Abstract
Despite the impact of cocaine's aversive effects on its abuse potential, the neurochemical basis of these aversive effects remains poorly understood. By blocking the reuptake of the monoamine neurotransmitters dopamine (DA), norepinephrine (NE) and serotonin (5-HT) into the presynaptic terminal, cocaine acts as a potent indirect agonist of each of these systems. The following studies attempted to assess the extent of monoaminergic mediation of cocaine's aversive effects using conditioned taste aversion (CTA) learning (Garcia, 1955). Specifically, Experiment 1 assessed the ability of selective monoamine transporter inhibitors, e.g., DAT (vanoxerine), NET (nisoxetine) and SERT (fluoxetine), to induce taste aversions (relative to cocaine). Only the NET inhibitor approximated the aversive strength of cocaine. Experiment 2 compared the effects of pretreatment of each of these transport inhibitors on the development of a cocaine-induced CTA. Pretreatment with nisoxetine and fluoxetine both attenuated cocaine-induced aversions in a manner comparable to that produced by cocaine itself. The DAT inhibitor was without effect. Combined, the results of these investigations indicate little or no involvement of dopaminergic systems in cocaine's aversive effects while NE appears to contribute most substantially, with a possible modulatory involvement by serotonin.
1. Introduction
Although cocaine has been reported to induce taste aversions under a variety of parametric conditions the biochemical basis of these aversions has not been determined (Ferrari et al., 1991; Goudie, 1978). Because cocaine is reported to inhibit the reuptake of a variety of monoamines, including dopamine (DA), norepinephrine (NE) and serotonin (5-HT), it is possible that activity at any one of these systems (or some combination) may be responsible for its aversive effects. In an attempt to assess the possible biochemical basis of cocaine's aversive effects, Freeman et al. (2007) examined the ability of a variety of relatively selective monoamine reuptake inhibitors to induce taste aversions in outbred, Sprague-Dawley rats. Specifically, rats were given a novel saccharin solution to drink and injected with varying doses (18-50 mg/kg) of the dopamine transport inhibitor (DAT) vanoxerine, the norepinephrine transport inhibitor (NET) desipramine or the serotonin transport inhibitor (SERT) clomipramine. Aversions induced by these compounds were compared to those induced by cocaine (at comparable doses). As expected, cocaine induced aversions in a dose-dependent manner. Aversions were also induced by all of the monoamine reuptake inhibitors, but only those induced by desipramine matched those induced by cocaine. That is, aversions at each dose tested were indistinguishable for cocaine and desipramine. Aversions induced by vanoxerine approximated those induced by cocaine only at the highest dose tested. Clomipramine-induced aversions relative to controls, but these aversions never matched those of cocaine. Given that the relatively selective NET inhibitor desipramine induced aversions comparable to those of cocaine, Freeman et al. suggested that increases in NE activity may primarily mediate the aversions induced by cocaine. The fact that both vanoxerine and clomipramine produced aversions (albeit with weaker potency and to a lesser degree) left open the role of DA and 5-HT in cocaine-induced aversions (see Hunt, Spivak and Amit, 1985).
In a further assessment of the possible role of NE in cocaine-induced aversions, Serafine and Riley (2009) examined the effects of preexposure to the NET inhibitor desipramine on cocaine-induced taste aversions. Such a procedure is a modification of the unconditioned stimulus (US) preexposure effect in taste aversion conditioning (for a review see Riley and Simpson, 2001). In this design, animals exposed to a drug (Drug A) prior to aversion conditioning with that same drug generally display a weaker taste aversion as a result. Although the basis of this attenuation remains unknown, it has been suggested to be a function of either associative (e.g., blocking) or non-associate (e.g., tolerance) factors (de Brugada et al., 2004; Elkins, 1974, Le Blanc and Cappell, 1974). Preexposure to Drug A is often reported to weaken aversions induced by Drug B. Such a cross-drug preexposure effect has been used to suggest that the two drugs share a common mechanism in inducing aversions (Fox et al., 2006; Kunin et al., 1999; Kunin et al., 2001). Such findings are independent of the underlying associative and nonassociative mechanism given that the similarities in the aversive stimulus properties of the preexposure drug and conditioning drug are the basis for either mechanism.
In the Serafine and Riley (2009) procedure, rats were given five exposures to cocaine, desipramine or vehicle every fourth day for a total of five exposures. Subjects were then given access to saccharin followed by an injection of cocaine. As expected, cocaine preexposure attenuated the acquisition of cocaine-induced taste aversions, an effect attributable to an adaptation or tolerance to cocaine's aversive effects during preexposure. Interestingly, preexposure to desipramine also attenuated cocaine-induced taste aversions and to the same degree as that produced by exposure to cocaine itself. Given the relative selectivity of desipramine as a NET inhibitor, the fact that desipramine preexposure attenuated cocaine-induced taste aversions is consistent with a role of NE in these aversions.
