Abstract
Lipopolysaccharide (LPS) binding protein (LBP) is an acute-phase protein that enhances the responsiveness of immune cells to LPS by virtue of its capacity to transfer LPS to CD14. To determine the role of LBP in the innate immune response to peritonitis, LBP gene-deficient (LBP−/−) and normal wild-type mice were intraperitoneally infected with Escherichia coli, the most common causative pathogen of this disease. LBP was detected at low concentrations in peritoneal fluid of healthy wild-type mice, and the local LBP levels increased rapidly upon induction of peritonitis. LBP−/− mice were highly susceptible to E. coli peritonitis, as indicated by accelerated mortality, earlier bacterial dissemination to the blood, impaired bacterial clearance in the peritoneal cavity, and more severe remote organ damage. LBP−/− mice displayed diminished early tumor necrosis factor alpha, interleukin-6, cytokine-induced neutrophil chemoattractant, and macrophage inflammatory protein 2 production and attenuated recruitment of polymorphonuclear leukocytes to the site of infection, indicating that acute inflammation was promoted by LBP. Locally produced LBP is an essential component of an effective innate immune response to E. coli peritonitis.
Acute bacterial peritonitis is a life-threatening condition characterized by the presence of bacteria in the otherwise germfree peritoneal cavity and almost invariably is caused by perforation of the intestines. Consequently, the most commonly encountered pathogens are enteric gram-negative bacteria, and Escherichia coli is found in up to 60% of cases (14). Despite advances in surgery and antimicrobial therapy, the mortality rate of peritonitis ranges from 30 to 50%. In sepsis that originates from peritonitis the mortality rate can be as high as 80% (9). The acute course of peritonitis makes instant initiation of host defense mechanisms mandatory.
The innate immune system enables the host to mount an immediate response to invading pathogens (17). Hence, the innate immune system is the central element of host defense in peritonitis. To recognize the presence of pathogens, innate immune cells express receptors that identify highly conserved pathogen-associated molecular structures (16). Lipopolysaccharide (LPS) is the major constituent of the outer cell wall of gram-negative bacteria and is the principal mediator of inflammatory responses to these pathogens. CD14, Toll-like receptor 4 (TLR4), and MD-2 make up the LPS receptor complex involved in the cellular recognition of and signaling by LPS (6, 10, 19, 25). The earliest events after LPS release require the transfer of LPS to immune cells. Spontaneous diffusion of LPS to cellular binding sites is very slow as a result of the amphiphile structure of LPS and its tendency to form aggregates in aqueous solution. LPS binding protein (LBP) greatly enhances the transfer of LPS from aggregates to the CD14-TLR4 receptor complex (23, 25). As a lipid transfer protein, LBP recognizes and binds the lipid A portion of LPS and augments the immune response to LPS up to 1,000-fold (15, 22, 26). LBP is an acute-phase protein that is synthesized principally in hepatocytes, and its production is greatly increased by stimulation with interleukin-1β (IL-1β) or IL-6 (20). Consequently, elevated LBP serum levels have been described for severe sepsis (2).
The biological significance of LBP was established when LBP gene-deficient (LBP−/−) mice were shown to be resistant to LPS toxicity and highly susceptible to Salmonella enterica serovar Typhimurium peritonitis (5, 11). Similar observations for Salmonella peritonitis were made with mice lacking CD14 or TLR4, emphasizing the importance of these three components of the LPS recognition machinery in this gram-negative infection (1). However, although these reports provided valuable information, an animal model having little clinical importance was used in the studies since Salmonella infections are food borne and almost never cause peritonitis. Attempts to investigate the in vivo contribution of LBP in a clinically relevant oral Salmonella infection did not reveal an important role for LBP (5). In addition, as an intracellular pathogen Salmonella relies on different host defense mechanisms than the extracellular pathogen E. coli relies on, which is shown by the fact that CD14−/− mice are protected against lethal effects in E. coli peritonitis but succumb to Salmonella peritonitis (1, 6). According to previous reports, CD14 deficiency was associated with an impaired polymorphonuclear leukocyte (PMN) influx in Salmonella peritonitis, whereas intense and early recruitment of PMNs protected mice in E. coli peritonitis (7, 27).
Our knowledge of the contribution of LBP to host defense against E. coli infection in general and against E. coli peritonitis in particular is very limited. Fierer et al. reported that there was no difference in survival between LBP−/− and wild-type mice after intraperitoneal (i.p.) injection of high doses of E. coli, but they did not show the actual data; the influence of LBP deficiency on local and systemic host responses was not reported by these authors (5). Le Roy et al. did not observe an effect on bacterial loads or cytokine release in mice treated with a blocking anti-LBP monoclonal antibody and intravenously challenged with either a high or low dose of E. coli (13). Nonetheless, there is evidence that LBP does play a role in E. coli peritonitis. Indeed, administration of high doses of exogenous LBP (100 μg) protected mice from lethality during E. coli peritonitis due to a proposed anti-inflammatory mechanism (12). In the present study we sought to determine the role of endogenously produced LBP in the early local and systemic host responses to abdominal sepsis caused by E. coli by using LBP−/− mice.
