Abstract
Programmes that provide no-fault compensation for an adverse event following vaccination have been implemented in 19 countries worldwide, the first in Germany in 1961 and the most recent in Hungary in 2005. We performed a review of these programmes and determined elements that were common to all of them: administration and funding, eligibility, process and decision-making, standard of proof, elements of compensation and litigation rights. Most programmes were administered by state or national governments except in Finland and Sweden where they are coordinated by pharmaceutical manufacturers. Although funding is usually from Treasury, Taiwan (China) and the United States of America impose a tax on vaccine doses distributed. Decisions on compensation are made using established criteria or assessed on a case-by-case basis, while the standard of proof required is usually less than that required for court cases. Benefits provided by programmes include medical costs, disability pensions and benefits for noneconomic loss and death. Most countries allow claimants to seek legal damages through the courts or a compensation scheme payout but not both. We conclude that a variety of programmes, based on ethical principles, have been successful and financially viable in developed countries throughout the world. We believe there is a strong argument for widespread implementation of these programmes in other developed countries.
ملخص
لقد نُفِذَت برامج تقديم التعويضات بدون إثبات الخطأ التالي للأحداث الضائرة المنسوبة إلى التلقيح في 19 بلداً حول العالم، كان أولها ألمانيا عام 1961، وأخرها المجر عام 2005. وقد أَجْرَى الباحثون مراجعة لهذه البرامج وحدّدوا العناصر الشائع تداولها في جميع البرامج مثل: الإدارة والتمويل، وشروط التأهل للتعويض، وعملية اتخاذ القرار، ومعيار الاستحقاقات، وعناصر التعويض، والحقوق في الدعاوى القضائية. وكانت أغلب البرامج تُدَار عن طريق الولاية أو الحكومة الوطنية ماعدا في فنلندا والسويد حيث يجري تنسيقها من قبل مُصَنّعيّ الأدوية. ومع أن التمويل يُقَدَم عادة من الخزانة، إلا أن الصين (مقاطعة الصين، تايوان) والولايات المتحدة الأمريكية تفرضان ضرائب على جرعات اللقاحات التي تم توزيعها. وتُتَخَذ قراراتُ التعويض عن طريق معايير ثابتة أو على أساس تقييم كل حالة على حدة، لكن معايير البيّنات المطلوبة عادة ما تكون أدنى مما هو مطلوب في الحالات المعروضة على المحاكم. وتشمل المزايا المُقَدَمة في البرامج التكاليف الطبية، ورواتب للإعاقات، وفوائد عن الخسائر غير الاقتصادية والوفاة. وتتيح أغلب البلدان للمدَّعين التماس الأضرار القانونية من خلال المحاكم أو عن طريق مخطط لسداد التعويض ولكن لا تسمح للاثنين معاً. واستنتج الباحثون أن مختلف البرامج، المستندة إلى مبادئ أخلاقية، كانت ناجحة وقابلة للتطبيق مالياً في البلدان المتقدمة في أنحاء العالم. ويعتقد الباحثون أن هناك حجة قوية لتنفيذ هذه البرامج على نطاق واسع في سائر البلدان المتقدمة.
Résumé
Les programmes qui fournissent une indemnisation sans égard à la faute dans le cas d’un événement négatif faisant suite à une vaccination ont été mis en place dans 19 pays du monde entier, le premier étant l’Allemagne en 1961 et le plus récent la Hongrie en 2005. Nous avons évalué ces programmes et déterminé les éléments qui leur étaient communs à tous : administration et financement, éligibilité, processus et prise de décision, norme de preuve, éléments d’indemnisation et droits relatifs aux litiges. La plupart de ces programmes sont gérés par les États ou les gouvernements nationaux, à l’exception de la Finlande et de la Suède où ils sont coordonnés par les fabricants pharmaceutiques. Bien que le financement provienne généralement du ministère des Finances, la Chine (province de Chine, Taïwan) et les États-Unis d’Amérique imposent une taxe sur les doses de vaccin distribuées. Les décisions quant à l’indemnisation sont prises à l’aide de critères définis ou évalués au cas par cas, alors que la norme de preuve requise est généralement inférieure à celle nécessaire pour les procès. Les avantages liés aux programmes comprennent les frais médicaux, les prestations d’invalidité et les avantages liés aux pertes non économiques et aux décès. La majorité des pays permet aux requérants de demander des dommages et intérêts légaux en faisant appel aux tribunaux, ou un dédommagement par un programme d’indemnisation, mais pas les deux. Pour conclure, différents programmes, reposant sur des principes éthiques, ont été couronnés de succès et viables du point de vue financier dans certains pays développés du monde entier. Nous pensons qu’il existe de bonnes raisons pour une vaste mise en œuvre de ces programmes dans d’autres pays développés.