From both the analysis of aversions induced by various monoamine transport inhibitors and the effects of desipramine preexposure on cocaine-induced taste aversions, NE appears to play the most prominent role in aversions induced by cocaine. The following experiments extended this analysis of the role of NE in cocaine-induced aversions to mice. The choice for extending this analysis to mice is twofold. First, species (and strain) differences have been reported in aversion learning and the effects of various manipulations on such learning (Caihol and Mormede, 2002; Jones et al., 2006). As such, it is unknown to what degree the work with rats generalizes to other rodent species. The interest in mice, however, extends beyond the demonstration of a possible species difference. Specifically, earlier work in knock-out mice assessing the role of various monoamines in the reinforcing effects of cocaine have implicated NE as a mediator of cocaine's aversive effects. Knock-out mice lacking the NET transporter display enhanced cocaine-induced place preferences, suggesting that in wildtype subjects (with intact transporters) NE may be aversive and, thus, counteracts the normally rewarding effects of DA activity (Hall et al., 2002; Hall et al., 2004; Xu et al., 2000).
In this context, the present experiments examined cocaine-induced taste aversions in mice and assessed the role of NE in these aversions. Specifically, Experiment 1 compared the aversive stimulus effects of highly selective inhibitors of DAT (vanoxerine), NET (nisoxetine) and SERT (fluoxetine) with that of cocaine (see Freeman et al., 2005). Experiment 2 examined the effects of preexposure to one of these three selective monoamine inhibitors (vanoxerine, nisoxetine and fluoxetine) on cocaine-induced taste aversions.
2. General Methods
2.1. Subjects
One hundred and sixty male ND4 Swiss-Webster albino mice purchased from Harlan Sprague-Dawley were used for Experiment 1 (n = 88) and Experiment 2 (n = 72). At the time testing began, all subjects weighed between 25-30g.
2.2. Apparatus/Housing
All subjects were individually housed in Plexiglas bins (44.5 × 23 × 20 cm) fitted with wire-grated tops. Subjects were maintained under a 12:12 LD cycle (lights on at 0800h) and at an ambient temperature of 23 °C. Har land Rat and Mouse Laboratory Diet was available ad libitum throughout the experiment. Water was available ad libitum until the experimental procedures were initiated (see below). All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003) and were approved by the American University Institutional Animal Care and Use Committee.
2.3. Drugs and Solutions
Cocaine HCl was generously provided by the National Institute on Drug Abuse. Nisoxetine HCl (LY-94939) and fluoxetine HCl were purchased from Sigma Pharmacueticals (St. Louis, MO), while vanoxerine dihydrochloride (GBR 12909) was provided by the Laboratory of Medicinal Chemistry at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). All drugs were dissolved in saline and administered subcutaneously (sc) in a concentration of 10 mg/ml (all doses are expressed as the salt). Saccharin (0.1% sodium saccharin, Sigma Chemical Co., St. Louis, MO) was prepared as a 1 g/l solution in tap water.
2.4. Conditioned Taste Aversion
Phase I: Habituation/Adaptation. Following 23 h of water deprivation, all subjects were allowed 1-h access to water. This procedure was repeated for 7 days, a point at which water consumption was stable for all subjects.
Phase II: Conditioning/Training. On Day 1 of this phase, subjects were allowed 1-h access to a novel saccharin solution during their scheduled fluid access. Immediately following saccharin presentation, subjects were injected sc with the particular drug under investigation, specified by experiment (see below). On the 3 water-recovery days following each conditioning trial, all subjects were allowed 1-h access to water without drug administration. This alternating procedure of conditioning/water recovery was repeated for a total of four complete cycles, resulting in a total of three drug administrations and four saccharin presentations.
