Abstract
Urinary nitrite and F2-isoprostanes, an index of oxidant stress, were elevated during chlamydial genital infection of mice. Enhancement of urinary nitrite and F2-isoprostanes was observed in phagocyte oxidase-deficient mice. Inhibition of inducible nitric oxide synthase reduced isoprostane excretion. We conclude that nitrogen radicals induce F2-isoprostane production and excretion during murine chlamydial genital infection.
Disease outcome subsequent to genital tract infection of female mice with Chlamydia trachomatis, mouse pneumonitis (MoPn) biovar, includes infertility and hydrosalpinx formation (8, 23). It has been speculated that exacerbated inflammation in susceptible strains of mice is responsible for the enhanced pathology, although the exact mechanisms involved remain enigmatic (6, 7).
Previously, it has been shown that inducible nitric oxide synthase (iNOS) is not essential to resolution of microbiologically detected infection in this model but plays a key role in eradicating latent infection and moderating disease outcomes (20-22). The later conclusions were supported by the observation that, in vivo, iNOS activity was inversely correlated with resistance to hydrosalpinx and infertility in wild-type mouse strains and also by the observation that chemical inhibition of iNOS activity or genetic deletion of iNOS (NOS2−/−) caused an increase in the rate of postinfection hydrosalpinx formation. In the same report, it was also noted that genetic deletion of phagocyte oxidase (PHOX) in mice (p47phox−/−) did not affect the course of infection but p47phox−/− mice were found to excrete significantly higher levels of nitrite in their urine and sustained significantly lower rates of chronic chlamydial disease as assessed by hydrosalpinx formation.
The protective effect of PHOX against chronic disease was reversed by chemical inhibition of iNOS activity in p47phox−/− knockout mice. It was concluded that while neither nitrogen nor oxygen radicals were essential to resolution of culture-proven infection in this model, iNOS activity served to mollify an unknown pathological effect. Though the results implicated a role for PHOX in oxidant stress and pathology in this model, protection from chronic disease by iNOS could not be completely attributed to inactivation of reactive oxygen species by nitric oxide and the resulting formation of peroxynitrite. This was because in the absence of both phagocyte oxidase and iNOS (i.e., in p47phox−/− mice treated with a chemical inhibitor of iNOS), a significant rate of hydrosalpinx still occurred.
Because of these findings, we became interested in assessing oxidant stress in vivo in this model. The prostaglandin F2-like compounds termed F2-isoprostanes are secondary end products of peroxidation of arachidonic acid and are formed independently of cyclooxygenases (16, 18). The measurement of F2-isoprostanes in culture supernatants as well as body fluids such as plasma, urine, and cerebrospinal fluid is a reliable and accurate index of direct free-radical-mediated lipid peroxidation and oxidative stress in several in vitro model systems as well as in animals and humans (5, 17).
Nitrogen and oxygen radicals and their coupling by-products (e.g., peroxynitrite) are known to induce the enzymatically generated prostaglandins (11, 13). Prostaglandins, in turn, may regulate iNOS activity (10, 14). In addition to enzymatic generation of prostaglandins, a role for iNOS in the nonenzymatic generation of F2-isoprostanes has been established (13). In that study, normal baseline levels of urinary F2-isoprostanes were significantly decreased in NOS2−/− mice compared to those in wild-type controls, indicating that iNOS may regulate F2-isoprostanes in vivo during homeostasis. Currently, little is known about the role of iNOS in the production of F2-isoprostanes during iNOS-mediated infectious or inflammatory conditions in vivo.
In the present study, we extended previous work related to the role of oxygen and nitrogen radicals in the development of chronic disease subsequent to chlamydial infection (e.g., hydrosalpinx formation) in the female mouse genital tract. Specifically, we hypothesized that measurement of urinary excretion of F2-isoprostanes subsequent to chlamydial infection could provide a noninvasive in vivo correlate of oxidative stress that would be predictive of disease outcome. To this end, we measured urinary excretion of F2-isoprostanes and nitrite in C57BL/6 wild-type, p47phox−/−, and p47phox−/− mice inhibited in nitric oxide production by the administration of the l-arginine analog NG-monomethyl-l-arginine (l-NMMA) and correlated this with rates of chronic disease in this model.
