Abstract
In recent years, operant discrimination training procedures have been used to teach giant African pouched rats to detect tuberculosis (TB) in human sputum samples. This article summarizes how the rats are trained and used operationally, as well as their performance in studies published to date. Available data suggest that pouched rats, which can evaluate many samples quickly, are sufficiently accurate in detecting TB to merit further investigation as a diagnostic tool.
Keywords: behavior analysis, discrimination training, tuberculosis, pouched rats, translational research
The article by Critchfield (2011) and the accompanying commentaries in this issue of The Behavior Analyst highlight the potential importance of translational research in behavior analysis. Also in this issue, Jones (2011) provides an informative illustration of this type of research, specifically the use of behavior-analytic strategies to teach dogs to detect explosives and to evaluate their performance in doing so. Jones focused on scent detection of remote explosives, in which samples that may or may not contain explosives are collected in one area and analyzed by animals located elsewhere. As he notes, dogs also are used operationally for the direct detection of land mines and other explosive remnants of war and are sometimes used to detect cancers in humans (e.g., Moser & McCulloch, 2010).
Like dogs, giant African pouched rats (Cricetomys gambianus) are used operationally to detect land mines; articles describing how these rats are trained and used have recently appeared in behavior-analytic journals (Poling, Weetjens, Cox, Beyene, & Sully, 2010, 2011; Poling, Weetjens, Cox, Mgode, et al., 2010;). Anti-Persoonsmijnen Ontmijnende Product Ontwikkeling (Anti-Personnel Landmine Detection Product Development, APOPO), a nonprofit humanitarian organization located in Morogoro, Tanzania, is responsible for the use of rats as mine-detection animals. APOPO is also exploring other humanitarian applications of scent-detecting rats. One that shows considerable promise is using the animals to detect tuberculosis (TB) in humans. The purpose of the present article is to provide a summary of APOPO's work in this area.
An Introduction to TB
Tuberculosis is an infection, often but not always of the lungs, caused by Mycobacterium tuberculosis. As data reported by the World Health Organization (WHO, 2008, 2009a, 2009b) make clear, it is a serious and common disease. According to the WHO, TB was responsible for the deaths of 1,770,000 people in 2007. About 2,000,000,000 people currently are infected with TB, and roughly 1 in 10 of them will become seriously ill with the disease. Most people infected with TB live in developing countries. The disease is especially common in sub-Saharan Africa, where it is the primary cause of death in people with HIV. Despite this, in 2008 only 1% of people with HIV were tested for TB. A major reason for the scarcity of TB testing in people with HIV, and in the population at large, is the unavailability of a cheap, fast, and accurate test.
The purpose of any TB detection system is to classify people into two discrete categories: those who have the disease and those who do not. The sensitivity of a given test refers to its ability to do the former, whereas its specificity refers to its ability to do the latter. At present, the technique most widely used to diagnose TB in the developing world is sputum smear microscopy (hereafter microscopy) (Dye, Watt, Bleed, Hosseini, & Raviglione, 2005; Steingart et al., 2006), a technique in use for decades and still recommended for low- and middle-income countries (e.g., Corbett et al., 2003; Dye et al., 2005). In microscopy, trained technicians view slides (typically magnified 100 times) that contain sputum samples stained by the Ziehl-Neelsen (or less often the Kinyoun) method (see Steingart et al., 2006), which makes Mycobacterium tuberculosis, an acid-fast bacillus, fairly easy to see and count. Microscopy has very good specificity, characteristically above 90% in published studies (Steingart et al., 2006). The sensitivity of the method, which is comprehensively reviewed by Steingart et al., varies widely in published studies, ranging from roughly 20% to 80%. Strategies for improving sensitivity have been developed, such as increasing the time that microscopists view slides (e.g., Ramsay et al., 2009), but low and variable sensitivity is a common problem in everyday applications in the developing world (Burgess et al., 2001; Hargreaves et al., 2001; Steingart et al., 2006). For example, a recent study in Nigeria revealed a sensitivity of only 23% (Ani et al., 2009). That is, fewer than one in four people with active TB were detected.