MATERIALS AND METHODS
Mice.
LBP−/− mice were generated as described previously and were backcrossed in a C57BL/6 background 11 times (26). Pathogen-free 9- to 11-week-old male C57BL/6 wild-type mice were purchased from Harlan Sprague-Dawley (Horst, The Netherlands). Age- and sex-matched mice were used in all experiments. The Animal Care and Use Committee of the University of Amsterdam (Amsterdam, The Netherlands) approved all experiments.
Stimulation of peritoneal macrophages and whole blood.
Wild-type mice (n = 8) were anesthetized by inhalation of isoflurane (Abbott Laboratories, Kent, United Kingdom), and peritoneal lavage was performed with 5 ml of sterile saline by using an 18-gauge needle. The lavage fluid was collected in sterile tubes and put on ice. Peritoneal macrophages were washed, counted, and resuspended in RPMI 1640 containing 1 mM pyruvate, 2 mM l-glutamine, penicillin, and streptomycin to a final concentration of 1 × 106 cells/ml. Cells were then cultured in 96-well microtiter plates (Greiner, Alphen a/d Rijn, The Netherlands) for 2 h and washed with RPMI 1640 to remove the nonadherent cells. Adherent monolayer cells were stimulated with heat-killed E. coli (O18:K1; 1 × 108 CFU/ml) or RPMI 1640 for 16 h. Stimulation was carried out in the presence or absence of 10% fetal calf serum (FCS) (Gibco, Detroit, Mich.). Supernatants were collected and stored at −70°C until they were assayed for tumor necrosis factor alpha (TNF-α) (the assay is described below). Blood was drawn from LBP−/− and wild-type mice after induction of deeper anesthesia by i.p. injection of 0.07 ml of FFM (0.315 mg of Fentanyl [Janssen, Beersen, Belgium] per ml, 10 mg of Fluanisone [Janssen] per ml, and 5 mg of Midazolam [Roche, Mijdrecht, The Netherlands] per ml) per g. Whole blood was diluted 1:5 in FCS-free RPMI 1640 and plated in 96-well plates, and stimulation was carried out as described above for peritoneal macrophages.
Induction of peritonitis.
Peritonitis was induced as described previously (21). In brief, E. coli O18:K1 was cultured in Luria-Bertani medium (Difco, Detroit, Mich.) at 37°C, harvested at the mid-log phase, and washed twice before inoculation. Mice were inoculated i.p. with 2 × 104 CFU of E. coli in 200 μl of sterile saline. The inoculum was plated on blood agar plates to determine viable counts.
Monitoring of mortality and enumeration of bacteria.
In survival studies, nine mice per treatment group were inoculated with E. coli, and mortality was assessed every 2 h. In separate studies, mice were sacrificed 4 or 16 h after infection; at these times, mice were anesthetized by inhalation of isoflurane, and peritoneal lavage was performed with 5 ml of sterile isotonic saline by using an 18-gauge needle. Lavage fluid was collected in sterile tubes and put on ice. After collection of the peritoneal fluid deeper anesthesia was induced by i.p. injection of 0.07 ml of FFM per g, as described above. After the abdomen was opened, blood was drawn from the lower caval vein, collected in sterile tubes containing heparin, and immediately placed on ice. Liver lobes were harvested and homogenized at 4°C in 4 volumes of sterile saline by using a tissue homogenizer (Biospec Products, Bartlesville, Okla.). The numbers of CFU were determined from serial dilutions of the peritoneal lavage fluid (PLF), liver homogenates, and blood plated on blood agar plates and incubated at 37°C for 16 h before colonies were counted.
Cell count counting and differential cell counting.
Cell counts, determined for each PLF sample stained with Türck's solution (Merck, Darmstadt, Germany), were determined with a hemocytometer (Türck counting chamber). The cells were then diluted to a final concentration of 105 cells/ml, and differential cell counting was performed with cytospin preparations stained with Giemsa stain.
Assays.
Murine LBP was measured by using a commercially available enzyme-linked immunosorbent assay (HyCult Biotechnology, Uden, The Netherlands) according to the manufacturer's instructions; the detection limit was 0.4 ng/ml. Cytokines and chemokines (TNF-α, IL-6, cytokine-induced neutrophil chemoattractant [KC], and macrophage inflammatory protein 2 [MIP-2]) were measured by using specific enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, Minn.) according to the manufacturer's instructions. The detection limits were 31 pg/ml for TNF-α, 16 pg/ml for IL-6, 12 pg/ml for KC, and 94 pg/ml for MIP-2. Alanine aminotransferase (ALAT) and creatinine activities were determined with commercially available kits (Sigma, St. Louis, Mo.) by using a Hitachi analyzer (Boehringer, Mannheim, Germany) according to the manufacturer's instructions.
Histology.