Mice with a targeted deletion in the cytosolic p47 PHOX gene (p47phox−/−), which is essential for superoxide production by NADPH oxidase, were obtained from a colony at the National Institute of Allergy and Infectious Diseases. The knockout in these mice was confirmed as described elsewhere (22). Wild-type C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine). A parallel set of mice from each experimental group was housed in rodent metabolism cages in order to collect urine for nitrite determination as described below.
For infection, mice were treated with DepoProvera (P4; Upjohn, Kalamazoo, Mich.) and inoculated intravaginally with 200 50% infectious doses (ID50) of HeLa 229-grown Chlamydia trachomatis MoPn, exactly as described elsewhere (3, 4). Shedding of viable chlamydiae from the lower urogenital tract was assessed by collection of cervical-vaginal swabs and culture in HeLa 229 cell monolayers for the quantification of inclusion-forming units (IFU) by indirect fluorescent microscopy (3, 4).
Necropsy was performed in animals at day 56 postinfection. At necropsy, hydrosalpinx formation was assessed by gross macroscopic or 10× microscopic observation as previously described (21, 23). Hydrosalpinx was assessed as a marker of chronic chlamydial disease and a surrogate marker of infertility in this model (23).
Inducible nitric oxide synthase activity in vivo in response to the infection was assessed as previously described (9, 20). Mice were housed in Plexiglas metabolism cages (Nalgene, Rochester, N.Y.), beginning 6 days prior to infection, and fed nitrate- and nitrite-free amino acid rodent chow (Ziegler Brothers, Gardners, Pa.) and sterile acidified Millipore water ad libitum. Some groups received either 50 mM l-NMMA (Cyclopps Corp., Salt Lake City, Utah) or 50 mM l-arginine (as a control) in their drinking water. Food and water were changed and replenished daily, and urine was collected daily in receptacles containing isopropanol (isopropanol-urine ratio, approximately 1:5) to suppress growth of bacterial and fungal contaminants. Urine samples were batch-assessed for nitrite content, an indicator of in vivo iNOS activity, by adaptation of the Greiss reaction (9, 20) and standardized according to creatinine content with a manual picric acid method (Sigma Aldrich, St. Louis, Mo.).
Urine F2-isoprostane concentrations were measured by gas chromatography-mass spectrometry as described previously and are expressed as nanograms per milligram of creatinine (13).
For statistical analysis, the mean nitrite and F2-isoprostane concentrations in urine over time in response to infection were compared with the Kruskal-Wallis one-way analysis of variance on ranks.
The infection course, urine nitrite levels, and rates of hydrosalpinx formation have been published previously (20-22). The C57BL/6 wild-type mice, p47phox−/− mice treated with l-arginine, and p47phox−/− mice chemically depleted of iNOS activity by the administration of l-NMMA were all capable of resolving their infections. The infection peaked at 4 to 7 days postinoculation, with a mean of 105 to 106 IFU per cervical-vaginal swab per mouse. All mice resolved the infection within 28 to 35 days. We did note that the p47phox−/− l-NMMA-treated mice resolved the infection more rapidly and sustained significantly lower IFU counts than either the p47phox−/− l-arginine-treated or the C57BL/6 wild-type mice (22). We have previously hypothesized that the shortened infection course in these mice was attributed to the dampening effect of oxygen and nitrogen radicals on T lymphocyte activity that is critical to resolution of infection in this model. Both oxygen and nitrogen radicals have been shown to dampen T-cell activity or induce apoptosis in T cells (1, 15, 19, 23).
