Abstract
Objective
Thrombus formation can occur in both macroscopic and microscopic blood vessels. In the brain, cerebral venous sinus thrombosis (CVST) and focal cortical infarctions can result from the formation of thrombi in these different sized vessels. In this study we define the relative contributions of three major pro- and anti-coagulation pathways (heparin-antithrombin, protein C, and tissue factor (TF)) in the thrombogenic responses that occur in large and small vessels of the brain.
Methods
CVST was induced by topical application of FeCl3 on the superior sagittal sinus (SSS), while photoactivation of fluorescein was used to induce thrombus formation in cerebral microvessels. Heparin, activated protein C (APC), and antibodies (Ab) to either APC or TF were used to assess thrombogenesis in wild type (WT) mice. Mutant mice that overexpress the endothelial protein C receptor (EPCR-tg) or with TF-deficiency in Tie2 expressing endothelial cells (LTFE) were also employed.
Results
Thrombus formation in the SSS of WT mice was attenuated by heparin and in EPCR-tg mice, while treatment with the APC Ab enhanced thrombogenesis. Arteriolar thrombosis was largely unresponsive to the interventions studied. However, in cerebral venules, thrombosis was inhibited by heparin and in EPCR-tg mice. TF Ab treatment also inhibited venular thrombosis, with a similar attenuation noted in LTFE mice.
Conclusion
Thrombin promotes while the activated protein C pathway blunts thrombus formation in an experimental model of CVST. Tissue factor involvement is more evident in cerebral microvascular thrombogenesis, with endothelial cell-associated TF mediating this response in venules, but not arterioles.
Keywords: Activated protein C, Brain, Heparin, Thrombosis, Tissue factor
Introduction
Cerebrovascular occlusion due to thrombosis is a major cause of morbidity and mortality 1. Consequently, thrombus formation in large (cerebral venous sinus) and small vessels (cerebral venule and arteriole) of the brain has been studied to better understand the underlying pathophysiology and to improve treatment options for conditions such as thrombotic stroke, focal cortical infarctions, and cerebral venous sinus thrombosis (CVST). A variety of different experimental approaches has been used to elicit thrombus formation in large and small blood vessels 2-4. Two widely used methods are based on producing vessel injury by topical application of a potent oxidizing agent (FeCl3) on the vessel surface or by focal activation of a photosensitive dye or fluorochrome within the vessel lumen 5, 6. These models have been used to demonstrate the efficacy of tissue plasminogen activator (tPA) and other drugs (e.g., aspirin) in the dissolution of existing thrombi or in the inhibition of clot formation. However, the relative contributions of different components of the coagulation and anticoagulation pathways to thrombus formation in the cerebral vasculature remain poorly understood.
The heparin-antithrombin system, the protein C pathway, and tissue factor (TF) pathway inhibitor system represent the three major anticoagulant mechanisms that function to prevent thrombosis 7, 8. Down-regulation of these natural anticoagulant mechanisms has been invoked to explain the thrombus formation that accompanies different disease states, including CVST and stroke 9, 10. For example, APC resistance may explain the increased risk for thrombus formation in both CVST and stroke in children 9, 11. Although heparin is widely used in the treatment of CVST, the contribution of this anticoagulant, as well as activated protein C and TF, to thrombus formation in either large or small blood vessels of the brain has not been previously evaluated.
The objective of this study was to define the relative contributions of three major pro- and anti-coagulation pathways (heparin-antithrombin system, protein C pathway, and TF pathway) in the thrombogenic responses that occur in large and small vessels of the brain. Ferric chloride and FITC photoactivation were used to induce thrombus formation in the superior sagittal sinus (SSS) and cerebral microvessels, respectively. The findings of this study reveal differing roles for coagulation-anticoagulation mechanisms in mediating thrombosis between large and small vessels and between arterioles and venules.