3. Experiment 1
Subjects were conditioned with one of three doses (18, 32 and 50 mg/kg) of vanoxerine, nisoxetine or fluoxetine. A vehicle group (0 mg/kg) received an injection of saline which was isovolumetric with the highest dose of drug. As a standard for comparison, an additional group of subjects was conditioned with a single dose of cocaine (18 mg/kg). The dose range used in this study matched those of the corresponding investigation by Freeman and colleagues (2005). As in Freeman's study vanoxerine was utilized as the DAT inhibiting drug, yet desipramine and clomipramine were replaced by nisoxetine and fluoxetine as the selective NET and SERT inhibitors (respectively). These compounds were found to be more commonly used within mouse literature related to the current investigations (Hall et al., 2002, 2004; Sora et al., 2001; Yamashita et al., 2006)
3.1. Statistical Analysis
Aversions produced by each dose of the five drugs were compared using an 11 × 4 Analysis of Variance (ANOVA). For this analysis the between-subjects variable was Group [saline, cocaine (18), vanoxerine (18,32,50), nisoxetine(18,32,50) or fluoxetine(18,32,50)] and the within-subjects variable was Trial (1-4). During follow-up analyses, one-way ANOVAs were used to compare the mean absolute saccharin consumption at each of the four conditioning trials. Pairwise comparisons were performed using Tukey's HSD post-hoc tests to identify specific group differences at each trial.
3.2. Results
The 11 × 4 Repeated Measures ANOVA revealed significant effects for Group [F (10, 72) = 7.96, p<.001] and Trial [F (3, 216) = 60.13, p<.001] as well as a significant Group × Trial interaction [F (30, 216) = 5.94, p<.001]. Follow-up analysis of Trial 1 found no significant differences [F (10, 75) = 1.30, p=.211]; however, subsequent analyses of Trial 2 [F (10, 75) = 3.95, p<.001], Trial 3 [F (10, 76) = 10.84, p<.001] and Trial 4 [F (10, 74) = 8.54, p<.001] revealed significant group differences.
Figure 1 illustrates the mean absolute saccharin consumption for subjects conditioned with each of the three doses of vanoxerine across all four conditioning trials. For comparison, groups injected with cocaine and vehicle are included as well. There were no significant group differences on the initial exposure to saccharin (Trial 1). On Trial 2, subjects injected with the 18 mg/kg dose of cocaine drank significantly less than controls (p<.01). Subjects injected with the lowest dose of vanoxerine (Van 18) drank significantly more than the cocaine-injected subjects. (p<.01), but did not differ from any other group. Subjects injected with 32 and 50 mg/kg vanoxerine (Van 32, Van 50) did not differ from any group. On Trial 3, subjects injected with cocaine drank significantly less than controls (p<.001). All groups injected with vanoxerine drank significantly more than the cocaine-injected group (p<.001, for all comparisons). Subjects injected with the highest dose (Van 50) now drank less than controls (p<.05). On Trial 4, the cocaine-injected subjects again consumed less than controls (p< .001). Vanoxerine-injected subjects drank significantly more than cocaine-injected subjects (p < .001), although none differed from controls or each other.
Figure 1.
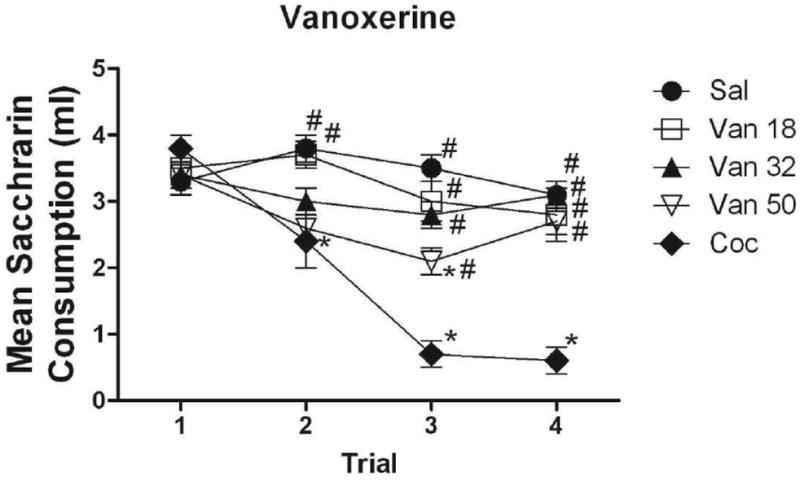
Mean (+/- SEM) absolute saccharin consumption for subjects conditioned with 18, 32 and 50 mg/kg of vanoxerine on Trials 1-4, along with saline and cocaine controls. *Denotes significant difference from subjects conditioned with saline #Denotes significant difference from subjects conditioned with cocaine.