Resumen
Diecinueve países de todo el mundo han puesto en marcha diversos programas para ofrecer a sus ciudadanos compensaciones sin admisión de responsabilidad por las reacciones adversas tras la vacunación. El primero de ellos fue Alemania, en 1961 y el más reciente, Hungría, en 2005. Hemos revisado estos programas y hemos determinado qué elementos son comunes a todos ellos: administración y financiación, elegibilidad, proceso y toma de decisiones, acervo probatorio, elementos de compensación y derechos de litigio. La mayoría de los programas estaban gestionados por el Estado o por los Gobiernos de cada país, excepto en los casos de Finlandia y Suecia, donde estaban coordinados por los laboratorios farmacéuticos. Si bien la financiación suele proceder del Erario Público, China (provincia de Taiwán) y Estados Unidos de América gravan impuestos sobre las dosis de vacunas distribuidas. Las decisiones sobre las compensaciones se adoptan siguiendo unos criterios establecidos o se evalúan en función de cada caso, mientras que el acervo probatorio necesario suele ser inferior al exigido para los casos que se elevan a los tribunales. Los beneficios que estos programas ofrecen incluyen gastos médicos, pensiones de invalidez y prestaciones por pérdidas no económicas y defunción. La mayoría de los países permite a los solicitantes reclamar daños y perjuicios ante los tribunales o un sistema de compensación monetaria, pero no ambas cosas. Hemos sacado en conclusión que, diversos países desarrollados en todo el mundo han aplicado con éxito varios programas económicamente viables basados en principios éticos. Creemos que hay razones más que suficientes para difundir la aplicación de estos programas en otros países desarrollados.
Резюме
Программы, предусматривающие компенсации во внесудебном порядке за ущерб здоровью, обусловленный вакцинацией, применяются в 19 странах мира, начиная с Германии, где такая программа была внедрена в 1961 году, и кончая Венгрией, где она осуществляется с 2005 года. Мы провели обзор этих программ и выявили элементы, являющиеся для них общими: управление и финансирование, право на получение компенсации, процедура подачи и рассмотрения заявления, уровень доказательств, элементы компенсации и право на возбуждение судебного иска. Управление программами в большинстве случаев осуществляет государство или местный орган власти, за исключением Финляндии и Швеции, где программы координируются производителями фармацевтической продукции. Хотя финансирование обычно производится из государственного бюджета, в Китае (Тайвань, провинция Китая) и в США действует налог на реализованные дозы вакцины. Решения о компенсации принимаются исходя из установленных критериев или на основе индивидуального рассмотрения каждого случая, причем уровень доказательств обычно ниже, чем при судебном разбирательстве. Пособия, выплачиваемые в рамках программ, включают в себя возмещение затрат на лечение, пенсию по инвалидности и пособие в связи с нефинансовым ущербом и в случае смерти. В большинстве стран заявителям разрешается требовать возмещения юридического ущерба в суде или путем получения компенсационных выплат, но не обоими этими способами. Мы приходим к выводу, что многочисленные программы, опирающиеся на морально-этические принципы, оказались успешными и продемонстрировали свою финансовую устойчивость в развитых странах мира. По нашему мнению, это убедительно свидетельствует о целесообразности широкого распространения этих программ также и в других развитых странах.
摘要
为疫苗接种后的不良反应提供无过失补偿的方案已经在世界范围内的19个国家实施,第一次是1961年在德国,而最近的一次是2005年在匈牙利。我们对这些方案进行审查并确定了所有这些方案的共同要素:管理和资金、资格、过程和决策、证明标准、补偿内容和诉讼权利。大多数方案都由国家或国家政府部门管理,但芬兰和瑞典除外,这两个国家由制药厂商协调。尽管资金通常来源于国库(如中国的台湾省),美国则向分配的疫苗剂量征税。运用确立的标准做补偿决定或视情况而定评估补偿,而所要求的证明标准通常少于诉讼案件所要求的证明标准。方案所提供的津贴包括医疗费用、伤残抚恤金以及对非经济损失和死亡的津贴。大多数国家都允许索赔人通过法庭寻求法定损害赔偿金或补偿计划支出,但不可同时要求两项赔偿或支出。我们得出的结论是:基于道德原则的各种方案在世界范围内的发展中国家是成功的,并且在经济上是可行的。我们相信这为该类方案在其他发展中国家的广泛实施提供了强有力的论据。
Introduction
The public health benefits of vaccination are clear. The World Health Organization estimates that, in 2008, more than 2.5 million deaths were prevented by vaccination.1 Immunization programmes have led to the eradication of smallpox, the elimination of measles and poliomyelitis in many regions, and substantial reductions in morbidity and mortality from Haemophilus influenzae type b, diphtheria, whooping cough and tetanus. However vaccines are not without risks and it is commonly accepted that, regardless of proper design, manufacture and delivery, adverse events occur following vaccination although serious adverse events are rare.2
At a population level, it is considered that these small risks are balanced by the benefits of widespread population immunization. However this means that an individual occasionally bears a significant burden for the benefit provided to the rest of the population. Although these vaccine-related adverse events occur occasionally due to negligence, more often there is no clearly attributable fault.