Materials and Methods
Mice
Male C57BL/6 (wild-type [WT] control strain) mice (Jackson Laboratories, ME), transgenic mice overexpressing the endothelial protein C receptor (EPCR-tg, Oklahoma Medical Research Foundation, OK), and mutant mice that are TF deficient in Tie2 expressing cells (LTFE) which do not contain TF in endothelium and hematopoietic cells (not published), or TF floxed control (Cre-/-) mice were used. The EPCR-tg and the LTFE were backcrossed onto C57BL/6 mice. A total of 76 WT (weight: 25.0 ± 0.3 g), 19 EPCR-tg (weight: 25.8 ± 0.8 g), 7 LTFE-tg (weight: 30.6 ± 1.0 g), and 6 Cre-/- (weight; 30.1 ± 0.8 g) mice were employed. All mice were housed under specific pathogen-free conditions in standard cages and fed standard laboratory chow and water. The experimental procedures employed were reviewed and approved by the Institutional Animal Care and Use Committee of LSU Health Sciences Center, and performed according to the criteria outlined by the National Institutes of Health.
Animal preparation
Mice were anesthetized with intraperitoneal pentobarbital (50 mg/kg), with supplemental doses (12.5 mg/kg) given as needed. The left femoral vein was cannulated for intravenous administration of fluorescein isothiocyanate (FITC)-dextran. Body temperature was maintained at 36.5-37.0 C during the experiment with a homeothermic blanket and monitored with a rectal temperature probe. The head of each mouse was fixed on the acrylic frame prior to creating the cranial window. Following skull fixation, a circular skin incision was made and a craniectomy was created 3 mm lateral and 2 mm posterior to the bregma. The exposed brain tissue was immersed in artificial cerebrospinal fluid and covered with a glass slide. Cerebral vessels were observed through the dura mater.
Intravital videomicroscopy
The mouse was moved onto the stage of an upright fluorescent microscope (Optiphot, Nikon, Japan) with a 20X water immersion objective lens (20X 0.40 WI, Nikon, Japan). The microscopic image was projected onto a monitor (PVM-2030, Sony, Japan) through a 3CCD video camera (DXC-390, Sony, Japan) and recorded using a DVD recorder (SR-MV50, JVC, NJ). A video timer (Time-Date Generator WJ-810, Panasonic, Japan) was connected to the monitor to record time and date. The diameters of the brain vessels were measured by video analysis software (Image J 1.37v, NIH, Public Domain software) on a personal computer (G5 Macintosh, Apple, CA).
Light/dye induced thrombosis
The procedure used to induce microvascular thrombosis by the light/dye method is described elsewhere 3, 12, 13. Briefly, after the preparation was stabilized, 10 mL/kg of 5% FITC-dextran (excitation: 495 nm, emission: 519 nm) (150,000 MW, Sigma, MO) was slowly injected intravenously. It was allowed to circulate for 10 min, and then venules and arterioles with diameters ranging between 30-45 μm were selected for study. A 100 μm length of vessel was epi-illuminated using a 175-W xenon lamp (Lambda LS, Sutter, CA) and a fluorescein filter cube (DM510 B-2A, Nikon, Japan). The average excitation power density was 0.5W/cm2. Epi-illumination was continuously applied to the vessels and thrombus formation was quantified by determining the time required for complete flow cessation for ≥ 60 sec (flow cessation time). One venule and one arteriole were subjected to thrombosis using this method.
SSS thrombosis
Following anesthesia and fixation of the skull (as described above), an elliptical skin incision was made to produce a narrow rectangular cranial window along the SSS (8mm) from the confluence of sinuses to a point just posterior to inferior cerebral vein (ICV). Care was taken to not injure the SSS and to minimize overheating brain tissue while drilling through the cranium. The exposed SSS was kept moist with phosphate buffered saline. Next, 50 μl of 1 % FITC-dextran (150,000 MW, Sigma, MO) was administered intravenously. Ten minutes after, a 1 mm2 square filter paper soaked with 40 % FeCl3 was placed (using an operating microscope) on the anterior part of SSS, where it runs anterior to the ICV. The filter paper was covered with a plastic film to avoid evaporation of the FeCl3 solution. Upon placing the FeCl3 on the SSS, the flow of blood in this vessel was observed using fluorescence intravital videomicroscopy to ensure occlusion of the anterior segment of the SSS. This procedure was needed because the ICV is well developed in mice, and blood flow from the SSS drains into the ICV only when the posterior part of SSS is occluded. The microscope, video monitor, and recording system used to observe the SSS were identical to that described above except for the objective lens (10X 0.25, Spencer, USA), light path (including neutral density filter), and video camera (C2400, Hamamatsu, Japan). Once the anterior SSS was occluded, a 1 mm2 square filter paper soaked with 10 % FeCl3 was placed on the posterior part of SSS, i.e., anterior to the confluence of cerebral venous sinuses. A timer was activated to measure the time to flow cessation, which could be discerned from the SSS at the edges of the filter paper. The time to flow cessation was determined when flow in the SSS ceased for a period ≥ 60 sec.