Lungs and livers for histology were harvested 4 or 16 h after infection, fixed in 4% formalin, and embedded in paraffin. Four-micrometer sections were stained with hematoxylin and eosin and analyzed by a pathologist who was blinded for groups. To score lung inflammation and damage, each entire left lung was screened for the following parameters: interstitial inflammation, intra-alveolar inflammation, edema, endothelialitis, bronchitis, pleuritis, and thrombus formation. To score liver injury, the following parameters were analyzed: formation of thrombi, hepatocellular necrosis, portal inflammation, and endothelialitis. Each parameter was graded on a scale from 0 to 3, as follows: 0, absent; 1, mild; 2, moderate; and 3, severe. The total injury score was expressed as the sum of the scores for all parameters; the maximum values were 21 for a lung and 12 for a liver.
Statistical analysis.
Differences between groups were calculated by using the Mann-Whitney U test. For survival analysis, a Kaplan-Meier analysis followed by a log rank test was performed. Values were expressed as means ± standard errors of the means. A P value of <0.05 was considered statistically significant.
RESULTS
LBP is detectable in PLF.
To investigate whether constitutively produced LBP is detectable in the peritoneal fluid of healthy uninfected mice and whether peritonitis induces local and systemic increases in the level of this protein, we measured LBP concentrations in PLF and plasma. In PLF, low levels of LBP were found in uninfected mice, and the levels increased 5-fold (4 h) and >30-fold (16 h) after induction of E. coli peritonitis (Table 1). The plasma LBP concentrations remained unaltered 4 h after induction of E. coli peritonitis and were modestly higher after 16 h compared to the levels in uninfected mice (Table 1). LBP was not detectable in PLF or plasma of LBP−/− mice. Hence, the peritoneal LBP concentrations increased earlier than the plasma LBP concentrations during E. coli peritonitis and the increases were more pronounced.
TABLE 1.
LBP concentrations in PLF and plasmaa
| Mice | LBP concn in:
|
|
|---|---|---|
| PLF (ng/ml) | Plasma (μg/ml) | |
| Uninfected control | 19.1 ± 2.7 | 8.6 ± 0.8 |
| 4 h after E. coli i.p. infection | 95.3 ± 27.5b | 10.8 ± 1.0 |
| 16 h after E. coli i.p. infection | 645.5 ± 117.1b,c | 30.9 ± 6.0d |
Mice (eight or nine mice per group) were infected with 2 × 104 CFU of E. coli i.p. and sacrificed after 4 and 16 h. The LBP concentrations in PLF and plasma were measured with an enzyme-linked immunosorbent assay. Samples of uninfected mice served as controls. The data are means ± standard errors of the means.
P < 0.005 for a comparison with the data for the uninfected control.
P < 0.0001 for a comparison with the data obtained 4 h after E. coli infection.
P < 0.05 for a comparison with the data for the uninfected control.
LBP is essential for responsiveness of peritoneal macrophages to heat-killed E. coli in vitro.
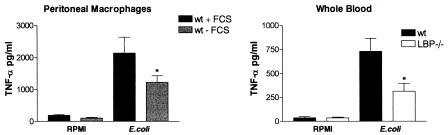
To obtain insight into the requirement for LBP in the peritoneal host response to E. coli, we determined the responsiveness of peritoneal macrophages to heat-killed bacteria. Freshly isolated peritoneal macrophages from wild-type mice, incubated under serum-free conditions, released smaller amounts of TNF-α upon stimulation with heat-killed E. coli than wild-type peritoneal macrophages supplemented with FCS (as a source of LBP) (P < 0.05) (Fig. 1). To ensure that these results were related to LBP and not to other proteins present in FCS, we repeated the experiments using whole blood derived from wild-type and LBP−/− mice stimulated in their own serum. The E. coli-induced release of TNF-α was diminished in blood from LBP−/− mice (P < 0.05) (Fig. 1). Hence, the responsiveness to E. coli depended on the presence of LBP.
FIG. 1.
Cellular responsiveness to E. coli depends on LBP. Peritoneal macrophages from wild-type mice (wt) were stimulated in the presence or absence of 10% FCS with heat-killed E. coli O18:K1 (1 × 108 CFU/ml) for 16 h. Blood was collected from wild-type and LBP−/− mice (n = 8) and stimulated for 16 h. The bars indicate means, and the error bars indicate standard errors of the means. An asterisk indicates that the P value is <0.05 for a comparison with the wild type (whole blood) or with the wild type supplemented with FCS (peritoneal macrophages).
Absence of LBP makes mice more susceptible to E. coli peritonitis.
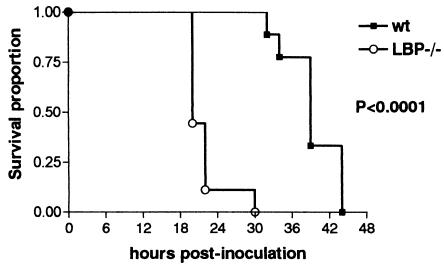
To study the contribution of endogenous LBP to the outcome of peritonitis in vivo, wild-type and LBP−/− mice (nine mice per strain) were inoculated with 2 × 104 CFU of E. coli i.p. and monitored for 5 days (Fig. 2). LBP−/− mice died earlier than their wild-type counterparts. Indeed, all LBP−/− mice succumbed to the bacterial challenge within 30 h, a time at which all of the wild-type mice were still alive. The last wild-type mouse died as late as 44 h after inoculation (P < 0.0001 for a comparison of LBP−/− and wild-type mice). Thus, the lack of LBP made mice more susceptible to peritoneal infection with E. coli.