Figure 2 illustrates the mean absolute saccharin consumption for subjects injected with each of the three doses of fluoxetine. As above, groups injected with cocaine and vehicle are included for comparison. No differences among groups were evident on Trial 1. On Trial 2, subjects injected with the lower doses of fluoxetine (Flu 18 and Flu 32) drank significantly more saccharin than the cocaine-injected subjects (p<.01, for all comparisons). There were no differences among the three fluoxetine-injected groups. Further, none of these groups differed significantly from the saline-injected controls. On Trial 3, all fluoxetine-injected groups drank significantly more than subjects injected with cocaine (p<.001). Subjects injected with the largest dose of fluoxetine (Flu 50) drank significantly less than controls (p<.05), while the lower dose groups (Flu 18, Flu 32) did not. The same patterns were evident on Trial 4 with the exception that no fluoxetine-injected group differed from controls on this trial. At no point did the three fluoxetine-injected groups differ significantly from each other.
Figure 2.
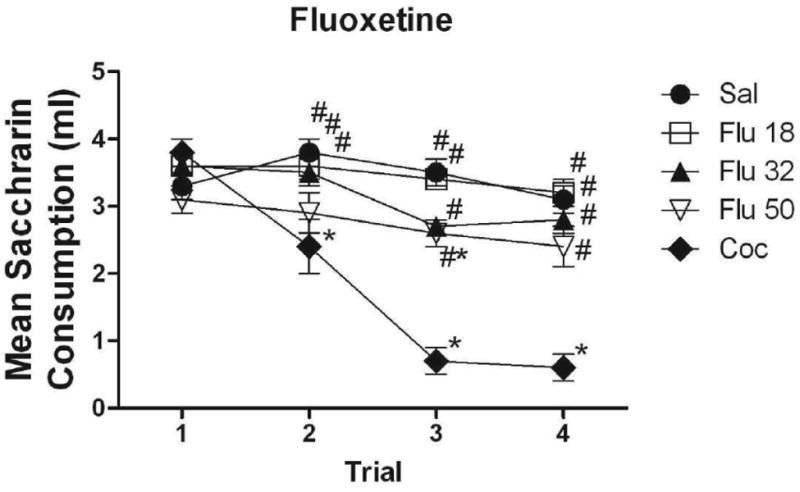
Mean (+/- SEM) absolute saccharin consumption for subjects conditioned with 18, 32 and 50 mg/kg of fluoxetine on Trials 1-4, along with saline and cocaine controls. *Denotes significant difference from subjects conditioned with saline #Denotes significant difference from subjects conditioned with cocaine.
Figure 3 illustrates saccharin consumption across all four conditioning trials for subjects in each of the three nisoxetine-injected groups. As above, groups injected with cocaine and vehicle are included for comparison. No differences among groups were evident on Trial 1. On Trial 2, none of the three groups injected with nisoxetine differed from saline- or cocaine-injected subjects. On Trial 3, subjects injected with the two lower doses of nisoxetine (Groups Nis 18 and Nis 32) consumed significantly more than the cocaine-injected subjects (p < .001 and p< .01, respectively). There were no differences in consumption between Groups Nis 50 and Coc. Both Groups Nis 32 and Nis 50 drank significantly less saccharin than controls on this trial (both p <.05, for all comparisons). On Trial 4, Groups Nis 18 and Nis 32 drank significantly more than Group Coc (both p < .01, for all comparisons), but failed to differ significantly from controls. Group Nis 50 did not differ from the cocaine-injected subjects and drank significantly less than the saline-injected controls (p<.01) and Group Nis 18 (p<.05).
Figure 3.
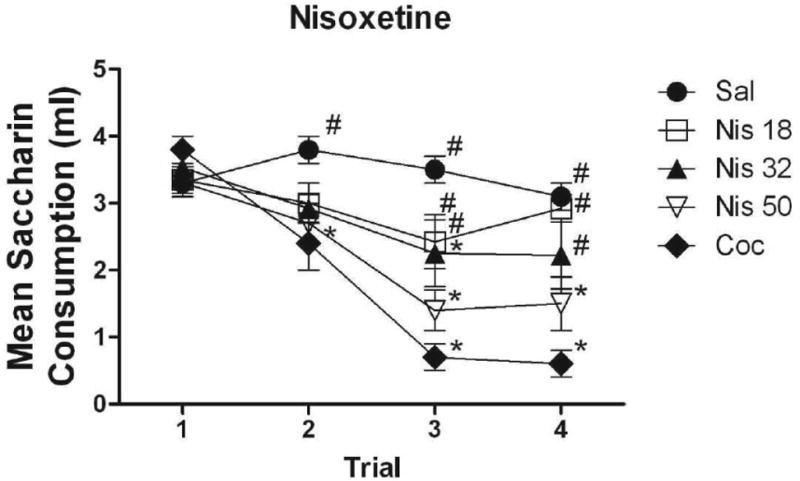
Mean (+/- SEM) absolute saccharin consumption for subjects conditioned with 18, 32 and 50 mg/kg of nisoxetine on Trials 1-4, along with saline and cocaine controls. *Denotes significant difference from subjects conditioned with saline #Denotes significant difference from subjects conditioned with cocaine.
