoshimura H. Sex difference in the oxidative metabolism of delta 9-tetrahydrocannabinol in the rat. Biochemical Pharmacology. 1991;41(8):1187–1194. doi: 10.1016/0006-2952(91)90657-q. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Block RI, Flaum M, Schultz SK, Boles Ponto LL, Watkins GL, et al. Acute marijuana effects on rCBF and cognition: a PET study. Neuroreport. 2000;11(17):3835–3841. doi: 10.1097/00001756-200011270-00047. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, et al. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology. 2002;26(6):802–816. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Block RI, Turner BM, Koeppel J, Magnotta VA, Ponto LB, et al. Marijuana alters the human cerebellar clock. Neuroreport. 2003;14(8):1145–1151. doi: 10.1097/00001756-200306110-00009. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jacobs A, Jr., Mialet JP, Yurgelun-Todd D, Gruber S. Evidence for a sex-specific residual effect of cannabis on visuospatial memory. Psychother Psychosom. 1997;66(4):179–184. doi: 10.1159/000289132. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Davison LA. Clinical Neuropsychology: Current Status and Applications. Hemisphere; New York: 1974. [Google Scholar]
- Rizzo M, Nawrot M, Zihl J. Motion and shape perception in cerebral akinetopsia. Brain. 1995;118(Pt 5):1105–1127. doi: 10.1093/brain/118.5.1105. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994;54(3):159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paton SM. Individual differences in smoking: gender and nicotine addiction. Nicotine Tob Res. 1999;1(Suppl 2):S153–157. doi: 10.1080/14622299050011991. discussion S165-156. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Krishnan GP, Vohs JL, O'Donnell BF. The effect of cannabis use and gender on the visual steady state evoked potential. Clin Neurophysiol. 2006;117(1):144–156. doi: 10.1016/j.clinph.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Sloan AW. Estimation of body fat in young men. J Appl Physiol. 1967;23(3):311–315. doi: 10.1152/jappl.1967.23.3.311. [DOI] [PubMed] [Google Scholar]
- Sloan AW, Burt AJ, Blyth CS. Estimating body fat in young women. J Appl Physiol. 1962;17:967–970. doi: 10.1152/jappl.1962.17.6.967. [DOI] [PubMed] [Google Scholar]
- Solowij N. Cannabis and cognitive functioning. Cambridge University Press; Cambridge ; New York: 1998. [Google Scholar]
- Suplita RL, 2nd, Eisenstein SA, Neely MH, Moise AM, Hohmann AG. Cross-sensitization and cross-tolerance between exogenous cannabinoid antinociception and endocannabinoid-mediated stress-induced analgesia. Neuropharmacology. 2008;54(1):161–171. doi: 10.1016/j.neuropharm.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SL, Tran V, Wagner EJ. Sex differences in the cannabinoid modulation of an A-type K+ current in neurons of the mammalian hypothalamus. J Neurophysiol. 2005;94(4):2983–2986. doi: 10.1152/jn.01187.2004. [DOI] [PubMed] [Google Scholar]
- Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84(1):1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol. 2001;430(1):41–47. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, S. O. o. A. S. Drug Abuse Warning Network, 2005: National estimates of Drug-Related Emergency Department Visits. Rockville, MD: 2007. [Google Scholar]
- Vecera SP, Rizzo M. Spatial attention: normal processes and their breakdown. Neurol Clin. 2003;21(3):575–607. doi: 10.1016/s0733-8619(02)00103-2. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Abesser M, Karcher J, Laser M, Kunos G. Coronary vasodilator effects of endogenous cannabinoids in vasopressin-preconstricted unpaced rat isolated hearts. J Cardiovasc Pharmacol. 2005;46(3):348–355. doi: 10.1097/01.fjc.0000175437.87283.f2. [DOI] [PubMed] [Google Scholar]
- Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther. 1983;34(3):352–363. doi: 10.1038/clpt.1983.179. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Matsunaga T, Narimatsu S, Yamamoto I, Yoshimura H. Sex difference in hepatic microsomal aldehyde oxygenase activity in different strains of mice. Res Commun Chem Pathol Pharmacol. 1992;78(3):373–376. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - 3rd Edition (WAIS-III) The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wetherington CL. Sex-gender differences in drug abuse: a shift in the burden of proof? Exp Clin Psychopharmacol. 2007;15(5):411–417. doi: 10.1037/1064-1297.15.5.411. [DOI] [PubMed] [Google Scholar]
- Wilson WH, Ellinwood EH, Mathew RJ, Johnson K. Effects of marijuana on performance of a computerized cognitive-neuromotor test battery. Psychiatry Research. 1994;51(2):115–125. doi: 10.1016/0165-1781(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Wiren KM, Hashimoto JG, Alele PE, Devaud LL, Price KL, Middaugh LD, et al. Impact of sex: determination of alcohol neuroadaptation and reinforcement. Alcohol Clin Exp Res. 2006;30(2):233–242. doi: 10.1111/j.1530-0277.2006.00032.x. [DOI] [PubMed] [Google Scholar]