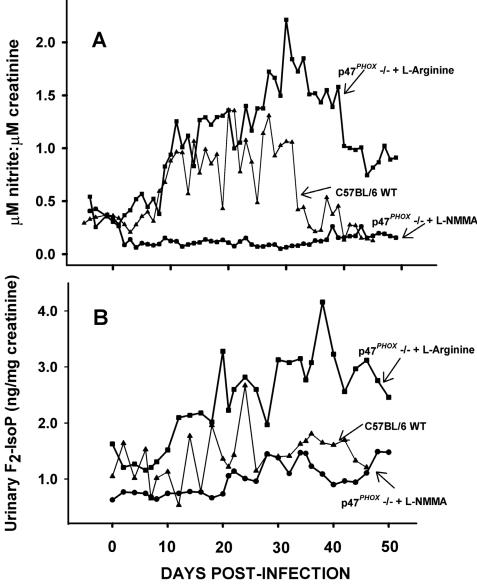
Figure 1 A shows the concentration of urinary nitrite excretion standardized to creatinine over the course of infection in p47phox−/− l-arginine-treated, p47phox−/− l-NMMA-treated, and C57BL/6 wild-type l-arginine-treated mice. These data are previously published and are replicated here only for clarification and ease of reference (22). These results indicate that in vivo iNOS activity in p47phox−/− mice was significantly elevated and protracted compared to that observed in wild-type mice. Accumulation of nitric oxide and, therefore, nitrite in the absence of a significant source of reactive oxygen intermediates to generate by-products such as peroxynitrite has been demonstrated previously (2). The administration of the iNOS inhibitor l-NMMA to p47phox−/− mice effectively blunted urine nitrite responses in these mice to a level below that in the C57BL/6 wild-type mice. This proved in vivo inhibition of iNOS activity by this treatment.
FIG. 1.
(A) Urine nitrite excretion in response to C. trachomatis urogenital infection in p47phox−/− and wild-type mice (reproduced from reference 22). Urine was collected daily from 6 days prior to infection until day 55 postinfection. Nitrite levels are standardized according to creatinine concentration as described in the text. The lines represent the data from the various groups as labeled in the figure. Significantly elevated excretion of nitrite was observed in the p47phox−/− l-arginine-treated mice compared to that in wild-type mice treated with l-arginine (P < 0.00001; Kruskal-Wallis one-way analysis of variance on ranks). This response was significantly blunted by treatment of the p47phox−/− mice with l-NMMA compared to the response in either the wild-type or p47phox−/− l-arginine-treated controls (P < 0.00001; Kruskal-Wallis one-way analysis of variance on ranks). (B) Excretion of F2-isoprostanes in urine in response to C. trachomatis urogenital infection in p47phox−/− and wild-type mice. Using separate aliquots of the same urine samples assessed for nitrite concentration as described for panel A, F2-isoprostane was assessed at 2-day intervals throughout the course of infection until day 48 (wild-type group) or 52 (p47phox−/− group) postinfection. F2-isoprostane levels were standardized to creatinine concentration. Lines represent data from the experimental groups as labeled in this panel and in panel A. Significantly elevated F2-isoprostane levels were observed in the p47phox−/− l-arginine-treated group compared to those in wild-type mice treated with l-arginine (P < 0.00001; Kruskal-Wallis one-way analysis of variance on ranks). This response was significantly blunted by treatment of the p47phox−/− mice with l-NMMA compared to the response in the p47phox−/− l-arginine-treated controls (P < 0.00001; Kruskal-Wallis one-way analysis of variance on ranks).
In panel B of Fig. 1, we show the novel finding of elevated urinary excretion of F2-isoprostanes subsequent to infection in the C57BL/6 wild-type mice. In p47phox−/− mice, this response was exacerbated concomitant with the observed elevated and protracted urine nitrite excretion, as described above. Chemical inhibition of iNOS activity in the p47phox−/− mice caused a concomitant blunting of urinary F2-isoprostane excretion. The data at day 0 in panel B of Fig. 1 also confirm previous observations that baseline levels of urinary F2-isoprostanes are decreased in p47phox−/− mice treated with l-NMMA compared to those in wild-type controls, indicating that iNOS regulates F2-isoprostanes in vivo during homeostasis (13). As with urine nitrite, F2-isoprostanes were standardized to urine creatinine to normalize for glomerular filtration rate variations among animals or groups of animals receiving different treatments.