Experimental protocols
To determine whether heparin, activated protein C (APC), and tissue factor contribute to small and large vessel thrombosis in the brain, the following experimental groups were studied using the photoactivation (arterioles & venules) and FeCl3 (SSS) thrombosis models: 1) control wild type (WT) mice, 2) WT mice receiving 100 IU/Kg (10) of heparin (heparin sodium, Abraxis, IL USA) 10 min. before thrombus induction (WT + hep), 3) WT mice receiving 10 μg/mouse (28) of murine activated protein C (mAPC, Oklahoma Medical Research Foundation, Oklahoma City OK) 10 min before thrombus induction (WT + APC), 4) WT mice receiving 120 μg/mouse (28) of rat anti-mouse APC monoclonal antibody (MPC1609, Oklahoma Medical Research Foundation, Oklahoma City, OK) 20 min. before thrombus induction (WT + APC Ab), 5) EPCR-tg mice, 6) WT mice receiving 20 mg/Kg (1) of a rat anti-mouse tissue factor monoclonal antibody 1H1 (TF Ab) 14 20 min before thrombus induction (WT + TF Ab), 7) LTFE mice, and 8) Cre-/- mice. In some experiments, we confirmed blood pressure and blood gases were not affected by each procedure.Figure 1 illustrates the specific sites within the coagulation/anticoagulation pathways that were targeted by our pharmacological and genetic interventions.
Figure 1. Targets of action of genetic and pharmacological interventions used to probe the role of components of the coagulation and anti-coagulation pathways.
TF, activated factor VII (VIIa), activated factor V (FVa) and activated factor X (FXa) complex is derived from extrinsic pathway, while platelet factor 3 (PF3), activated factor VIII (FVIIIa), FVa, and FXa complex are derived from the intrinsic pathway. These complexes (prothombin activating complex (PAC); surrounded by broken lined square) generate thrombin from prothrombin. TF act as an initial activator after vessel injury after binding FVIIa. LTFE mice do not contain TF in endothelium and hematopoietic cells. Protein C is activated with through the interactions of thrombomodulin (TM), protein S and EPCR binding. APC inactivates FVa and FVIIIa, which is a component of PAC. Heparin acts as an enhancer of antithrombin, which blocks both thrombin activity and other coagulation factors to generate fibrin.
Statistics
Each group was compared with its wild type control counterpart. Statistical difference was determined by a two-tailed t-test. All analyses were performed using Statview software 4.5 (Abacus Concepts Inc.). All values are expressed as means ± SE, and statistical significance was set at P<0.05.
Results
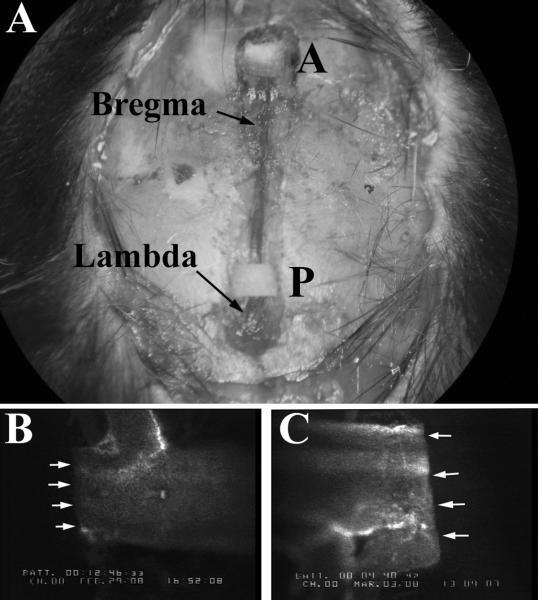
Figure 2 presents an image of a cranial window preparation (panel A), illustrating the location of the filter paper used to occlude the anterior (A) and posterior (P) segments of the SSS. Angiograms derived from each vessel segment are shown in panels B & C. Flow cessation in the SSS was readily apparent in this experimental model of CVST. Similarly, photoactivation-induced thrombus formation in cerebral arterioles and venules was readily discerned using intravital videomicroscopy. The average diameters of the vessels exposed to thrombus formation in this study were: 453.2 ± 4.8 μm, 32.7 ± 0.6 μm, and 35.1 ± 0.4 μm for SSS, arterioles, and venules, respectively.