Without evidence of clear negligence, it is difficult to obtain compensation through traditional legal mechanisms. Recognizing this, several countries have implemented vaccine-injury compensation programmes.3 These programmes reflect a belief that it is fair and reasonable that a community that is protected by a vaccination programme accepts responsibility for and provides compensation to those who are injured by it. In 1999, Evans conducted a thorough review of 13 compensation programmes.3 We aimed to update this review examining similar programme elements to those described both by Evans and by Mariner in her 1985–6 study.4
Search strategy
We used a meta-search engine (Supersearch MetaLib®) to identify key published resources on vaccine-injury compensation schemes. Databases searched were: Web of Science®, Scopus v.4 (Elsevier), Medline (ISI), CINAHL®Plus (EBSCO), PsycINFO® (CSA), PubMed, Academic Search Premier (EBSCO), Expanded Academic ASAP (Gale), JSTOR, LegalTrac (Gale) and Law Journal Library (Hein).
Keywords entered were vaccine AND injury AND compensation; “vaccine injury”; vaccine AND damage AND compensation; vaccine AND compensation; “vaccine policy”; “vaccine injury” AND international; and “vaccine injury” AND [country name]. We scanned reference lists of key full text papers. We used citation tracking in PubMed, Google Scholar, ScienceDirect and EBSCOhost to forward track key papers and identify articles cited in mainstream journals. We performed a grey literature search in Google using the same keywords. We searched web pages of international organizations, bilateral agencies, nongovernmental organizations, consultancy firms and universities involved in funding, delivering or evaluating immunization services. We perused national government web sites to find details of specific country’s schemes. Finally we contacted key individuals involved in vaccine compensation programmes throughout the world.
Evolution of programmes
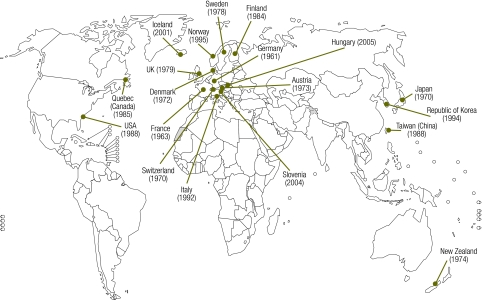
We identified 19 countries with current vaccine compensation schemes (Fig. 1). In 1953, the German Supreme Court ruled that people who were injured by compulsory vaccination (in this case smallpox) were entitled to compensation. Germany enacted a compensation programme in 1961.5 France implemented a similar scheme in the 1960s.6 Concern over injuries caused by medicines and the inadequacies of traditional litigation processes increased after the thalidomide tragedy in the 1960s. In the 1970s, concerns over adverse events related to diphtheria–tetanus–pertussis vaccination led to programmes being established in Austria,7 Denmark,8 Japan,9 New Zealand,10 Sweden11 Switzerland12and the United Kingdom of Great Britain and Northern Ireland (UK).13 In the 1980s, Taiwan (China),14 Finland,15 the United States of America (USA)16 and Quebec (Canada)17 implemented programmes. Italy,18 Norway19 and the Republic of Korea20 followed in the 1990s. The most recently implemented programmes are those in Hungary,21 Iceland22 and Slovenia.23
Fig. 1.
Countries and provinces that have introduced vaccine-injury compensation schemes (including year of introduction)
UK, United Kingdom of Great Britain and Northern Ireland; USA, United States of America.
Since Evans’ review3 there have been several political and socioeconomic shifts that have affected vaccine-injury compensation. Thirteen of the schemes reviewed in this paper are based in Europe, where many countries have since integrated vaccine liability as part of a more comprehensive no-fault approach to medical accidents.24 Furthermore, there is interest in harmonizing health policy within the European Union, illustrated by a recent proposal for a pan-European compensation scheme for injuries caused by defective products.25 France has moved away from a court-based compensation scheme to an administrative system and, in the United Kingdom, there has been discussion of an alternative general medical accidents’ liability scheme.26,27 There has also been significant public pressure in other countries, including Australia,28,29Canada30 and Ireland,31,32 to establish similar schemes. Recently, China has shown interest in a no-fault compensation scheme for vaccine injuries.33 To date there are no schemes that cover developing countries.
Arguments for schemes
Arguments supporting vaccine-injury compensation include political and economic pressures, litigation threats, increasing confidence in population-based vaccine programmes and ensuring sustainability of vaccine supply. However, compensation schemes are also based on underlying principles of fairness and justice.
If there is no formal compensation scheme, the only source of compensation is through the courts, usually under the law of tort. Tort law requires a claimant to prove that he or she has suffered a wrong due to another person’s negligence or deliberate harm. The problem with this process, in the case of vaccination, is that there is often no clearly negligent party. A court-based approach to compensation can be inequitable and unpredictable, resulting in high monetary awards for some, while those who do not seek legal recourse receive nothing.