Figure 4 illustrates saccharin consumption on the final exposure to saccharin (Trial 4) for all groups (Van 18, Van 32, Van 50, Flu 18, Flu 32, Flu 50, Nis 18, Nis 32, Nis 50, Sal, Coc). At 18 and 32 mg/kg, there were no significant differences among the three drug conditions (i.e., Van, Nis, Flu). At 50 mg/kg, the vanoxerine and fluoxetine groups did not differ from one another; however, subjects injected with the highest dose of nisoxetine drank significantly less than those injected with saline or vanoxerine (p<.05).
Figure 4.
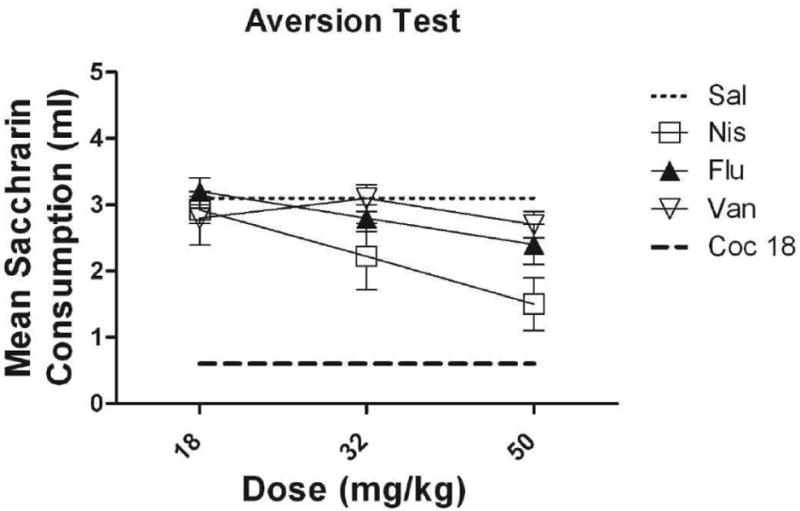
Mean (+/- SEM) absolute saccharin consumption for subjects conditioned with 18, 32, and 50 mg/kg of vanoxerine, fluoxetine and nisoxetine on Trial 4
4. Experiment 2
Subjects were randomly assigned to receive preexposure to one of five possible compounds; saline (isovolumetric with the highest dose), cocaine (18 mg/kg), vanoxerine (50 mg/kg), nisoxetine (32 mg/kg) and fluoxetine (50 mg/kg). The highest dose of vanoxerine and fluoxetine (50 mg/kg) employed in Experiment 1 was used in Experiment 2. Although effects produced by this dose never approximated that of cocaine, aversions were evident on at least one trial at this dose for each drug, indicating a behaviorally active dose. The specific choice of 32 mg/kg nisoxetine was based on the fact that in Experiment 1, effects induced by this dose were comparable to those induced by 50 mg/kg fluoxetine and vanoxerine. Finally, 18 mg/kg cocaine was chosen because unpublished work from this laboratory (see Randall- Thompson, 2005) has shown that this dose of cocaine produces intermediate aversions comparable to those produced by the abovementioned doses of vanoxerine, nisoxetine and fluoxetine. During the drug preexposure phase in Experiment 2, each subject received a sc injection of one of the aforementioned drugs approximately 5 h after daily water access (1 h). The drug was given every other day for a total of 10 days, resulting in a total of five drug injections. Fluid intake was monitored throughout this period. During the conditioning phase of Experiment 2, subjects were allowed 1-h access to a novel saccharin solution during their scheduled fluid-access period. Immediately following saccharin access, half of the subjects were injected sc with 32 mg/kg cocaine HCl. This dose of cocaine was selected because it has been shown to produce a moderate aversion in mice, allowing for the possibility of potentiation or attenuation by drug preexposure. The remaining subjects were injected with equivolume saline. Similar to the procedure used during Experiment 1, all subjects received a total of four saccharin-drug (or vehicle) pairings with three water-recovery days between each pairing.