FIG. 2.
Accelerated mortality in LBP−/− mice. Survival was monitored in wild-type (wt) and LBP−/− mice (nine mice per strain) after i.p. infection with 2 × 104 CFU of E. coli. The P value is the value for the difference between survival data as determined by the log rank test.
LBP is important for preventing early bacterial dissemination.
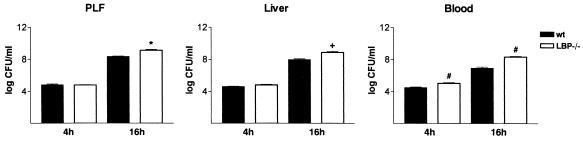
To investigate how LBP contributes to the host defense in acute peritonitis, we determined the numbers of bacteria in the PLF, liver, and blood 4 and 16 h after i.p. infection with E. coli. By 4 h after induction of peritonitis significantly higher numbers of bacteria were obtained from blood samples from LBP−/− mice than from blood samples from wild-type animals (3.2 × 104 ± 0.7 × 104 CFU/ml for wild-type mice and 11.2 × 104 ± 2.3 × 104 CFU/ml for LBP−/− mice; P = 0.007) (Fig. 3). The numbers of CFU for both the PLF and liver samples did not differ for the two strains at this early time point (Fig. 3). Sixteen hours after induction of peritonitis considerably higher bacterial burdens were found in the PLF, liver, and (analogous to the findings obtained after 4 h) blood samples from the LBP−/− animals. For the PLF samples the levels were 2.2 × 108 ± 0.3 × 108 and 13.7 × 108 ± 3.7 × 108 CFU/ml for the wild-type and LBP−/− mice, respectively (P = 0.0002); for the liver samples the levels were 0.9 × 108 ± 0.2 × 108 and 7.4 × 108 ± 3.3 × 108 CFU/ml, respectively (P < 0.0001); and for the blood samples the levels were 0.4 × 108 ± 0.1 × 108 and 3.6 × 108 ± 1.4 × 108 CFU/ml, respectively (P = 0.006) (Fig. 3). Therefore, LBP contributes to the host's capacity to control early bacterial dissemination and local peritoneal bacterial clearance.
FIG. 3.
Impaired bacterial clearance in LBP−/− animals: numbers of CFU in PLF, liver, and blood 4 and 16 h after i.p. infection with E. coli of wild-type (wt) and LBP−/− mice. The bars indicate means and the error bars indicate standard errors of the means for eight or nine mice per strain at each time. The results are representative of the results of two independent experiments. A plus sign, an asterisk, and a number sign indicate that the P values are <0.0001, <0.0005, and <0.01, respectively, for a comparison with the data for wild-type mice.
LBP deficiency is associated with impaired PMN influx.
Having shown that LBP contributes to containment of the infection within the peritoneal cavity, we next asked which factors might be involved in the early spread of E. coli. Given that PMN influx to the site of infection is a hallmark of the innate immune response, we determined leukocyte counts in the PLF. Four hours after the induction of peritonitis, the PMN influx was found to be moderately diminished in LBP−/− mice compared with the influx in wild-type mice (P < 0.05) (Table 2). The difference became even more evident at later times, when a remarkably reduced number of PMNs was observed in LBP−/− animals (P < 0.0005 for a comparison with the wild type) (Table 2).
TABLE 2.
Peritoneal cell counts and differentials in wild-type and LBP−/− mice with E. coli peritonitisa
| Time (h) | Mice | Total no. of cells × (104/ml) | No. of PMNs × (104/ml) | No. of macrophages × (104/ml) |
|---|---|---|---|---|
| 4 | Wild type | 52 ± 10.2 | 39.2 ± 6.9 | 12.8 ± 3.5 |
| 4 | LBP−/− | 47 ± 4.8 | 22.2 ± 3.6b | 24.8 ± 4.6b |
| 16 | Wild type | 409 ± 56.1 | 343.7 ± 52.3 | 53.8 ± 11.2 |
| 16 | LBP−/− | 143 ± 30.3b | 109.8 ± 21b | 41.9 ± 8.7 |
Cell counts for peritoneal lavage samples 4 and 16 h after i.p. infection with E. coli. The values are means ± standard errors of the means for eight or nine mice per strain at each time.
P < 0.05 for a comparison with the data for wild-type mice.
LBP is important for appropriate early cytokine-chemokine responses.