In the USA, before 1987, those injured by vaccines had no choice but to take their chances in the court system and seek recovery for their injuries directly from the manufacturer.34 Without a compensation system, it became difficult for vaccine manufacturers to predict their exposure to lawsuits. Accordingly, manufacturers and their insurers increased prices based on worst-case estimates.35 This led to exponential price rises, vaccine shortages and a reduction in vaccine research. Furthermore, several small vaccine manufacturers left the market.35
A vaccine-injury compensation scheme removes the uncertainty of tort liability for manufacturers and provides a more fair, efficient and stable approach for injured parties. Litigation is an expensive and restricted avenue that is inaccessible for many vaccine recipients. Furthermore, compensation schemes avoid the polarization of drug companies against vaccine recipients through litigation and the associated negative media coverage.36
Many countries that have implemented compensation schemes have done so as an expression of community solidarity.4 Ethicist Michelle Mello argues that solidarity means members of a community do not bear the risks of vaccination alone.37 Vaccine injuries can be severe and complex, and are often suffered by children who require a lifetime of care and may not qualify for other benefits under accident insurance schemes.3 In a vaccination programme, the injured and uninjured pay unequal shares of the social cost of producing the social good of herd immunity.37 Mello argues that, in line with principles of fairness and solidarity, mechanisms are needed to prevent the uninjured (unintentionally) “free-riding” on the injured.
Common programme elements
From our review of current schemes, we identified six elements common to all schemes: administration and funding, eligibility, process and decision-making, standard of proof, elements of compensation and litigation rights. These elements are similar to those used by Evans in his 1999 review.3 Key aspects of these elements are summarized in Table 1 for six developed countries with established compensation programmes, selected to demonstrate the variety of approaches to these programmes.
Table 1. Common elements of vaccine-injury compensation schemes for six selected countries.
| Common elements | Finland | Germany | New Zealand | Sweden | United Kingdom of Great Britain and Northern Ireland | United States of America |
|---|---|---|---|---|---|---|
| Administration and funding | Manufacturers, distributors, suppliers tax | General revenue of the Lander (state governments) | ACC earner’s account (funded by personal income tax) and ACC non-earner’s account (funded by government) | Percentage levy of member manufacturers’ annual sales | National treasury | Flat-rate tax paid by manufacturers for every childhood vaccine sold |
| Eligibility/vaccines covered | All | All recommended vaccines (child and adult) | All | All | Recommended childhood vaccines, adult influenza, armed forces | Recommended childhood vaccines, adult influenza, armed forces |
| Process and decision-making | Finnish Pharmaceutical Insurance Pool claims officer | Internal Lander office of social recompensation (medical expert consultation) | Review by ACC Treatment Injury Advisory Committee | Claims manager with Zurich insurance | Evaluation by appointed medical officer | Vaccine injury table or proving “causation in fact”a |
| Standard of proof | Balance of probabilitiesb | Probable cause | Balance of probabilitiesb | “Preponderant probability” | Balance of probabilitiesb | Balance of probabilitiesb |
| Elements of compensation | Unreimbursed medical costs, disability pension, noneconomic loss, death benefits | Medical costs, disability pension, funeral costs | Medical costs, disability pension, death benefits | Unreimbursed medical costs, disability pension, death benefits | Lump sum payment | Non-reimbursed medical expenses, lost wages, noneconomic loss, future care costs, death, legal fees |
| Litigation rights | No | Limited | No | No | Yes | If compensation accepted, no further civil claim allowed |
| Claims compensated | 2004–2008: 45 vaccine claims | 1961–2001: ~4000 claims | 1992–2000: 77/293 | Vaccine-specific data not available | 2005: 4/106 | 2008: 145/226 (non-autism claims)c |
ACC, Accident Compensation Corporation.
a “Causation in fact” considers an injury to be proven if these 3 criteria are met: (i) a medical theory causally connecting the vaccination and the injury; (ii) a logical sequence of cause and effect showing that the vaccination was the reason for the injury; and (iii) a showing of a proximate temporal relationship between vaccination and injury.
b Balance of probabilities mean that there is a “preponderance of evidence” or more evidence than not to suggest the vaccine caused the injury. This is a lower burden of proof than “beyond reasonable doubt".
c Between 2001 and 2010 more than 5600 claims were made for autism or autism spectrum disorder caused by measles–mumps–rubella vaccine and thimerosal-containing vaccines. These cases were consolidated into the Omnibus Autism Proceedings. After several test cases, no causal link has been found between autism and the vaccines, however a large number of cases have not yet been formally dismissed or withdrawn from the courts. Given the separate process that has been established for this large number of claims, this data is listed separately in the claims data published by the National Vaccine Injury Compensation Programme in the United States of America.38
Administration and funding
Most compensation schemes are government enacted and run. This usually occurs at a national level, but in Germany and Switzerland the programme is administered by the state (or canton). Quebec, the only province in Canada with a vaccine-injury compensation scheme, administers its programme through the Provincial Ministry of Health and Social Services.