Figure 2. Measurement of thrombosis in the superior sagittal sinus (SSS).
(Panel A) view through the cranial window; P designates the FeCl3 soaked filter paper lying on the posterior segment of the SSS, A designates the filter paper lying on the anterior segment. Panel B depicts an angiogram of a flow cessation determination in the posterior segment of the SSS. Panel C is an angiogram of the plugging in the anterior segment of SSS after exposure to 40% FeCl3.
Figure 3 summarizes the effects of heparin treatment on thrombus formation in the SSS and in cerebral venules. Heparin significantly prolonged the time to flow cessation in both the macroscopic and microscopic venous vessels. Arterioles were not influenced by heparin treatment (data not shown).
Figure 3. Effects of heparin on thrombin formation in the superior sagittal sinus (SSS) and cerebral venules.
Panel A: WT (n = 10), WT + hep (n = 9). Panel B: WT (n = 10), WT + hep (n = 7). *designates p<0.05 versus WT; **. P<0.01 versus WT.
Figure 4 shows the responses of the SSS and cerebral venules to thrombus formation under conditions of altered APC availability. Administration of exogenous murine APC did not alter thrombus formation in the SSS (panel A), cerebral venules (panel D), or arterioles (data not shown). Immunoblockade of endogenous APC with an anti-murine APC antibody enhanced the thrombosis response in the SSS (panel B) but had no effect on either venules (panel E) or arterioles. Overexpression of EPCR, which enhances the activation of endogenous protein C to APC, significantly prolonged the time to flow cessation in the SSS (panel C) and cerebral venules (panel F), but not in arterioles.
Figure 4. Role of the protein C pathway in thrombus formation within the superior sagittal sinus (SSS) and cerebral venules.
Panel A, B, C: WT (n = 10), WT + APC (n = 10), WT + APC Ab (n = 7), EPCR-tg (n = 8). Panel D, E, F: WT (n = 10), WT + APC (n = 7), WT + APC Ab (n = 5), EPCR-tg (n = 11). * designates p<0.05 versus WT; **. P<0.01 versus WT.
The responses of the SSS, venules, and arterioles to thrombosis following treatment with a TF blocking antibody are summarized in Figure 5. The TF Ab significantly prolonged the time to flow cessation in arterioles and venules, but not in the SSS. To assess the contribution of endothelial- vs peripheral blood cell-associated TF to the thrombosis response in arterioles and venules, we compared the time to flow cessation between mutant mice expressing low TF in Tie2-positive cells and their control counterparts (Figure 6). These experiments revealed that tissue factor deficiency in endothelial or hematopoietic cells prolonged thrombus formation in venules, but not in arterioles.
Figure 5. Effects of tissue factor (TF) immunoneutralization on thrombus formation in the superior sagittal sinus (SSS), venules, and arterioles.
Panel A: WT (n = 10), WT + TF Ab (n = 5). Panel B, C: WT (n = 10), WT + TF Ab (n = 6). * designates p<0.05 versus WT; **. P<0.01 versus WT.
Figure 6. Effect of endothelial tissue factor deficiency on thrombus formation in cerebral arterioles and venules.
Panel A and B: Cre-/- (n = 6), LTFE (n = 7). *designates p<0.05 versus Cre-/-.