4.1. Statistical Analyses
Differences in water consumption among groups during drug preexposure were analyzed by a 5 × 10 ANOVA with the between-subjects variable of Preexposure Drug (vanoxerine, nisoxetine, fluoxetine, saline and cocaine) and the within-subjects variable of Preexposure Day (1-10). Differences in saccharin consumption over conditioning for subjects injected with cocaine or saline were analyzed using a 10 × 2 × 4 ANOVA with the between-subjects variables of Preexposure Drug (vanoxerine, nisoxetine, fluoxetine, saline and cocaine), Conditioning Drug (saline and cocaine) and the within-subjects variable of Trial (1-4). Follow-up analyses were performed separately for the cocaine- and saline-conditioned animals because these were the only specific comparisons of relevance. The mean absolute saccharin consumption among groups was compared on each conditioning trial using one-way ANOVAs, and Tukey's HSD post-hoc tests were used in order to identify individual group differences at each trial.
4.2 Results
The ANOVA on fluid consumption during preexposure (see Figure 5) revealed a significant effect of Preexposure Day [F (7, 29) = 2.87, p<.05], but no significant effect of Preexposure Drug [F (4, 35) = 1.95, p=.12] and no significant Preexposure Drug × Preexposure Day interaction [F (28,128) = 1.95, p=.07].
Figure 5.
Mean water consumption for subjects preexposed to saline, cocaine, fluoxetine, nisoxetine or vanoxerine
The ANOVA on saccharin consumption during conditioning revealed significant effects of Trial [F (3, 207) = 49.27, p<.001], Preexposure Drug [F (4, 69) = 4.12, p<.01] and Conditioning Drug [F (1, 69) = 104.95 p<.001]. In addition, there were significant Trial × Preexposure Drug [F (12, 207) = 2.62, p<.01], Trial × Conditioning Drug [F (3, 207) = 29.84, p<.01] and Trial × Preexposure Drug × Conditioning Drug [F (12, 207) = 2.12, p<.05] interactions. There was no significant Conditioning Drug × Preexposure Drug effect [F (4, 69) = 2.04, p=.10]. Concerning the Conditioning Drug main effect, the ANOVA found that overall, subjects conditioned with cocaine consumed significantly less than those conditioned with saline (p<.001). Planned comparisons (Trial 4) of subjects preexposed to the same drug yet conditioned with different drugs (saline vs. cocaine) revealed that across all preexposure drugs, groups conditioned with cocaine consumed significantly less saccharin than those conditioned with saline (Sal-Sal vs. Sal-Coc, p<.001; Coc-Sal vs. Coc-Coc, p<.01; Nis-Sal vs. Nis-Coc, p<.01; Flu-Sal vs. Flu-Coc, p<.01; Van-Sal vs. Van-Coc, p<.001).
Specific preexposure drug comparisons at each trial were also made among subjects within each conditioning group (saline and cocaine). The upper panel of Figure 6 illustrates the mean absolute saccharin consumption across the four conditioning trials for all drug-preexposed subjects conditioned with 32 mg/kg cocaine. There were no significant group differences on Trial 1. On Trial 2, neither the vanoxerine, nisoxetine nor cocaine-preexposed groups differed in their consumption from the saline-preexposed group. The fluoxetine-preexposed subjects drank significantly more saccharin than saline- and vanoxerine-preexposed subjects (p<.01, for all comparisons) on this trial. On Trial 3, there were no significant differences among subjects preexposed to vanoxerine, cocaine and saline. Fluoxetine-preexposed subjects continued to differ from controls (p<.05). On this trial, the nisoxetine-preexposed group (p<.05) consumed significantly more saccharin than saline-preexposed subjects. On Trial 4, subjects preexposed to vanoxerine and nisoxetine did not differ significantly from the saline-preexposed group. On this trial, subjects preexposed to cocaine and fluoxetine drank significantly more than saline-preexposed subjects (p<.05, for all comparisons).
Figure 6.