In Scandinavian countries, vaccine-injury compensation is part of broad no-fault compensation schemes for both medical treatment and medicines. In Denmark and Norway, this is administered by the Department of Health, whereas the Finnish and Swedish schemes are voluntary for pharmaceutical companies and are not operated by the government. After the thalidomide disaster in the early 1960s, the international pharmaceutical industry, operating in Sweden, collaborated with the insurance industry and government to establish a Swedish vaccine-injury compensation scheme, to which pharmaceutical companies and importers voluntarily pay contributions.36,39 Similarly, in Finland concerns about litigation and justice led to the government proposing a statutory scheme, even drafting an Act, but pharmaceutical manufacturers instead formed the Finnish cooperative for the indemnification of medicine-related injuries and negotiated with the insurance sector to establish its own voluntary scheme.15 In Norway, although the scheme is government run, it is also funded by contributions from the pharmaceutical industry. In New Zealand, there is no separate administrative entity to address vaccine injuries. Instead these are covered by the broad Accident Compensation Corporation,40 which is a statutory corporation that provides no-fault compensation for any personal injury and death caused by accident.
The source of funding for vaccine-injury compensation schemes largely reflects where decision-making power lies. Several countries finance their programmes from national, state or municipal treasuries or, in the case of Japan, a mixture of each. Finland, Norway and Sweden use a manufacturers’ levy. New Zealand’s scheme is financed from several sources including levies on employers, employees and motor vehicle owners, government funding and investment returns.
Taiwan (China) and the USA retain centralized government control over their schemes, which are funded from a vaccine tax. In Taiwan (China), a tax of one New Taiwan dollar (US$ 0.034) per vaccine dose is paid by the manufacturer or importer of the vaccine. In the USA, the tax is US$ 0.75 per dose.
In most countries, the compensation schemes are a secondary source of funding for medical and disability expenses. In general, patients receive primary support from the national public or private insurers. The compensation schemes can be relatively modest in size and not need to cover the full range of expenses that might be considered in a tort or product liability case.
Eligibility
As noted previously by Evans, there is considerable variation in the vaccines covered by compensation schemes. Some schemes cover only mandatory or recommended vaccines, while others cover all licensed medicines. The United Kingdom and USA cover childhood vaccines, adult influenza and vaccines given to the armed forces.41,42 In Italy, compensation is only payable for injuries from one of five mandatory vaccines or from non-mandated vaccines required for travel or employment.18 Other countries determine eligibility based on occupation (e.g. health care worker), indication (e.g. travel), citizenship and time elapsed between the vaccine and a claim.
All the schemes have threshold injury or disability criteria that need to be met before claiming compensation. In New Zealand, an injury has to be “severe” to be eligible for compensation.40 In England, compensation is paid when there is greater than 60% disability.41 Similarly, in Finland to be eligible for compensation an injury must result in a loss of functional ability for at least 14 days.15 German law only specifies that the injury must exceed a “normal post-vaccinal reaction", however supplemental payments are conditional on disability existing for at least 6 months.7
Evans describes four broad categories of benefits that are provided by vaccine-injury compensation schemes.3 These are: medical costs, disability pensions, coverage for noneconomic loss and death benefits. With the exception of the United Kingdom with its lump sum payment of 120 000 British pounds sterling, all schemes cover medical expenses, disability pensions and death benefits.41 These payments are usually proportional to the severity of the vaccine injury. Some countries also cover noneconomic loss including “pain and suffering” and compensation to family. The USA also compensates both successful and unsuccessful claimants for reasonable legal costs.
Process
All countries, except Finland and Sweden, have passed legislation to enact their compensation schemes and government departments operate the programmes in most countries. Most schemes require claims to be filed with an administrative body that makes initial eligibility and compensation decisions on claims. Many countries use an administrative process for deciding compensation eligibility and payment amounts. These schemes usually have an internal review process, with the option of external review if a claim is deemed complex or contentious. Proponents of these schemes believe this administrative approach is less adversarial, has lower costs, lessens the need to apportion blame and maximizes the opportunity for those with genuine vaccine injuries to receive just compensation.
While the procedures for filing a claim in the USA are modelled quite closely on the civil litigation process, the scheme includes a process for pre-determining causation if a vaccine injury is included on its vaccine injury table.42 This process presumes causation if any injury listed in the table occurs within a specified time frame after vaccination. For example, if anaphylaxis occurrs within 4 hours of hepatitis B vaccine administration, it is presumed due to the vaccine. While an alternate mechanism exists for injuries which fall outside the table specifications, most claims have been for “on-table” injuries.43 All countries examined have a formalized appeal process for claimants. In some places, including Scandinavia and the USA, appeals can be lodged disputing the size of the compensation payment. Some countries impose time-limits on lodging an appeal.
Vaccine-injury compensation schemes aim to streamline the process of receiving compensation. Most countries prioritize the timely resolution of claims, although the processing time varies depending on the size of the scheme and whether the scheme is part of a broader no-fault programme. In New Zealand, the Accident Compensation Corporation has 9 months to make a decision.10 In the USA, a claim decision takes an average 2–3 years.44 In France, the Office National d’Indemnisation des Accidents Medicaux has a statutory responsibility to process claims within 6 months.27
Standard of proof
No-fault vaccine-injury compensation programmes are based on the premise that the adverse outcome is not attributable to a specific individual or industry but due to an unavoidable risk associated with vaccines. A problem for all compensation schemes is determining whether there is a causal relationship between a vaccine and a specific injury. The method by which causation is proven in tort law can be quite different from the accepted method of establishing causation in science and epidemiology. The most commonly accepted criteria for establishing epidemiological causation are the Bradford Hill criteria.45 While they do not provide a definitive checklist for assessing causality,46 these criteria provide a framework for separating causal and non-causal explanations of observed associations. Despite its importance, there is no single, clear consensus on the definition of causation.