Discussion
The goal of this study was to determine whether interventions that target some of the major pro- and anti-coagulation pathways alter thrombus formation in large and small blood vessels of the brain. A novel method was developed to simulate the clinical problem of CVST, which involved the assessment of FeCl3-induced thrombus formation in the SSS of wild type and mutant mice. This model is relatively easy to perform and allows for in vivo quantification of thrombus formation in veins with a narrow range of diameters. CVST is associated with mixed cell thrombi that are composed of platelets, leukocytes, fibrin and red blood cell 15, which can be recapitulated in experimental animals after exposure of vessels to FeCl3 2, 16.
Our findings with the CVST model (summarized in Table 1) reveal thrombus formation can be significantly delayed by treatment of wild type mice with heparin. Heparin is used clinically not only to treat CVST in order to prevent expansion of the thrombus after, but also as prophylaxis against relapse 10, 17-20. While some reports describe the effect of heparin on recanalization of CVST, no effort has been previously made to assess its effectiveness on thrombus formation in an experimental model of CVST 21, 22. Our positive findings with heparin in experimental CVST support its clinical use and confirm the central role of thrombin in producing the mixed thrombi that are characteristic of CVST and FeCl3-induced thrombosis.
TABLE 1.
Summary of the resultsa
| Pro- or anticoagulant system and model | Time to flow cessation | |||
|---|---|---|---|---|
| Light/dye model | FeCl3 model | |||
| Venule | Arteriole | SSS | ||
| Heparin-antithrombin system | ||||
| WT + hep | [uparrow] | [rarrow] | [uparow] | |
| Protein C pathway | ||||
| WT + APC | [rarrow] | [rarrow] | [rarrow] | |
| WT + APC Ab | [rarrow] | [rarrow] | [downarrow] | |
| EPCR-tg | [uparrow] | [rarrow] | [uparrow] | |
| TF pathway | ||||
| WT + TF Ab | [uparrow] | [uparrow] | [rarrow] | |
| LTFE | [uparrow] | [rarrow] | NE | |
SSS, superior sagittal sinus; WT, wild-type; hep, heparin; APC, activated protein C; Ab, antibody; EPCR, endothelial protein C receptor; tg, transgenic; TF, tissue factor; LTFE, low-tissue factor-expressing; [uparrow], prolongation; [rarrow], no change; [downarrow], shortening of time to flow cessation; NE, not examined.
Our study also provides novel insights into the protective role of the protein C anticoagulant pathway in experimental CVST. This pathway is initiated by thrombin binding to thrombomodulin (TM), with the subsequent activation of protein C by the thrombin-TM complex and the endothelial protein C receptor (EPCR) 7, 23. Activated protein C (APC) interacts with protein S to inactivate factors Va and VIIIa, thereby exerting an anticoagulant effect. Our findings in the experimental CVST model indicate that treatment of wild type mice with an APC blocking antibody enhances SSS thrombosis while EPCR-tg mice exhibit an attenuated thrombogenic response. Hence, the availability of endogenous APC appears to be a significant determinant of the rate of thrombus formation in the SSS. This observation may be relevant to clinical CVST in view of reports that describe an association between an impaired protein C pathway and the risk for development of CVST 10. Our analysis of thrombus formation in microvessels revealed a somewhat different pattern of involvement of the different coagulant/anticoagulant pathways. Cerebral arterioles appeared to be largely unresponsive to the different interventions evaluated, including heparin. Since the APC blocking antibody and over-expression of EPCR have been shown to effectively alter light/dye-induced thrombus formation in arterioles of cremaster muscle 24, the absence of any change in thrombus formation with these interventions in the present study suggests cerebral arterioles may not express a robust protein C pathway. This is supported by reports describing low TM levels in the brain 25, 26.
Although two different methods (FeCl3 & photoactivation) were used to promote thrombus formation in the SSS and cerebral venules, the responses to the different anticoagulant interventions exhibited some significant similarities, i.e., both heparin & EPCR overexpression delayed the thrombus development in large and microscopic veins. However, some differences were noted, including an unresponsiveness of cerebral venules to the APC blocking A similar unresponsiveness of light/dye-induced thrombus formation to APC blocking antibody has been previously described in cremaster muscle venules 24.