Pouched Rats as TB Detectors
In the hope of developing a viable alternative to or adjunct for microscopy, APOPO is exploring the use of African giant pouched rats (Cricetomys gambianus) to detect the presence of TB. These large and long-lived rats, which are native to much of Africa and have an excellent sense of smell, detect TB by sniffing sputum samples. They are trained to respond consistently in one way (pause) if the sample contains the TB bacillus (is positive) and respond in another way (not pause) if the sample does not contain the bacillus (i.e., is negative). Each rat can test hundreds of samples each day, allowing inexpensive testing.
APOPO's goal is to produce rats that consistently emit an easily observed indicator response when they smell a sputum sample that contains M. tuberculosis and not to emit this response at other times. In essence, this is a signal-detection task (Green & Swets, 1966), in which the odor uniquely associated with M. tuberculosis constitutes a signal and all other odors constitute noise. The rats' task is to respond only to the signal. This is accomplished by training the rats in an operant stimulus discrimination task in which the designated indicator response, pausing for at least 5 s at a hole immediately above a sputum sample, is reinforced when the sputum sample is known to contain M. tuberculosis but not when the sample does not contain the bacillus. Such differential reinforcement establishes the odor of TB as a discriminative stimulus (SD) that reliably evokes the operant response (pausing), which rarely occurs in its absence.
In signal-detection terminology, emitting the indicator (operant) response when the signal (SD) is present on a given trial is termed a hit and emitting that response when the signal is not present is termed a false alarm. Indicating that the signal is not present on a given trial, either by withholding the response indicating a signal (as in our procedure) or by emitting another response (as in procedures used by others), is termed a correct rejection if the signal is not present and a miss if the signal is present. Hits and correct rejections are correct responses, whereas false alarms and misses are incorrect. In operational TB systems it is highly desirable to have a high rate of hits and a relatively low rate of false alarms.
By dividing total hits by total hits plus total misses and multiplying the result by 100%, one can produce a quantitative (i.e., percentage) measure of the sensitivity of the rats, or of any other TB diagnostic. In essence, as noted previously, sensitivity summarizes how good the test is at detecting patients with TB. Specificity, in contrast, refers to how good the test is at detecting patients who do not have the disease. A percentage measure of sensitivity can be determined by dividing total correct rejections by total correct rejections plus total false alarms and multiplying by 100%. As noted previously, an ideal diagnostic has high sensitivity and high specificity. In addition, if the diagnostic is to be useful in developing countries, where TB is most common and the gravest problem, it also has to be affordable and produce results quickly with minimal reliance on equipment (WHO, 2009a).
Major organizations concerned with TB as a worldwide health issue, including the Stop TB Partnership, the Foundation for Innovative Diagnostics, and the WHO, strongly emphasize that an evidence-based approached to TB diagnosis is essential (Pai, Ramsay, & O'Brien, 2008). That is, high-quality research, typically reported in peer-refereed journal articles, must document the value of new diagnostic techniques before they are widely adopted. APOPO recently has published three studies describing the use of rats as TB detectors.
In the first study, Weetjens, Mgode, Machang'u, et al. (2009) described a procedure in which pouched rats were trained in a rectilinear cage to sniff each of 10 holes. A small pot containing a sputum sample taken from a patient at a direct observation of treatment short course (DOTS) TB center in Dar es Salaam, Tanzania, was placed immediately below each hole. DOTS centers are open to all citizens with respiratory difficulties and are intended to detect and provide treatment for TB. The rats were trained by operant conditioning to pause for at least 5 s at holes where the sputum sample was positive for TB (as confirmed by other methods) but not to pause at holes where the sputum sample was negative. In essence, the training procedure involved differential reinforcement, arranged so that rats (which were mildly food deprived) received food (bananas mixed with food pellets, both mashed) only when they paused for the required time (5 s) at holes with positive samples. The training is equivalent to that used to establish the odor of 2,4,6-trinitrotoluene (TNT), the primary explosive in most land mines, as an SD for mine-detection rats, except that the odor of sputum samples that contain the TB bacillus is established as the SD. We detail how rats are trained to detect TNT (and land mines) elsewhere (Poling, Weetjens, Cox, Beyene, Bach, et al., 2010; Poling, Weetjens, Cox, Beyene, & Sully, 2010) and summarize how they are trained to detect TB below.