In tort litigation the defendant, or defective product, is on trial for “causing” a specific individual’s or group’s adverse outcome. A direct link must be established between the particular action of that defendant or product and the adverse outcome. Legal causation is deterministic and requires proof of an allegation.
In general, most compensation schemes offer a more liberal approach to standard of proof than the legal standard. For instance, the Swedish general drug injury compensation scheme requires a “preponderant probability” that an injury was caused by a drug. While apparently reluctant to define this specifically, commentators interpret this as a “slightly more than 50%” chance of a drug having caused an injury.36
In New Zealand, vaccine injuries were previously considered “medical misadventures".47 This was taken in practice to mean a “medical error” or “medical mishap". Although both forms of accident were eligible for compensation, the distinction required the Accident Compensation Corporation to investigate whether a vaccine injury was caused by an error or was an adverse outcome of a correctly delivered vaccine. This concept of “medical misadventure” was later replaced with the concept of “treatment injury”.10 This reflects a more genuine no-fault system, ensuring compensation for injured vaccine recipients regardless of whether the injury is judged avoidable or not. Similarly, in the USA, proof of the level required in the law courts is not necessary to access compensation. One of the key goals of the scheme was simplification of the compensation system for all parties. It was felt that requiring legal causation to be proved would be overly time consuming and laborious.
Litigation rights
To ensure that compensation schemes remain attractive to claimants, they must offer a compensation payment and process that is more appealing than the tort or litigation system. Most countries legislate that claimants can seek either damages through the courts or a compensation scheme payout but not both. Other countries, such as Denmark and the United Kingdom, adjust compensation payments if damages have been received through the courts.
Conclusion
Vaccine-injury compensation programmes are increasingly regarded as an important component of successful vaccination programmes. They have been used for the past 50 years to ensure that individuals who are adversely affected in the interests of protecting the whole community are adequately compensated and cared for. There are a variety of schemes with different structures and approaches in use throughout the world. The schemes function most efficiently when they operate alongside well established, comprehensive national social welfare systems. In these countries, vaccine-injury compensation schemes have been found to have a relatively low administrative cost, especially compared to civil litigation cases.36,48
In the first decade of the 21st century, acceptance of vaccine-injury compensation has grown. Schemes are being enacted beyond industrialized Europe and North America. The importance of these schemes, based on ethical principles, has been stressed by parent groups, and claimants have reported satisfaction in having received compensation through a streamlined process.49,50 Apart from the reluctance of governments to move away from the adversarial approach to providing compensation, we believe there is a strong argument for widespread implementation of these programmes in other developed countries.
Competing interests:
None declared.
References
- 1.State of the world's vaccines and immunization, 3rd ed. Geneva: World Health Organization; 2009. Available from: http://whqlibdoc.who.int/publications/2009/9789241563864_eng.pdf [accessed 15 March 2011].
- 2.Collet JP, MacDonald N, Cashman N, Pless R. Monitoring signals for vaccine safety: the assessment of individual adverse event reports by an expert advisory committee. The Advisory Committee on Causality Assessment. Bull World Health Organ. 2000;78:178–85. [PMC free article] [PubMed] [Google Scholar]
- 3.Evans G. Vaccine-injury compensation programmes worldwide. Vaccine. 1999;17(Suppl 3):S25–35. doi: 10.1016/S0264-410X(99)00291-1. [DOI] [PubMed] [Google Scholar]
- 4.Mariner WK. Compensation programmes for vaccine-related injury abroad: a comparative analysis. St Louis Univ Law J. 1987;31:599–654. [PubMed] [Google Scholar]
- 5.Protection against infection act (Infektionsschutzgesetz - IfSG), 1045; 20 July 2000 Berlin: Federal Law Gazette; 2000. Available from: http://www.rki.de/cln_162/nn_217358/EN/Content/Prevention/Inf__Dis__Surveillance/inf__dis__down.html?__nnn=true [accessed 15 March 2011].
- 6.Dispositif de règlement amiable des dommages imputables à des vaccinations obligatoires (in French). Paris: Office National d'Indemnisation des Accidents Médicaux; 2004. Available from: http://www.oniam.fr/vaccinations.php [accessed 15 March 2011].
- 7.Act for the compensation of vaccination damages (Impfschadengesetz, BGBI nr. 371/1973) (in German). Vienna: Bundeskanzleramt; 1973. Available from: http://www.ris.bka.gv.at/Ergebnis.wxe?Suchworte=371%2F1973&x=0&y=0&Abfrage=Gesamtabfrage [accessed 15 March 2011].
- 8.The Danish Liability for Damages Act. Copenhagen: Patientforsikringen; 2010. Available from: http://www.patientforsikringen.dk/en/Love-og-Regler/Lov-om-klage-og-erstatningsadgang/Behandlingsskader.aspx [accessed 15 March 2011].