TF, a key mediator of the initiation phase of thrombogenesis 27, 28, activates coagulation by binding to and activating factor VII. TF is mainly produced and expressed by monocytes, neutrophils, platelets, and the vessel wall, including adventitia or media. Microparticles (0.05 to 1.0 um) shed from monocytes and platelets are also a rich source of TF that can initiate coagulation. We noted a significant delay in thrombus formation following TF antibody treatment in cerebral arterioles and venules, but not in the SSS. Whether the different role of TF in large and small vessels reflects the levels of expression in the different vessel populations or is the result of the different models (FeCl3 vs photoactivation) used to elicit thrombus formation is unclear. However, a novel finding was the significant differences in thrombus formation between mice that genetically express low levels of TF in vascular endothelial and hematopoietic cells (LTFE) and their control counterparts with venules, but not significant with arterioles, exhibiting a prolongation of thrombus formation. These results suggest endothelial cell and/or hematopoietic cell TF likely accounts for the protective effect of the TF immunoneutalization against thrombosis in cerebral venules, while blood cell- and/or microparticle-associated TF mainly contributes to the arteriolar thrombosis response. These responses may be unique to the cerebral circulation since previous reports ascribe a dominant role for vessel wall-associated TF in arterial thrombosis while blood cell (or microparticle)-associated TF plays a major role in venous thrombosis 28. Additional work is needed to define the specific cell populations that account for TF-mediated thrombosis in cerebral arterioles.
This study provides the first quantitative estimates of thrombus formation in an experimental model of CVST and provides novel insights into the coagulant/anticoagulant mechanisms that modulate thrombus formation in the SSS and in cerebral arterioles and venules. Our findings reveal differing roles for heparin, activated protein C, and TF in mediating/attenuating thrombosis between large and small vessels, and between arterioles and venules of the brain. These observations may bear on the clinical utility of targeting specific components of the coagulation/anticoagulation pathways for treatment of CVST or the cerebral microvascular thrombosis associated with ischemic stroke.
Grant Information / Other Acknowledgments
Supported by funds from the Malcolm Feist Cardiovascular Endowment, a grant from the National Heart Lung and Blood Institute (HL26441) and Leducq International Network Against Thrombosis (LINAT) awarded by the Leducq Foundation, Paris. CTE is an investigator of the Howard Hughes Medical Institute.
Biography
This manuscript by Nagai, et al. investigates the relative contributions of three major pro- and anti-coagulation pathways including heparin-antithrombin, protein C, and tissue factor (TF) and the thrombogenic responses that occur in large and small vessels in the brain. Thrombosis was induced in the superior sagittal sinus by topical application of ferric chloride or by FITC photoactivation within the vessel lumen. Their objective was to elucidate the relative contributions of the above stated pathways in the cerebral vasculature.
In their experiment, the three pathways were studied by using both the ferric chloride method on the SSS and photoactivation method on arterioles and venules by comparing mice in the following groups: (1) control wild type (WT) mice versus WT treated with heparin prior to thrombus induction, (2) control WT versus WT receiving activated protein C (APC), control WT versus WT receiving rat anti-mouse APC monoclonal antibody, and control WT versus transgenic mice overexpressing endothelial protein C receptor (EPCR), (3) control WT versus WT receiving tissue factor monoclonal antibody, (4) tissue factor (TF) deficient mutant mice versus TF floxed control mice.
Comparisons between groups were made by quantifying time required for complete flow cessation. This was done via observation through video microscopy and epi-illumination. However, the explanation of their quantification method needs to be further clarified. It is unclear whether “flow cessation time” means complete flow cessation for a total of 60 seconds or 60 seconds from first observed flow cessation. Since the discussion section states, “this model is relatively easy to perform and allows for in vivo quantification of thrombus formation” the explanation of the quantification method should be more clearly expressed to assess feasibility of its reproducibility.
This is an interesting and potentially useful paper that provides a novel experimental model. This may provide valuable insight into defining differing roles of pro- and anti-coagulation pathways in cerebral vasculature. This may have future implications on clinical treatment targets for not only cerebral venous sinus thrombosis, but also any area of related to ischemic stroke.