Training Rats to Detect TB
All of APOPO's rats come from its breeding colony. At a young age the rat pups begin to interact with humans, and when the rats are 3 to 6 weeks of age trainers regularly handle them; expose them to a wide range of sights, sounds, and smells; and hand-feed them preferred foods like bananas and peanuts. Training then begins. The first step is clicker training, which occurs in a metal cage. In this process, trainers repeatedly present food to rats through a plastic tube attached to a syringe, and the rats soon learn to approach the trainers when the click sounds, because doing so produces food. As noted, the food presented is mashed banana mixed with crushed commercial rat chow (to increase nutritional value). To increase the reinforcing effectiveness of food, the rats receive a major portion of their food during the daily training sessions, which last about half an hour. They are food deprived for 17 hr when training starts, which serves as an establishing operation for food as a reinforcer (Laraway, Snycerski, Michael, & Poling, 2003).
After being repeatedly paired with food, the click is established as an SD for approaching the trainer as well as a conditioned reinforcer. When this is accomplished, discrimination training begins. The first step in discrimination training is teaching the rat to pause when it smells a sputum sample that contains the TB bacillus (i.e., a positive sample). Sputum samples are provided by patients who visit DOTS centers to seek diagnosis and treatment of respiratory ailments. Each patient typically provides two or three samples, taken at different times. A segment of each sample is analyzed by microscopy, and the remainder is placed in a small plastic pot and frozen for subsequent evaluation by APOPO's rats. A microscopist at the organization at which the sample is obtained prepares and analyzes the slide, and his or her evaluation of each sample is recorded. Samples are evaluated as negative (no M. tuberculosis present) or positive (M. tuberculosis present) and if the latter as a few bacteria (AFB), +1, +2, or +3, depending on the number of bacteria present (see Fujika, 2005). At APOPO's laboratory, the frozen samples are thawed and autoclaved to kill M. tuberculosis and other microorganisms. Samples designated as TB positive by microscopists are used as positive training samples, and samples designated as TB negative by microscopists are used as negative training samples.
The first step of discrimination training is accomplished in a small metal cage with a hole in the center of the floor. A plastic pot containing a sputum sample deemed positive by a microcroscopist is presented immediately below the hole, and food is immediately presented when the rat places its nose in or just above the hole. This typically occurs quickly, but if not shaping is used to engender the response. Over time, a progressively longer period of pausing at the hole is required for reinforcement. The final criterion is 5 s. When this criterion is met reliably, discrimination training begins.
Discrimination training at APOPO historically has been done manually, with data recorded and reinforcers delivered by the trainers. They begin the second step of training in a metal cage with three holes in the floor. Pots that contain sputum samples positive for TB are placed below half of the holes, on average, and pots containing negative samples are placed below the other half. Pausing for 5 s above positive samples is reinforced, and pausing at holes above negative samples has no programmed consequences. Training continues in this manner, with 60 to 90 pots presented each day, until the hit rate for an individual rat is above 80% and the false alarm rate is below 5%. When this criterion is met, it is trained in a 10-hole cage.
The final training step for TB detection rats is conducted in a 10-hole cage (205 cm long by 55 cm wide by 55 cm high). In this step, each rat is exposed to 50 to 100 samples per day, of which 5% to 20% are known positives and the rest are known negatives. Animals are trained until their hit rate is consistently above 80% and their false alarm rate is below 5%. When a rat meets this criterion, it becomes an operational animal and can participate in TB detection studies.
Research Findings
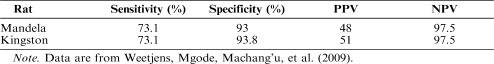
In the study by Weetjens, Mgode, Machang'u, et al. (2009), two rats trained as just described evaluated samples that were determined through culturing, which is the gold standard for TB detection (Reid & Shah, 2009), as TB positive or TB negative. The status of these samples was unknown to trainers. Over 7 days, the rats were exposed to 817 test sputum samples, of which 67 were TB positive. Both rats were exposed to the same samples. Their performance is depicted in Table 1, which shows their sensitivity (percentage correct identifications of samples known to be positive) and their specificity (percentage correct identifications of samples known to be negative). Also shown are their positive predictive value (PPV) and their negative predictive value (NPV). PPV is defined as the number of true positives divided by the number of true positives plus false positives. Similarly, NPV is defined as the number of true negatives divided by the number of true negatives plus false negatives. Both rats had sensitivities of 73%, which is well above the usual sensitivity of microscopy (Steingart et al., 2006), and acceptably high specificities, PPVs, and NPVs.
Table 1.