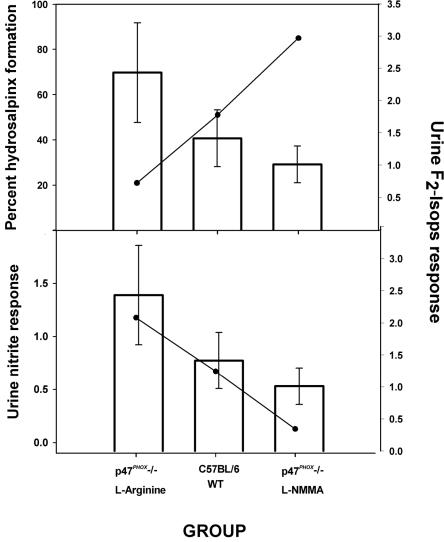
Figure 2A summarizes the present data and shows an inverse correlation of the mean cumulative urine F2-isoprostane level (day 6 postinfection through day 48) with the rate of hydrosalpinx formation, an indicator of chronic chlamydial disease in this model. Similarly, Fig. 2B shows that the mean cumulative F2-isoprostane levels (day 6 postinfection through day 48) were positively correlated with mean cumulative urine nitrite levels in response to the infection. We conclude that elevation of F2-isoprostane levels directly correlates with iNOS activity in vivo and is inversely associated with chronic chlamydial disease in this model.
FIG. 2.
Urinary excretion of F2-isoprostanes correlates inversely with the rate of hydrosalpinx formation and directly with urine nitrite excretion. (A) The mean rates of hydrosalpinx formation in the various experimental groups are summarized and shown here as a line graph. The mean urine F2-isoprostane level (day 6 through day 48 postinfection) was calculated for each group and is displayed as the open bars. A negative correlation between F2-isoprostane levels and the rate of hydrosalpinx formation was observed when the mean F2-isoprostane levels and rates of hydrosalpinx were compared among the groups (correlation coefficient = −0.95204). (B) The mean urine nitrite excretion (also days 6 through 48 postinfection) was calculated for each experimental group from the data displayed in panel A and is shown here as the solid line graph in panel B. The bar graph in panel B is the same as that in panel A and represents mean urine F2-isoprostane excretion from day 6 to day 48 postinfection. A positive correlation was found between the mean urine nitrite excretion during the infection and urine F2-isoprostane excretion (correlation coefficient = 0.8843).
Herein we present the novel finding of elevated in vivo F2-isoprostane levels in response to a chlamydial infection. Furthermore, we have established that iNOS-generated nitrogen radicals in this model can mediate inflammatory production of F2-isoprostanes. This was proved by the finding of positive correlation between urine nitrite excretion and F2-isoprostane levels in response to chlamydial infection in both C57BL/6 wild-type and p47phox−/− mice and confirmed by the blunting of the F2-isoprostane response when iNOS activity was inhibited by the administration of l-NMMA in p47phox−/− mice.
In numerous other models, F2-isoprostanes have been found to be a reliable and accurate measure of in vivo oxidative stress, although the exact mechanism of their generation has been unclear. For example, elevated F2-isoprostane levels correlate nicely with disease severity in animal models of oxidant stress (e.g., CCl4 and diquat intoxication) as reviewed previously (16, 18). Similarly, F2-isoprostane levels are elevated in human inflammatory diseases mediated by oxidant stress, such as Alzheimer's disease, asthma, cardiovascular disease, type 1 and 2 diabetes, systemic lupus erythematosus, Crohn's disease, and others, as reviewed previously (5).
In the present model, elevated iNOS activity may be involved in microbicidal eradication of persistent chlamydiae from affected tissues, eliminating a source of antigenic stimulus that would drive chronic inflammatory responses, as has been hypothesized elsewhere (21). An alternative hypothesis would be that iNOS mollifies overzealous inflammatory responses, such as those mediated by T lymphocytes (1, 15, 19, 24), or may modulate the activity of metalloproteinases, thereby reducing proteolytic dissolution of tissue (12). In either circumstance, F2-isoprostanes could be hypothesized to be noninvasive markers for a protective immunological or inflammatory response in vivo.