Amy Lee, M.D., Ralph Dacey, M.D., St. Louis, Missouri
The authors carry out a series of cleverly-designed experiments that seek to delineate, perhaps for the first time, the role of 3 antithrombotic pathways in human cerebral venous thrombotic disease. The pathways are the heparin-antithrombin 3, protein C-thrombin-thrombomodulin and tissue factor pathways. Mouse transgenic and other molecular manipulations are used to alter these pathways and determine the effects on superior sagittal sinus thrombosis in mice and on photothrombosis of mouse arterioles and venules.
The results suggest that the antithrombin 3 and protein C pathways are involved in large venous sinus thrombosis whereas the tissue factor pathway is less important. This fits with clinical data showing the former are associated with cerebral venous thrombosis. The results with tissue factor are complicated, however, by differences between brain and systemic vessels.
The conclusions here could be that the mouse models reflect human disease, which seems supported by some of the results. Maybe more specific treatments could be designed based on the results, with the goal to increase efficacy and decrease side effects such as iatrogenic hemorrhaging. The limitations, however, are that one does not know the relative contributions of these pathways to thrombosis and antithrombosis in mice and humans. Second, to what extent the manipulations inhibited the pathways involved is uncertain. Several methods were used to inhibit the protein C and tissue factor pathways and the results were not the same for each method. Is this because of incomplete inhibition of the pathway? Finally, activating thrombosis by light or oxidant stress, as done here, may not be the same as precipitating factors in human cerebral venous thrombosis. Thus, more work is needed and one has to consider whether some fundamental advance is going to be made in a lower species or if this can only be made by studying humans or a closely allied species.
R. Loch Macdonald, M.D., Ph.D., Toronto, Ontario
Footnotes
Financial Disclosure: Dr. Esmon holds patents and licenses dealing with the protein C system, Dr. Kirchhofer is employed by Genentech, Inc, and the remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008 May 10;371(9624):1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Pinel C, Wice SM, Hiebert LM. Orally administered heparins prevent arterial thrombosis in a rat model. Thromb Haemost. 2004 May;91(5):919–926. doi: 10.1160/TH03-08-0527. [DOI] [PubMed] [Google Scholar]
- 3.Rumbaut RE, Slaff DW, Burns AR. Microvascular thrombosis models in venules and arterioles in vivo. Microcirculation. 2005 Apr-May;12(3):259–274. doi: 10.1080/10739680590925664. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell KA, Dyck RH. Induction of reproducible focal ischemic lesions in neonatal mice by photothrombosis. Dev Neurosci. 2005 Mar-Aug;27(2-4):121–126. doi: 10.1159/000085983. [DOI] [PubMed] [Google Scholar]
- 5.Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 1990 Nov 15;60(4):269–280. doi: 10.1016/0049-3848(90)90106-m. [DOI] [PubMed] [Google Scholar]
- 6.Rosenblum WI, El-Sabban F. Platelet aggregation in the cerebral microcirculation: effect of aspirin and other agents. Circ Res. 1977 Mar;40(3):320–328. doi: 10.1161/01.res.40.3.320. [DOI] [PubMed] [Google Scholar]
- 7.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005 Nov;131(4):417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 8.Esmon CT. The impact of the inflammatory response on coagulation. Thromb Res. 2004;114(5-6):321–327. doi: 10.1016/j.thromres.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Boekholdt SM, Kramer MH. Arterial thrombosis and the role of thrombophilia. Semin Thromb Hemost. 2007 Sep;33(6):588–596. doi: 10.1055/s-2007-985755. [DOI] [PubMed] [Google Scholar]