Performance of Two Rats Relative to Culturing
Weetjens, Mgode, Machang'u, et al. (2009) also compared rats' evaluation of sputum samples to evaluations made by trained microscopists at the DOTS center. Summary results were presented for 16 rats that evaluated 2,597 sputum samples, of which 345 were smear positive. The mean sensitivity of these rats was 87.9% and the mean specificity was 93.3%. More detailed analysis of the performance of three animals yielded comparable results. Like comparisons to the results of culturing, comparison to the results of microscopy suggest that Cricetomys are accurate enough in detecting TB to be of practical value.
The results reported by Weetjens, Mgode, Machang'u, et al. (2009a) were sufficiently promising that the Tanzanian Ministery of Health allowed the rats to be used for second-line screening of sputum samples provided by DOTS center patients. An article by Weetjens, Mgode, Davis, et al. (2009) reports the initial results of this pilot project, in which rats analyzed sputum samples collected from four DOTS centers in Dar es Salaam, Tanzania, from January 2008 to May 2009. In this study, sputum smears were first analyzed by DOTS microscopists, then by the rats. Positive indications by the rats on samples evaluated as negative by the microscopists were confirmed by a second microscopy. Samples from 15,041 patients were evaluated by microscopy at the DOTS centers and by the rats. The DOTS centers found TB in 1,838 of the patients (12.2%), whereas the rats detected the disease in 2,415 patients (16.1%). The cases detected by rats but missed by DOTS centers (N = 577) increased TB detection by 31.4%, which is statistically, and more important, clinically significant.
Poling, Weetjens, Cox, Mgode, et al. (2010) reported data on evaluations of 23,101 sputum samples collected from 10,523 patients. The samples were evaluated first by DOTS center microscopists and then by the rats. Microscopists identified 2,487 sputum samples and 1,403 different patients (13.3% of the total patients) as TB positive. The rats verified these findings, identifying 2,274 of these samples and 1,335 of the patients as TB positive. The rats also identified as positive an additional 927 TB samples deemed negative at the DOTS centers that were found in a second microscopy to contain the bacillus. These samples came from 620 patients not previously diagnosed with TB; therefore, the rats' evaluations increased the case detection rate by 44%. Further, the rats evaluated many samples quickly and economically. The authors concluded that the use of pouched rats for TB detection in developing countries certainly warrants further research.
These articles provide evidence of proof of principle with respect to using rats to detect human TB. They also illustrate the possibility of conducting significant medical research in a developing country while depending heavily on local labor (over 90% of APOPO's employees are Tanzanians) and focusing on sustainable, humanitarian development. Moreover, and important in the present context, they provide an example of what might be construed as translational research, in which strategies commonly used to establish stimulus control in basic research with animals were used to train pouched rats to perform a valuable service for humans. At present, however, the use of Cricetomys to detect human TB is a promising but not proven technology, and additional research is needed to ascertain its true value. That research affords significant challenges and opportunities for behavior analysts and other scientists.
Behavior Analysis and Future Research with TB Detecting Rats
As noted, any useful TB detection technology must have sufficiently high sensitivity and specificity to be of clinical value. Put differently, and in terms perhaps more familiar to behavior analysts, such technology must have adequate accuracy. As Johnston and Pennypacker (2009) point out, accuracy refers to “the extent to which observed values approximate to the events that actually occurred” (p. 355) and is quantified by comparing the values yielded by the detection system of interest (e.g., APOPO's rats) to the best system comparison system available. It is widely recognized that culturing is the best system for detecting TB in humans (Reid & Shah, 2009) and the results reported by Weetjens, Mgode, Machang'u, et al. (2009) suggest that the sensitivity and specificity of rats' evaluations of sputum samples are reasonably high when compared to the results of culturing. These results reflect evaluations of only 67 culture-positive samples evaluated by two rats, however. Further comparison of rats' evaluations of sputum samples relative to evaluation by culturing are badly needed and are planned for early 2011. Culturing is, however, slow (it typically takes about 6 weeks for M. tuberculosis to reproduce sufficiently in culture medium to be very easy to detect) and relatively expensive. Moreover, it is not easy to culture TB under the conditions characteristic of laboratories in the developing world, and doing so requires some expertise in microbiology. It is for these reasons that APOPO has not yet published a large-scale evaluation of rats' performance relative to culturing.