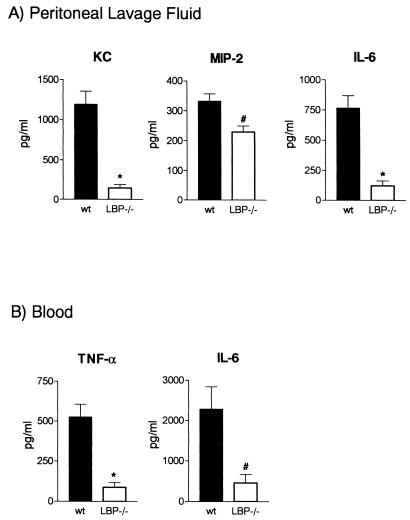
The factors that account for PMN attraction to the site of infection involve cytokines and chemokines produced by both macrophages and PMNs. To investigate which mediators are influenced by the absence of LBP, we measured the levels of IL-6, TNF-α, KC, and MIP-2, all of which are factors that are known to play important roles in host defense against peritonitis. In accordance with impaired PMN attraction, the peritoneal KC and MIP-2 concentrations were markedly reduced in LBP−/− mice 4 h after induction of peritonitis (Fig. 4A). Likewise, the IL-6 levels in peritoneal fluid were lower in LBP−/− animals (Fig. 4A). The levels of these mediators were not different for the two mouse strains 16 h after induction of peritonitis (data not shown). The peritoneal concentrations of TNF-α, which was undetectable 4 h postinfection, were significantly higher in LBP−/− mice after 16 h, which probably reflected the severe systemic infection that mice suffered from by then (116 ± 22.1 pg/ml in wild-type mice and 254 ± 44.8 pg/ml in LBP−/− mice; P < 0.05). As a measure of the early systemic inflammatory response, the TNF-α and IL-6 concentrations in plasma were determined 4 h after induction of peritonitis and were found to be strongly decreased in LBP−/− animals (Fig. 4B).
FIG. 4.
Decreased CXC chemokine and cytokine concentrations in LBP−/− mice. The protein levels of KC, MIP-2, and IL-6 were measured in PLF (A) and the TNF-α and IL-6 concentrations were measured in plasma of wild-type (wt) and LBP−/− mice (eight or nine mice per strain) 4 h after i.p. infection with E. coli (2 × 104 CFU). The bars indicate means, and the error bars indicate standard errors of the means. An asterisk and a number sign indicate that the P values are <0.0005 and <0.005, respectively, for a comparison with the data for wild-type mice.
More severe multiple organ failure in LBP−/− mice.
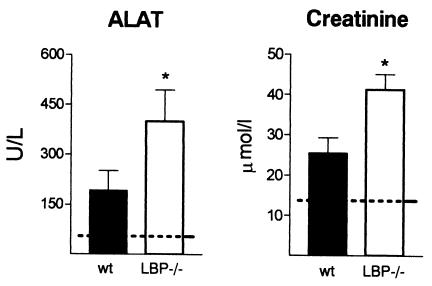
In line with the finding that there was earlier and increased bacterial dissemination in LBP−/− mice, these mice displayed more severe remote organ damage, as assessed by histology and clinical chemistry. The number of thrombi, the extent of hepatocellular necrosis, and the portal inflammation in the liver, as well as the degree of inflammation in the lungs, were greater in LBP−/− mice than in wild-type mice; the inflammation scores determined as described in Materials and Methods were 3.5 ± 0.5 for wild-type mice and 7.5 ± 0.7 for LBP−/− mice for liver samples (P = 0.002) and 5.0 ± 0.5 for wild-type mice and 6.8 ± 0.5 for LBP−/− mice for lung samples (P < 0.05) (Fig. 5). Multiple organ failure is a serious complication of severe sepsis. To investigate whether septic peritonitis was associated with multiple organ failure, we measured biochemical parameters for liver injury (ALAT) and kidney failure (creatinine) 16 h after i.p. infection with E. coli. Higher elevations of ALAT and creatinine were detected in LBP−/− animals, indicating that there was more severe organ damage in these mice than in the wild-type mice (P < 0.05) (Fig. 6).
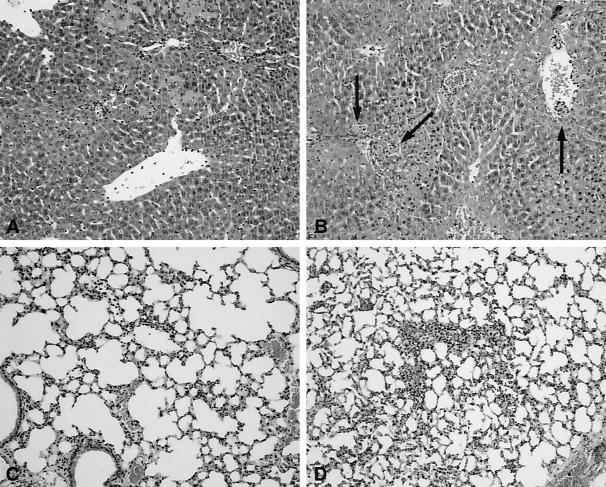
FIG. 5.
Liver and lung injuries were more severe in LBP−/− mice: representative histological images of livers (A and B) and lungs (C and D) from wild-type (A and C) and LBP−/− (B and D) mice 16 h after i.p. infection with E. coli (2 × 104 CFU). In the livers of LBP−/− mice (B) numerous thrombi (arrows) were observed together with inflammation and hepatocellular necrosis. Lung inflammation was also more pronounced in LBP−/− mice (D) than in wild-type mice. The images are representative of the images for eight or nine mice per strain. The sections were stained with hematoxylin and eosin. Magnification, ×10.