- 10.Masuhr F, Mehraein S, Einhaupl K. Cerebral venous and sinus thrombosis. J Neurol. 2004 Jan;251(1):11–23. doi: 10.1007/s00415-004-0321-7. [DOI] [PubMed] [Google Scholar]
- 11.Rahemtullah A, Van Cott EM. Hypercoagulation testing in ischemic stroke. Arch Pathol Lab Med. 2007 Jun;131(6):890–901. doi: 10.5858/2007-131-890-HTIIS. [DOI] [PubMed] [Google Scholar]
- 12.Rumbaut RE, Randhawa JK, Smith CW, Burns AR. Mouse cremaster venules are predisposed to light/dye-induced thrombosis independent of wall shear rate, CD18, ICAM-1, or P-selectin. Microcirculation. 2004 Apr-May;11(3):239–247. doi: 10.1080/10739680490425949. [DOI] [PubMed] [Google Scholar]
- 13.Anthoni C, Russell J, Wood KC, et al. Tissue factor: a mediator of inflammatory cell recruitment, tissue injury, and thrombus formation in experimental colitis. J Exp Med. 2007 Jul 9;204(7):1595–1601. doi: 10.1084/jem.20062354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirchhofer D, Moran P, Bullens S, Peale F, Bunting S. A monoclonal antibody that inhibits mouse tissue factor function. J Thromb Haemost. 2005 May;3(5):1098–1099. doi: 10.1111/j.1538-7836.2005.01253.x. [DOI] [PubMed] [Google Scholar]
- 15.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008 Feb 21;451(7181):914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockyer S, Kambayashi J. Demonstration of flow and platelet dependency in a ferric chloride-induced model of thrombosis. J Cardiovasc Pharmacol. 1999 May;33(5):718–725. doi: 10.1097/00005344-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Masuhr F, Einhaupl K. Treatment of cerebral venous and sinus thrombosis. Front Neurol Neurosci. 2008;23:132–143. doi: 10.1159/000111375. [DOI] [PubMed] [Google Scholar]
- 18.Ferro JM, Canhao P. Acute treatment of cerebral venous and dural sinus thrombosis. Curr Treat Options Neurol. 2008 Mar;10(2):126–137. doi: 10.1007/s11940-008-0014-0. [DOI] [PubMed] [Google Scholar]
- 19.Ferro JM, Canhao P. Complications of cerebral vein and sinus thrombosis. Front Neurol Neurosci. 2008;23:161–171. doi: 10.1159/000111377. [DOI] [PubMed] [Google Scholar]
- 20.Azin H, Ashjazadeh N. Cerebral venous sinus thrombosis--clinical features, predisposing and prognostic factors. Acta Neurol Taiwan. 2008 Jun;17(2):82–87. [PubMed] [Google Scholar]
- 21.Kim DE, Schellingerhout D, Jaffer FA, Weissleder R, Tung CH. Near-infrared fluorescent imaging of cerebral thrombi and blood-brain barrier disruption in a mouse model of cerebral venous sinus thrombosis. J Cereb Blood Flow Metab. 2005 Feb;25(2):226–233. doi: 10.1038/sj.jcbfm.9600023. [DOI] [PubMed] [Google Scholar]
- 22.Rottger C, Madlener K, Heil M, et al. Is heparin treatment the optimal management for cerebral venous thrombosis? Effect of abciximab, recombinant tissue plasminogen activator, and enoxaparin in experimentally induced superior sagittal sinus thrombosis. Stroke. 2005 Apr;36(4):841–846. doi: 10.1161/01.STR.0000157663.43209.a2. [DOI] [PubMed] [Google Scholar]
- 23.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007 Apr 15;109(8):3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida H, Russell J, Stokes KY, Yilmaz CE, Esmon CT, Granger DN. Role of the protein C pathway in the extraintestinal thrombosis associated with murine colitis. Gastroenterology. 2008 Sep;135(3):882–888. doi: 10.1053/j.gastro.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii H, Salem HH, Bell CE, Laposata EA, Majerus PW. Thrombomodulin, an endothelial anticoagulant protein, is absent from the human brain. Blood. 1986 Feb;67(2):362–365. [PubMed] [Google Scholar]
- 26.Wong VL, Hofman FM, Ishii H, Fisher M. Regional distribution of thrombomodulin in human brain. Brain Res. 1991 Aug 9;556(1):1–5. doi: 10.1016/0006-8993(91)90540-c. [DOI] [PubMed] [Google Scholar]
- 27.Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006 Mar-Apr;36(2):104–107. doi: 10.1016/j.bcmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007 Aug;27(8):1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]