We had originally hypothesized that elevated urine F2-isoprostane levels would be an indicator of oxidative stress induced by PHOX-derived oxygen radicals and thereby provide a measure of in vivo PHOX activity similar to that of urine nitrite resulting from iNOS activity in vivo. We had also hypothesized that this would be positively correlated with the formation of hydrosalpinx and, therefore, development of chronic chlamydial disease in this model. However, we were surprised to find that elevated iNOS activity positively correlates with protection from chronic disease sequelae (i.e., hydrosalpinx formation and infertility). It would appear that this might be at least one example where iNOS-mediated oxidative stress in vivo correlates with a positive disease outcome. In this regard, it would be interesting to determine if F2-isoprostane levels are elevated during pelvic inflammatory disease or other inflammatory diseases of chlamydial etiology and whether this could be used as a noninvasive indicator of chlamydial disease outcome in humans.
Acknowledgments
This work was supported in part by Public Health Service grants AI49354 (to K.H.R.), AI19782 (to G.I.B.), GM15431 (to J.D.M.), CA77839 (to J.D.M.), and DK48831 (to J.D.M.). J.D.M. is the recipient of a Burroughs Wellcome Fund Clinical Scientist Award in Translational Research.
Editor: F. C. Fang
REFERENCES
- 1.Allione, A., P. Bernabei, M. Bosticardo, S. Ariotti, G. Forni, and F. Novelli. 1999. Nitric oxide suppresses human T lymphocyte proliferation through IFN-gamma-dependent and IFN-gamma-independent induction of apoptosis. J. Immunol. 163:4182-4191. [PubMed] [Google Scholar]
- 2.Clark, S., M. Coffey, R. Maclean, P. Collins, M. Lewis, A. Cross, and V. O'Donnell. 2002. Characterization of nitric oxide consumption pathways by normal, chronic granulomatous disease and myeloperoxidase-deficient human neutrophils. J. Immunol. 169:5889-5896. [DOI] [PubMed] [Google Scholar]
- 3.Cotter, T. W., Q. Meng, Z. Shen, Y. Zhang, H. Su, and H. D. Caldwell. 1996. Protective efficacy of major outer membrane protein specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4704-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter, T. W., G. S. Miranpuri, K. H. Ramsey, C. E. Poulsen, and G. I. Byrne. 1997. Reactivation of chlamydial genital tract infection in mice. Infect. Immun. 65:2067-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cracowski, J. L., T. Durand, and G. Bessard. 2002. Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends Pharmacol. Sci. 23:360-366. [DOI] [PubMed] [Google Scholar]
- 6.Darville, T., C. W. Andrews, K. K. Laffoon, W. Shymasani, L. R. Kishen, and R. G. Rank. 1997. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect. Immun. 65:3065-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darville, T., C. W. Andrews, Jr., J. D. Sikes, P. L. Fraley, L. Braswell, and R. G. Rank. 2001. Mouse strain-dependent chemokine regulation of the genital tract T helper cell type 1 immune response. Infect. Immun. 69:7419-7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De La Maza, L. M., S. Pal, A. Khamesipour, and E. M. Peterson. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect. Immun. 62:2094-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granger, D. L., J. B. Hibbs, and L. M. Broadnax. 1991. Urinary nitrate excretion in relation to murine macrophage activation: influence of dietary l-arginine and oral NG-monomethyl-l-arginine. J. Immunol. 146:1294-1302. [PubMed] [Google Scholar]
- 10.Kelner, M. J., and S. F. Uglik. 1995. Superoxide dismutase abolishes the platelet-derived growth factor-induced release of prostaglandin E2 by blocking induction of nitric oxide synthase: role of superoxide. Arch. Biochem. Biophys. 322:31-38. [DOI] [PubMed] [Google Scholar]