- 9.Cabinet order no. 197 on implementation of immunization law (in Japanese). Tokyo: National Diet Library; 1948. Available from: http://hourei.ndl.go.jp [accessed 15 March 2011].
- 10.Accident Compensation Act 1972, s.2 § amended by Accident Compensation Amendment Act 2001. Wellington: New Zealand Legislation; 2001; Available from: http://www.legislation.govt.nz [accessed 15 March 2011].
- 11.The Undertaking (Indemnity rules). Stockholm: Lakemedelsforsakringen (LFF);2009; Available from: http://www.lakemedelsforsakringen.se/default.asp?id=10822&ptid= [accessed 15 March 2011].
- 12.Federal law from 18 December 1970 on communicable disease control (Epidemics act) 818.101 Berne: Federal Authorities of the Swiss Confederation;1970. Available from: http://www.admin.ch/ch/d/sr/c818_101.html [accessed 15 March 2011].
- 13.The Regulatory Reform (Vaccine Damage Payments Act 1979) Order 2002, Statutory Instrument 2002 no. 1592 London: Office of Public Sector Information; 2002. Available from http://www.opsi.gov.uk/si/si2002/20021592.htm [accessed 15 March 2011].
- 14.Communicable Disease Act, article 30: Regulations governing revenues and expenditures, safeguarding and utilization of the compensation fund for vaccine injuries Taipei: Department of Health of the Executive Yuan; 2001. Available from: http://dohlaw.doh.gov.tw/Chi/EngContent.asp?msgid=46&KeyWord= [accessed 15 March 2011].
- 15.Finnish Pharmaceutical Insurance Pool [Internet site]. Helsinki: Finnish Pharmaceutical Insurance Pool; 2011. Available from: http://www.lvp.fi/www/page/lvp_www_2090 [accessed 15 March 2011].
- 16.National Childhood Vaccine Injury Act. 1986; 42 USC 300aa-11 et seq Washington, DC:Department of Health and Human Services; 1986. Available from: http://www.hrsa.gov/vaccinecompensation/authorizinglegislation.pdf [accessed 15 March 2011].
- 17.Public Health Protection Act, RSQ, chapter S-2.2. Quebec City: Government of Quebec; 2011. Available from: http://www2.publicationsduquebec.gouv.qc.ca/dynamicSearch/telecharge.php?type=2&file=/S_2_2/S2_2_A.html [accessed 15 March 2011].
- 18.Law no. 238 of 25 July 1997, modifications and additions to the Law no. 210 of February 25 1992, relating to compensation to those harmed by mandatory vaccinations, blood transfusions and blood products (in Italian). Rome: Ministry of Health; 1997. Available from: http://www.normattiva.it [accessed 15 March 2011].
- 19.Patient Injury Act of 15 June 2001 no. 53 (in Norwegian). Oslo: Ministry of Health; 2001. Available from: http://www.lovdata.no/all/nl-20010615-053.html [accessed 15 March 2011].
- 20.Prevention of Contagious Diseases Act, Law 6162 (in Korean). Seoul: Korean Centers for Disease Control and Prevention; 2009. Available from: http://www.glin.gov/view.action?glinID=70100 [accessed 15 March 2011].
- 21.Boncz I, Sebestyen A. Compensation for vaccine injury in Hungary. Lancet. 2006;367:1144. doi: 10.1016/S0140-6736(06)68504-8. [DOI] [PubMed] [Google Scholar]
- 22.Act on patient insurance no. 111/2000 Reykjavik: Ministry of Welfare; 2000. Available from: http://eng.velferdarraduneyti.is/acts-of-Parliament/nr/20366 [accessed 15 March 2011].
- 23.Zanoni G, Berra P, Lucchi I, Ferro A, O'Flanagan D, Levy-Bruhl D, et al. Vaccine adverse event monitoring systems across the European Union countries: time for unifying efforts. Vaccine. 2009;27:3376–84. doi: 10.1016/j.vaccine.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 24.Dute J, Faure MG, Koziol H, editors. Tort and insurance law, vol 8: No-fault compensation in the health care sector. New York: Springer; 2004. [Google Scholar]
- 25.Calderini M, Cantamessa M, Palamigiano A. Analysis of the economic impact of the development risk clause as provided by directive 85/374/EEC on Liability for Defective Products. Rome: Fondazione Roselli; 2004 Available from: http://www.fondazionerosselli.it/User.it/index.php?PAGE=Sito_en/centri_ricer1&unit_id=14&rice_id=231 [accessed 15 March 2011].
- 26.Donaldson L. Making amends: a consultation paper setting out proposals for reforming the approach to clinical negligence in the NHS: a report by the Chief Medical Officer. London: Department of Health; 2003. [Google Scholar]
- 27.Gromb S, Dupon M. L'indemnisation des accidents de vaccination. Med Mal Infect. 2009;39:809–14. doi: 10.1016/j.medmal.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Isaacs D. Should Australia introduce a vaccine-injury compensation scheme? J Paediatr Child Health. 2004;40:247–9. doi: 10.1111/j.1440-1754.2004.00357.x. [DOI] [PubMed] [Google Scholar]
- 29.Report of the Select Committee on Immunisation and Vaccination Rates in Children Perth; Parliament of Western Australia; 1999.