To conduct such a study requires substantial funding, even though costs are low in Tanzania. APOPO has succeeded in securing sufficient funds to evaluate rats as a TB detection tool in large part because a good, cheap detection technique is clearly needed and deemed by wealthy institutions and individuals to be worthy of financial support. Poling (2010) suggested that behavior analysts should “focus on a disease,” that is, work in an area of concern to many people, and APOPO's TB detection work has certainly done so. It is important to emphasize, however, that the idea of using rats for detecting TB (and land mines) came from Bart Weetjens, a product developer and Buddhist monk (and not a behavior analyst) and that many people worked on the project. Critchfield (2011) rightly notes that “outside the behavioral sciences, the problem of limited individual expertise often is solved through collaboration of individuals with different types of skills (e.g., Mace & Critchfield, 2010)” (p. 10). Skill in behavior analysis alone is not sufficient for evaluating TB detection by rats, but such skill can be of considerable value. Mastery of the principles of operant and respondent conditioning, expertise in research designs with small numbers of subjects, and creative problem solving are components of a competent behavior analyst's repertoire that are useful in many contexts.
Consider, for example, that TB positive training samples currently are sputum samples deemed to contain M. tuberculosis by DOTS center microscopists. Microscopists fail to detect the bacteria in many samples that actually contain it, especially when few bacteria are present (Steingart et al., 2006); hence, some training samples deemed negative are actually TB positive. This probably increases the level of what appear to be false positives, because rats may be identifying bacteria that microscopists missed in some training samples, which they deemed negative and were so considered by APOPO. Moreover, the vast majority of positive training samples contain relatively high bacterial counts; that is, they are rated as +2 or +3. The intensity of the training stimulus often influences the shape of the generalization gradient in discrimination training (e.g., Poling, Simmons, & Appel, 1978), and it is probable that use of far fewer AFB training samples than +2 or +3 training samples affects the likelihood that rats will detect operational AFB samples. An understanding of operant stimulus control calls attention to the variables just described and knowledge of research strategies with small numbers of subjects suggests strategies for evaluating whether these variables actually affect TB detection and, if the effect is harmful, how to control them. Such work is planned for the coming year, and at least three behavior analysts (Durgin, Mahoney, and Poling) will be involved in it.
REFERENCES
- Ani A, Okpe S, Akambi M, Ejelionu E, Ykubu B, Owolodum O, et al. Comparison of a DNA based PCR method with conventional methods for the detection of M. tuberculosis in Jos, Nigeria. Journal of Infection in Developing Countries. 2009;3:470–475. doi: 10.3855/jidc.420. [DOI] [PubMed] [Google Scholar]
- Burgess A.L, Fitzgerald D.W, Severe P, Joseph P, Noel E, Rastogi N, et al. Integration of tuberculosis screening at an HIV voluntary counseling and testing centre in Haiti. Aids. 2001;15:1875–1879. doi: 10.1097/00002030-200109280-00018. [DOI] [PubMed] [Google Scholar]
- Corbett E.L, Watt C.J, Walker N, Maher D, Williams B.G, Raviglione M.C, et al. The growing burden of tuberculosis: Global trends and interactions with the HIV epidemic. Archives of Internal Medicine. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- Critchfield T. Translational contributions of the experimental analysis of behavior. The Behavior Analyst. 2011;34:3–17. doi: 10.1007/BF03392227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C, Watt C.J, Bleed D.M, Hosseini S.M, Raviglione M.C. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. Journal of the American Medical Association. 2005;293:2767–2775. doi: 10.1001/jama.293.22.2767. [DOI] [PubMed] [Google Scholar]
- Fujika A. AFT microscopy training. Tokyo: Research Institute of Tuberculosis; 2005. [Google Scholar]
- Green D.M, Swets J.A. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Hargreaves N.J, Kadzakumanja O, Phiri S, Nyangulu D.S, Salaniponi F.M.L, Harries A.D, et al. What causes smear-negative pulmonary tuberculosis in Malawi, an area of high HIV seroprevalence? International Journal of Tuberculosis and Lung Disease. 2001;5:113–122. [PubMed] [Google Scholar]