- 11.Landino, L. M., B. C. Crews, M. D. Timmons, J. D. Morrow, and L. J. Marnett. 1996. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc. Natl. Acad. Sci. USA 93:15069-15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda, H., T. Okamota, and T. Akaike. 1998. Hum. matrix metalloprotease activation by insults of bacterial infection involving proteases and free radicals. Biol. Chem. 379:193-200. [DOI] [PubMed] [Google Scholar]
- 13.Marnett, L. J., T. L. Wright, B. C. Crews, S. R. Tannenbaum, and J. D. Morrow. 2000. Regulation of prostaglandin biosynthesis by nitric oxide is revealed by targeted deletion of inducible nitric-oxide synthase. J. Biol. Chem. 275:13427-13430. [DOI] [PubMed] [Google Scholar]
- 14.Milano, S., F. Arcoleo, M. Dieli, R. D'Agostino, P. D'Agostino, G. De Nucci, and E. Cillari. 1995. Prostaglandin E2 regulates inducible nitric oxide synthase in the murine macrophage cell line J774. Prostaglandins 49:105-115. [DOI] [PubMed] [Google Scholar]
- 15.Millar, A. E., J. Sternberg, C. McSharry, X. Q. Wei, F. Y. Liew, and C. M. Turner. 1999. T-cell responses during Trypanosoma brucei infections in mice deficient in inducible nitric oxide synthase. Infect. Immun. 67:3334-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrow, J. D. 2000. The isoprostanes: their quantification as an index of oxidant stress status in vivo. Drug Metab. Rev. 32:377-385. [DOI] [PubMed] [Google Scholar]
- 17.Morrow, J. D., and L. J. Roberts. 1997. The isoprostanes: unique bioactive products of lipid peroxidation. Prog. Lipid Res. 36:1-21. [DOI] [PubMed] [Google Scholar]
- 18.Morrow, J. D., and L. J. Roberts. 2002. The isoprostanes: their role as an index of oxidant stress status in human pulmonary disease. Am. J. Respir. Crit. Care Med. 166:S25-S30. [DOI] [PubMed] [Google Scholar]
- 19.Nabeshima, S., M. Nomoto, G. Matsuzaki, K. Kishihara, H. Taniguchi, S. Yoshida, and K. Nomoto. 1999. T-cell hyporesponsiveness induced by activated macrophages through nitric oxide production in mice infected with Mycobacterium tuberculosis. Infect. Immun. 67:3221-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsey, K. H., G. S. Miranpuri, C. E. Poulsen, N. B. Marthakis, L. M. Braune, and G. I. Byrne. 1998. Inducible nitric oxide synthase does not affect resolution of murine chlamydial genital tract infections or eradication of chlamydiae in primary murine cell culture. Infect. Immun. 66:835-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsey, K. H., G. S. Miranpuri, I. M. Sigar, S. Ouellette, and G. I. Byrne. 2001. Chlamydia trachomatis persistence in the female mouse genital tract: inducible nitric oxide synthase and infection outcome. Infect. Immun. 69:5131-5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramsey, K. H., I. M. Sigar, S. V. Rana, J. Gupta, S. M. Holland, and G. I. Byrne. 2001. Role for inducible nitric oxide synthase in protection from chronic Chlamydia trachomatis urogenital disease in mice and its regulation by oxygen free radicals. Infect. Immun. 69:7374-7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su, H., R. Messer, W. Whitmire, E. Fischer, J. C. Portis, and H. D. Caldwell. 1998. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable chlamydiae. J. Exp. Med. 188:809-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Veen, R. C., T. A. Dietlin, F. M. Hofman, L. Pen, B. H. Segal, and S. M. Holland. 2000. Superoxide prevents nitric oxide-mediated suppression of helper T lymphocytes: decreased autoimmune encephalomyelitis in nicotinamide adenine dinucleotide phosphate oxidase knockout mice. J. Immunol. 164:5177-5183. [DOI] [PubMed] [Google Scholar]