- 30.Kutlesa NJ. Creating a sustainable immunization system in Canada - the case for a vaccine-related injury compensation scheme. Health Law J. 2004;12:201–42. [PubMed] [Google Scholar]
- 31.O'Halloran M. Harney supports a no-fault compensation scheme. Irish Times, 3 March 2009. Available from: http://www.irishtimes.com/newspaper/health/2009/0303/1224242142398.html [accessed 15 March 2011].
- 32.Vaccine Damage Steering Group. Report of Vaccine Damage Steering Group Dublin: Department of Health and Children; 2009. Available from: http://www.dohc.ie/publications/vaccine_damage.html [accessed 15 March 2011]. [Google Scholar]
- 33.Gourley S, Chen Y, Bass S, Austin S. China - compensation for drug and device-related injuries. In: PLC Cross-border Life Sciences Handbook 2008/09. London: Practical Law Company; 2009. Available from: http://www.sidley.com/files/Publication/44f59dfe-70d9-4a9a-8554-bf25e4589885/Presentation/PublicationAttachment/a6c89483-8f6b-46b1-992e-c16c8ec946e6/PLC_China_Compensation_Dec2008.pdf [accessed 15 March 2011].
- 34.Hensen R. Inoculated against recovery: a comparative analysis of vaccine-injury compensation in the United States and Great Britain. Tulsa Journal of Comparative and International Law. 2007-2008;15:61. [Google Scholar]
- 35.Evans G, Levine EM, Saindon EH. Legal issues (chapter 75). In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines, 5th ed. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 36.Hodges C. Nordic compensation schemes for drug injuries. J Consum Policy. 2006;29:143–75. doi: 10.1007/s10603-006-9003-4. [DOI] [Google Scholar]
- 37.Mello MM. Rationalizing vaccine-injury compensation. Bioethics. 2008;22:32–42. doi: 10.1111/j.1467-8519.2007.00590.x. [DOI] [PubMed] [Google Scholar]
- 38.National Vaccine Injury Compensation Programme. Statistics Reports, 20 October 2009 Washington, DC: Department of Health and Human Services; 2009. Available from: http://www.hrsa.gov/vaccinecompensation/statistics_report.htm [accessed 15 March 2011].
- 39.Oldertz C. Security insurance, patient insurance and pharmaceutical insurance in Sweden. Am J Comp Law. 1986;34:635–56. doi: 10.2307/840326. [DOI] [Google Scholar]
- 40.Accident Compensation Corporation [Internet site]. Available from: http://www.acc.co.nz/index.htm [accessed 15 March 2011].
- 41.Vaccine Damage Payments Unit [Internet site]. Available from: http://www.direct.gov.uk/en/MoneyTaxAndBenefits/BenefitsTaxCreditsAndOtherSupport/Disabledpeople/DG_10018714 [accessed 15 March 2011].
- 42.National Vaccine Injury Compensation Programme Washington, DC: Department of Health and Human Services; 2011. Available from: http://www.hrsa.gov/vaccinecompensation/covered_vaccines.htm [accessed 15 March 2011].
- 43.Evans G. The National Vaccine Injury Compensation Programme (VICP): what's new? In: National Immunization Conference, Nashville, Tennessee,14 May 2004 Available from: http://cdc.confex.com/cdc/nic2004/recordingredirect.cgi/id/1495 [accessed 15 March 2011].
- 44.National Vaccine Injury Compensation Programme. Statistics Reports, 3 January 2011 Washington, DC: Department of Health and Human Services; 2011. Available from: http://www.hrsa.gov/vaccinecompensation/statistics_report.htm [accessed 15 March 2011].
- 45.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips CV, Goodman KJ. The missed lessons of Sir Austin Bradford Hill. Epidemiol Perspect Innov. 2004;1:3. doi: 10.1186/1742-5573-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Royal Commission to inquire into and report upon workers’ compensation. Compensation for personal injury in New Zealand: report of the Royal Commission of Inquiry. Wellington: Government Printing; 1967. Available from: http://www.library.auckland.ac.nz/data/woodhouse/ [accessed 15 March 2011].
- 48.Plotkin SA. Lessons learned concerning vaccine safety. Vaccine. 2001 Oct 15; 20 Suppl 1:S16-9; discussion S1. [DOI] [PubMed]
- 49.Balinska MA. Vaccination in tomorrow's society. Lancet Infect Dis. 2003;3:443–7. doi: 10.1016/S1473-3099(03)00674-1. [DOI] [PubMed] [Google Scholar]
- 50.Advisory Commission on Childhood Vaccines. Transcript of meeting notes, 18 November 2008. Washington, DC: Department of Health and Human Services; 2008. Available from: http://www.hrsa.gov/vaccinecompensation/accv.htm [accessed 15 March 2011]. [Google Scholar]