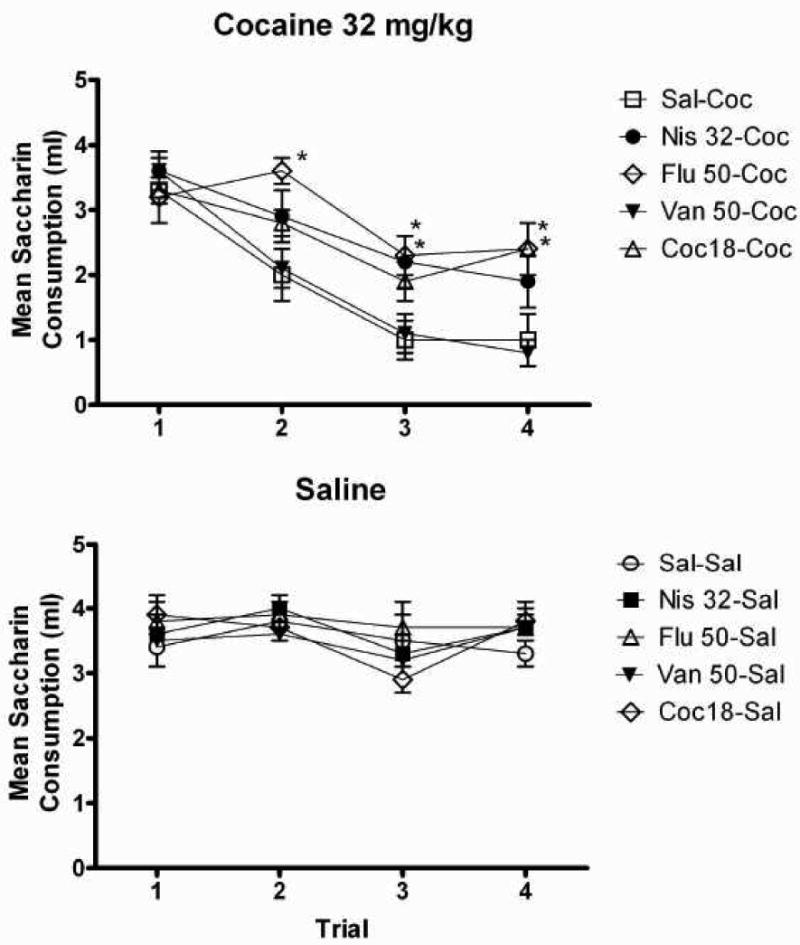
Mean (+/- SEM) absolute saccharin consumption for subjects conditioned with 32 mg/kg of cocaine (Upper panel) or saline (Lower panel) following preexposure to various doses of either saline, cocaine, fluoxetine, nisoxetine or vanoxerine. *Denotes significant difference from the saline-preexposed group
The lower panel of figure 6 illustrates mean absolute saccharin consumption for subjects injected with saline during conditioning. The 5 × 4 Repeated-Measures ANOVA revealed a significant main effect of Trial [F (3, 26) = 5.89, p<.01] where consumption decreased over sessions. There was no significant effect of Preexposure Drug [F (4, 34) = .525, p=.63] and no significant Trial × Preexposure Drug interaction [F (12, 93) = .678, p=.72].
5. Discussion
These studies assessed the monoaminergic mediation of cocaine's aversive effects. Specifically, Experiment 1 compared the strength of the aversive effects of three relatively selective monoamine transport inhibitors (nisoxetine, vanoxerine and fluoxetine). Experiment 2 used the preexposure procedure to identify which of the three selective monoamine inhibitors most closely matched the aversive stimulus properties of cocaine through its ability to attenuate the acquisition of cocaine-induced taste aversions.
From the comparison of aversions induced by the various monoamine reuptake inhibitors (Experiment 1), it appears that individual blockage of the DA or 5-HT transporters has minimal aversive effects. Specifically, vanoxerine and fluoxetine induced taste aversions only at the highest dose of each drug and only on one trial. Lower doses of each compound did not differ from saline on any of the four conditioning trials. The fact that vanoxerine was a weak aversive agent is not surprising in light of research demonstrating that DAT inhibition is the property of cocaine most likely responsible for its positive hedonic value (Roberts et al., 1999), although drugs can have both rewarding and aversive effects (Ettenberg and Geist, 1991; Parker, 1995). These findings also agree with previous research with rats within this preparation demonstrating that this compound lacks strong aversive stimulus effects (Freeman et al., 2005; Howell and Bryd, 1991; Roberts, 1993). Consistent with its relative weak ability to induce aversions in Experiment1, preexposure to vanoxerine in Experiment 2 had no influence on the development of cocaine-induced CTA, i.e., animals preexposed to vanoxerine and conditioned with cocaine displayed aversions comparable to those displayed in conditioned animals preexposed to saline. Although DAT inhibitors can often substitute for cocaine in behavioral assessments of reward, these findings suggest that the affective experience of enhanced DA transmission is not the molecular mechanism responsible for cocaine-induced taste aversions (Koob, 1992; Rothman and Glowa, 1995).
It should be noted, however, that despite similarities in the effects of cocaine and vanoxerine on DA reuptake, pharmacological differences between the two compounds complicate any conclusions about their relative strength as aversive agents (Grill et al. 1988; Refahi-Lyamani et al., 1995; Tella, et al., 1995; Wilson et al., 1994). For example, vanoxerine has a larger molecular weight than cocaine (523.49 vs. 339.82), meaning that equivalent doses of these two drugs result in more cocaine molecules in the system in comparison to vanoxerine (Bauhmann et al., 2002). Binding assays and audioradiological methods have also shown that vanoxerine differs from cocaine in its absorption and metabolism, as well as in its affinity for and disassociation from DAT. Vanoxerine also has a much slower onset of action and metabolism, making it a much longer lasting drug than cocaine (Grill et al. 1988; Refahi-Lyamani et al., 1995; Tella, et al., 1996; Wilson et al., 1994). This complication allows for the possibility that the differences in the observed ability of these two drugs to induce taste aversions (and to display generalization in the preexposure preparation) may be due to differences in drug pharmacokinetic or pharmacodynamic factors.