FIG. 6.
Liver and kidney injuries were more pronounced in LBP−/− mice. ALAT (liver injury) and creatinine (kidney failure) concentrations were measured in plasma of wild-type (wt) and LBP−/− mice 16 h after i.p. infection with E. coli (2 × 104 CFU). The dotted lines indicate the mean values obtained from plasma of uninfected mice. The bars indicate means and the error bars indicate standard errors of the means for eight or nine mice per strain. An asterisk indicates that the P value is <0.05 for a comparison with the data for wild-type mice.
DISCUSSION
Peritonitis is a life-threatening condition that is frequently associated with systemic dissemination of bacteria and septic shock. Host defense in peritonitis is a classical domain of the innate immune system as a rapid response to invading pathogens is essential for the host to survive. In this study we showed that LBP is a crucial component in the early phase of host defense against E. coli peritonitis. We found that endogenous LBP is essential for the clearance of E. coli, presumably via LBP's contribution to the early initiation of the inflammatory response, such as the production of cytokines and chemokines and the (subsequent) attraction of PMNs to the peritoneal cavity. The delayed inflammatory response in the absence of LBP led to more severe signs of systemic infection, including multiple organ failure and accelerated mortality.
Low levels of LBP were readily detected in peritoneal fluid of uninfected wild-type mice, which established the local presence of LBP within the peritoneal cavity. i.p. infection with E. coli induced a faster increase in peritoneal LBP concentrations than in plasma LBP concentrations. We hypothesized that the local, endogenous LBP might have biological functions in peritoneal host defense and found that there was an association with the initiation of the inflammatory response to E. coli. Lamping et al. determined that there was a protective, anti-inflammatory role for an exogenously administered high dose of LBP (100 μg) in d-galactosamine-sensitized mice inoculated with LPS and in E. coli peritonitis by showing that the mortality rates in these mice were reduced (12). Since a high dose of LBP was associated with reduced serum IL-6 and TNF-α levels upon injection of LPS, these authors postulated that high concentrations of LBP provide protection by diminishing LPS toxicity. However, it is questionable whether such high LBP concentrations are ever reached under physiological conditions. Moreover, the beneficial mechanism of administration of a high dose of LBP in E. coli peritonitis was not investigated. In the present study we found that physiological LBP levels had advantageous effects in E. coli peritonitis and attributed this to LBP's contribution to an effective proinflammatory host response. We believe that our model adequately reflects the biological situation found at the onset of peritonitis. High, acute-phase LBP levels occur later, when IL-1- and IL-6-induced LBP synthesis has been initiated, and usually peak after 24 h. It is tempting to speculate that low concentrations of LBP are essential for the initiation of an inflammatory response, whereas higher LBP concentrations represent an important anti-inflammatory mechanism to ameliorate the overwhelming inflammation seen at later times during septic peritonitis. Focusing on initial events following i.p. infection with E. coli, we observed that LBP−/− mice were less able to clear bacteria from the site of infection and that there was earlier and more pronounced systemic dissemination. To determine how LBP is related to the antimicrobial host defense in E. coli peritonitis, we first investigated the cellular influx into the peritoneal cavity, and we found markedly reduced numbers of infiltrating PMNs in LBP−/− mice. It has been shown that in peritonitis bacterial clearance depends on the attraction of PMNs to the site of infection and that the CXC chemokines KC and MIP-2 are major contributors to PMN recruitment (5, 7, 24). Accordingly, we found significantly lower KC and MIP-2 concentrations in the PLF of mice lacking LBP, which might explain the inadequately low number of PMNs in LBP−/− mice infected with E. coli. In line with our findings, Fierer et al. observed a similar reduction in peritoneal CXC chemokines and a decreased PMN influx in LBP−/− mice 3 h after i.p. infection with Salmonella (5). Since i.p. administration of casein or the gram-positive pathogen Staphylococcus aureus induced comparable chemokine releases and PMN influxes in wild-type and LBP−/− mice, it is likely that the lack of LBP specifically impairs the gram-negative bacterium- or LPS-induced release of chemokines and subsequent PMN attraction.
During Salmonella peritonitis TNF-α is another factor that contributes to the attraction of PMNs to the peritoneal cavity, and LBP−/− mice could be rescued from Salmonella-induced lethality by exogenously administered TNF-α (8, 27). Similarly, in the absence of LBP, we found that peritoneal macrophages and whole blood had a reduced capacity to release TNF-α upon stimulation with E. coli in vitro. Moreover, plasma TNF-α concentrations were substantially lower in LBP−/− mice after induction of peritonitis in vivo. These observations add to the notion that LBP contributes considerably to an effective early inflammatory response during E. coli peritonitis.