- Johnston J.M, Pennypacker H.S. Strategies and tactics of behavioral research (3rd ed.) New York: Routledge; 2009. [Google Scholar]
- Jones B.M. Applied behavior analysis is ideal for the development of a land mine detection technology using animals. The Behavior Analyst. 2011;34:55–73. doi: 10.1007/BF03392235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraway S, Snycerski S, Michael J, Poling A. Motivating operations and terms to describe them: Some further refinements. Journal of Applied Behavior Analysis. 2003;36:407–414. doi: 10.1901/jaba.2003.36-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace F.C, Critchfield T.S. Translational research in behavior analysis: Historical traditions and imperative for the future. Journal of the Experimental Analysis of Behavior. 2010;93:293–312. doi: 10.1901/jeab.2010.93-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, McCulloch M. Canine scent detection of human cancers: A review of methods and accuracy. Journal of Veterinary Behavior. 2010;5:145–152. [Google Scholar]
- Pai M, Ramsey A, O'Brien R. Evidence-based tuberculosis diagnosis. PLoS Medicine. 2008;5:e156. doi: 10.1371/journal.pmed.0050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling A. Looking to the future: Will behavior analysis survive and prosper? The Behavior Analyst. 2010;33:7–17. doi: 10.1007/BF03392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling A, Simmons M.A, Appel J.B. Morphine and shock detection: Effects on shock intensity. Communications in Psychopharmacology. 1978;2:333–336. [PubMed] [Google Scholar]
- Poling A, Weetjens B.J, Cox C, Beyene N, Bach H, Sully A. Teaching giant African pouched rats to find land mines: Operant conditioning with real consequences. Behavior Analysis in Practice. 2010;3:19–25. doi: 10.1007/BF03391761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling A, Weetjens B, Cox C, Beyene N, Sully A. Using giant African pouched rats (Cricetomys gambianus) to detect land mines. The Psychological Record. 2010;60:715–727. [Google Scholar]
- Poling A, Weetjens B.J, Cox C, Beyene N.W, Bach H, Sully A. Using trained pouched rats to detect land mines: Another victory for operant conditioning. Journal of Applied Behavior Analysis. 2011;44:351–355. doi: 10.1901/jaba.2011.44-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling A, Weetjens B, Cox C, Mgode G, Jubitana M, Kazwala R, et al. Using giant African rats to detect tuberculosis: 2009 findings. American Journal of Tropical Medicine and Hygiene. 2010;83:1308–1310. doi: 10.4269/ajtmh.2010.10-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay A, Cuevas L.E, Mundy C.J.F, Nathanson C.-M, Chirambo P, et al. New policies, new technologies: Modelling the potential for improved smear microscopy services in Malawi. PLoS ONE. 2009;4((11)):e7760. doi: 10.1371/journal.pone.0007760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M.J.A, Shah N.S. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infectious Diseases. 2009;9:173–184. doi: 10.1016/S1473-3099(09)70043-X. [DOI] [PubMed] [Google Scholar]
- Steingart K.R, Henry M, Ng V, Hopewell P.C, Ramsay A, Cunningham J, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: A systematic review. Lancet Infectious Diseases. 2006;6:570–581. doi: 10.1016/S1473-3099(06)70578-3. [DOI] [PubMed] [Google Scholar]
- Weetjens B.J, Mgode G.F, Davis B.K, Cox C, Beyene N.W. Global forum update on research for health (Vol. 6, pp. 39–42) Geneva: Global Forum for Health Research; 2009. African giant rats for tuberculosis detection: A novel diagnostic technology. [Google Scholar]
- Weetjens B.J, Mgode G.F, Machang'u R.S, Kazwala R, Mfinanga G, Lwilla F, et al. African pouched rats for the detection of pulmonary tuberculosis in sputum samples. International Journal of Tuberculosis and Lung Disease. 2009;13:737–743. [PubMed] [Google Scholar]
- World Health Organization. Global tuberculosis control: Epidemiology, strategy, financing. Geneva: WHO Press; 2008. [Google Scholar]
- World Health Organization. Pathways to better diagnostics for tuberculosis: a blueprint for the development of TB diagnostics by the new diagnostics working group of the Stop TB Partnership. 2009a. Retrieved from http://www.stoptb.org/wg/tb_hiv/assets/documents/Fact%20 sheet%20HIV%20TB%20for%20IAS%20FINAL.pdf.
- World Health Organization. TB/HIV facts. 2009b. Retrieved from http://www.stoptb.org/wg/new_diagnostics/assets/documents/BluePrintTB_annex_web.pdf.