Our finding that LBP promotes acute inflammation in E. coli peritonitis matches results of studies of Salmonella peritonitis (5, 8, 11). Although it remains to be determined exactly how PMNs contribute to host defense against Salmonella, an intracellular pathogen, these cells seem to play a role in the Salmonella peritonitis model, with LBP as an important effector molecule. However, previous studies of E. coli peritonitis did not reveal a vital role for LBP (5, 13). Differences in the E. coli strain used may have had an effect. Whereas most investigators used E. coli O111:B4, we used a more virulent, encapsulated E. coli O18:K1 strain. Differences in host responses to these two strains have been reported previously. Although the peritoneal PMN influx depended on LBP when mice were challenged with heat-killed E. coli O111:B4, protection against lethality by LBP could not be demonstrated after intravenous or i.p. administration of live E. coli O111:B4 in vivo (5, 13). In addition, anti-CD14 antibodies did not impair the outcome in E. coli O111:B4-infected mice but decreased bacterial clearance in E. coli O18:K1-challenged rabbits (13, 18). Moreover, C3H/HeJ mice, which are not responsive to LPS due to a point mutation in the tlr4 gene, are highly susceptible to E. coli O18:K1 infection but not to E. coli O111:B4 infection (4, 7). Pretreatment with murine TNF-α and IL-1 led to restored bacterial killing and could rescue E. coli O18:K1-treated C3H/HeJ mice from lethality (3, 4). Together with the present observations, these data imply that there are different host response mechanisms for these two E. coli strains. The strong dependence of E. coli O18:K1 on LBP, CD14, and TLR4 seems to rely on the greater need for additional stimuli, like TNF-α or IL-1, in order to enable phagocytes to kill bacteria. Accordingly, we observed impaired bacterial clearance and attenuated TNF-α production in LBP−/− mice.
Host defense in peritonitis is a delicate balance between proinflammatory pathways intended to eliminate bacteria and anti-inflammatory pathways intended to prevent systemic inflammation (21). Any imbalance in pro- or anti-inflammatory mediators might prove to be harmful. In the present study, the inadequate onset of inflammation in LBP−/− mice led to early systemic dissemination and increased bacterial outgrowth, which in turn instigated systemic inflammation and multiple organ failure. We therefore concluded that LBP is indispensable for the innate immune response in E. coli O18:K1 peritonitis and that its presence at physiological levels is mandatory for adequate initiation of host response mechanisms.
Acknowledgments
This work was supported by grants from the Fonds zur Foerderung der wissenschaftlichen Forschung in Oesterreich (FWF) (to S.K.) and The Netherlands Research Organization (to S.F.) and by NIH grants GM54060, DK50305, and RR14466 (to D.T.G).
We thank I. Kop and J. Daalhuisen for expert technical assistance.
Editor: B. B. Finlay
REFERENCES
- 1.Bernheiden, M., J. M. Heinrich, G. Minigo, C. Schutt, F. Stelter, M. Freeman, D. Golenbock, and R. S. Jack. 2001. LBP, CD14, TLR4 and the murine innate immune response to a peritoneal Salmonella infection. J. Endotoxin Res. 7:447-450. [PubMed] [Google Scholar]
- 2.Blairon, L., X. Wittebole, and P. F. Laterre. 2003. Lipopolysaccharide-binding protein serum levels in patients with severe sepsis due to gram-positive and fungal infections. J. Infect. Dis. 187:287-291. [DOI] [PubMed] [Google Scholar]
- 3.Cross, A., L. Asher, M. Seguin, L. Yuan, N. Kelly, C. Hammack, J. Sadoff, and P. Gemski, Jr. 1995. The importance of a lipopolysaccharide-initiated, cytokine-mediated host defense mechanism in mice against extraintestinally invasive Escherichia coli. J. Clin. Investig. 96:676-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross, A. S., J. C. Sadoff, N. Kelly, E. Bernton, and P. Gemski. 1989. Pretreatment with recombinant murine tumor necrosis factor alpha/cachectin and murine interleukin 1 alpha protects mice from lethal bacterial infection. J. Exp. Med. 169:2021-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fierer, J., M. A. Swancutt, D. Heumann, and D. Golenbock. 2002. The role of lipopolysaccharide binding protein in resistance to Salmonella infections in mice. J. Immunol. 168:6396-6403. [DOI] [PubMed] [Google Scholar]
- 6.Haziot, A., E. Ferrero, F. Kontgen, N. Hijiya, S. Yamamoto, J. Silver, C. L. Stewart, and S. M. Goyert. 1996. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity 4:407-414. [DOI] [PubMed] [Google Scholar]
- 7.Haziot, A., N. Hijiya, S. C. Gangloff, J. Silver, and S. M. Goyert. 2001. Induction of a novel mechanism of accelerated bacterial clearance by lipopolysaccharide in CD14-deficient and Toll-like receptor 4-deficient mice. J. Immunol. 166:1075-1078. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich, J. M., M. Bernheiden, G. Minigo, K. K. Yang, C. Schutt, D. N. Mannel, and R. S. Jack. 2001. The essential role of lipopolysaccharide-binding protein in protection of mice against a peritoneal Salmonella infection involves the rapid induction of an inflammatory response. J. Immunol. 167:1624-1628. [DOI] [PubMed] [Google Scholar]
- 9.Holzheimer, R. G., K. H. Muhrer, N. L'Allemand, T. Schmidt, and K. Henneking. 1991. Intraabdominal infections: classification, mortality, scoring and pathophysiology. Infection 19:447-452. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 11.Jack, R. S., X. Fan, M. Bernheiden, G. Rune, M. Ehlers, A. Weber, G. Kirsch, R. Mentel, B. Furll, M. Freudenberg, G. Schmitz, F. Stelter, and C. Schutt. 1997. Lipopolysaccharide-binding protein is required to combat a murine gram-negative bacterial infection. Nature 389:742-745. [DOI] [PubMed] [Google Scholar]
- 12.Lamping, N., R. Dettmer, N. W. Schroder, D. Pfeil, W. Hallatschek, R. Burger, and R. R. Schumann. 1998. LPS-binding protein protects mice from septic shock caused by LPS or gram-negative bacteria. J. Clin. Investig. 101:2065-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Roy, D., F. Di Padova, Y. Adachi, M. P. Glauser, T. Calandra, and D. Heumann. 2001. Critical role of lipopolysaccharide-binding protein and CD14 in immune responses against gram-negative bacteria. J. Immunol. 167:2759-2765. [DOI] [PubMed] [Google Scholar]
- 14.Lorber, B., and R. M. Swenson. 1975. The bacteriology of intra-abdominal infections. Surg. Clin. N. Am. 55:1349-1354. [DOI] [PubMed] [Google Scholar]
- 15.Martin, T. R., J. C. Mathison, P. S. Tobias, D. J. Leturcq, A. M. Moriarty, R. J. Maunder, and R. J. Ulevitch. 1992. Lipopolysaccharide binding protein enhances the responsiveness of alveolar macrophages to bacterial lipopolysaccharide. Implications for cytokine production in normal and injured lungs. J. Clin. Investig. 90:2209-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medzhitov, R., and C. Janeway, Jr. 2000. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 173:89-97. [DOI] [PubMed] [Google Scholar]
- 17.Medzhitov, R., and C. Janeway, Jr. 2000. Innate immunity. N. Engl. J. Med. 343:338-344. [DOI] [PubMed] [Google Scholar]
- 18.Opal, S. M., J. E. Palardy, N. Parejo, and R. L. Jasman. 2003. Effect of anti-CD14 monoclonal antibody on clearance of Escherichia coli bacteremia and endotoxemia. Crit. Care Med. 31:929-932. [DOI] [PubMed] [Google Scholar]
- 19.Schromm, A. B., E. Lien, P. Henneke, J. C. Chow, A. Yoshimura, H. Heine, E. Latz, B. G. Monks, D. A. Schwartz, K. Miyake, and D. T. Golenbock. 2001. Molecular genetic analysis of an endotoxin nonresponder mutant cell line: a point mutation in a conserved region of MD-2 abolishes endotoxin-induced signaling. J. Exp. Med. 194:79-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schumann, R. R., C. J. Kirschning, A. Unbehaun, H. P. Aberle, H. P. Knope, N. Lamping, R. J. Ulevitch, and F. Herrmann. 1996. The lipopolysaccharide-binding protein is a secretory class 1 acute-phase protein whose gene is transcriptionally activated by APRF/STAT/3 and other cytokine-inducible nuclear proteins Mol. Cell. Biol. 16:3490-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sewnath, M. E., D. P. Olszyna, R. Birjmohun, F. J. ten Kate, D. J. Gouma, and T. van Der Poll. 2001. IL-10-deficient mice demonstrate multiple organ failure and increased mortality during Escherichia coli peritonitis despite an accelerated bacterial clearance. J. Immunol. 166:6323-6331. [DOI] [PubMed] [Google Scholar]
- 22.Tobias, P. S., K. Soldau, and R. J. Ulevitch. 1989. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J. Biol. Chem. 264:10867-10871. [PubMed] [Google Scholar]
- 23.Tobias, P. S., K. Soldau, and R. J. Ulevitch. 1986. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J. Exp. Med. 164:777-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walley, K. R., N. W. Lukacs, T. J. Standiford, R. M. Strieter, and S. L. Kunkel. 1997. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect. Immun. 65:3847-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 26.Wurfel, M. M., B. G. Monks, R. R. Ingalls, R. L. Dedrick, R. Delude, D. Zhou, N. Lamping, R. R. Schumann, R. Thieringer, M. J. Fenton, S. D. Wright, and D. Golenbock. 1997. Targeted deletion of the lipopolysaccharide (LPS)-binding protein gene leads to profound suppression of LPS responses ex vivo, whereas in vivo responses remain intact. J. Exp. Med. 186:2051-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang, K. K., B. G. Dorner, U. Merkel, B. Ryffel, C. Schutt, D. Golenbock, M. W. Freeman, and R. S. Jack. 2002. Neutrophil influx in response to a peritoneal infection with Salmonella is delayed in lipopolysaccharide-binding protein or CD14-deficient mice. J. Immunol. 169:4475-4480. [DOI] [PubMed] [Google Scholar]