Differences in the physiochemical factors that influence drug effects become less of a concern when interpreting the results of nisoxetine and fluoxetine. Not only do these compounds have a molecular weight similar to that of cocaine, their general pharmacodynamics and pharmacokinetics closely parallels that of cocaine (de Ponti, 2004; Zhou, 2004). Like vanoxerine, only the highest dose of fluoxetine produced a statistically significant aversion, and even here only on a single trial. This observation is consistent with the relatively weak aversions induced by other SERT inhibitors (e.g., clomipramine and fluvoxamine), although other studies using fluoxetine have produced aversions comparable in strength to that of the emetic LiCl (Freeman et al., 2005; Gommans et al., 1998; Oliver et al., 1999; Prendergast, et al., 1996). Despite being a relatively weak aversive conditioning agent (Experiment 1), preexposure to fluoxetine resulted in an attenuation of a subsequent cocaine-induced CTA greater than that produced when cocaine itself was used as the preexposure drug (Experiment 2). This suggests that SERT inhibition closely resembles the neurochemical mechanism underlying cocaine's aversive properties. In some areas of the brain, serotonin transmission has inhibitory control over dopaminergic transmission; therefore, SERT inhibition may be aversive due to its antagonism of the rewarding effects of enhanced dopamine transmission (Rocha et al., 2005; Rothman et al., 2005). Although fluoxetine's aversive effects are not sufficiently strong or salient to act as a significant aversion-inducing agent (Experiment 1), there appears to be little discrimination between them and those of cocaine. Although the present studies do not allow a resolution for the fact that fluoxetine failed to induce strong aversions yet attenuated the subsequent acquisition of cocaine-induced aversions, it is interesting to note that such effects have been reported with morphine. For example, preexposure to morphine at doses that fail to induce aversions can attenuate the acquisition of aversions induced by higher doses of morphine (Martin et al., 1988). Also, Hunt and colleagues (1985) reported that a low dose of morphine that alone could not condition an aversion maintained a previously established morphine-induced aversion. That is, once an aversion was induced by an intermediate dose of morphine, this aversion was maintained when the taste was repeatedly paired with the dose of morphine previously reported to be ineffective in the aversion design. It is clear that the ability of a compound to induce aversions is not always correlated with other behavioral actions in the aversion design, e.g., preexposure effect or maintenance. Again, the basis for these dissociations and their relevance to underlying mediation of aversive effects remains to be determined.
Although the role of 5-HT remains ambiguous, the results of both studies suggest that noradrenergic activity is the primary mediator of cocaine's aversive effects (Hall et al., 2002). In Experiment 1, the NET inhibitor nisoxetine produced the most potent taste aversion of all three selective monoamine transporter inhibitors. Both the 32 and 50 mg/kg doses of nisoxetine produced significant suppression in saccharin consumption, and they did so on multiple trials. Although the aversive potency of nisoxetine failed to match that of cocaine (given that a higher dose was required in order to achieve comparable avoidance of saccharin), the preexposure effect produced by the two drugs was statistically indistinguishable (see Experiment 2). These findings are consistent with other work suggesting that NET inhibition produces significant negative affect, although the nature of this is not known. Norepinephrine-induced sympathetic nervous system activation and anxiogenesis may be the basis of the aversive effects of NET inhibition. Human cocaine users often report significant anxiety and jitteriness during use (Yang et al., 1992). HPA axis hyperactivity has been related to noradrenergic system disruptions possibly as the result of significant noradrenergic innervation of the hypothalamus. Accordingly, NE has modulatory control over a number of hypothalamic releasing hormones including, corticotrophin-releasing factor (Everitt and Hokfelt, 1990; Mokrani et al., 1997), although the exact behavioral and affective consequences of corticotripin-releasing factor (CRF) remain unclear. Interestingly, exogenous CRF is capable of eliciting both taste preference and aversions in rats (Heinriches et al., 1991).
Although there is considerable internal agreement across these assessments, it should be noted that recent work by Freeman et al. (2008) investigating the role of NE in cocaine-induced taste aversions fails to support the abovementioned conclusion. Specifically, Freeman et al. reported that rats injected with selective α1 and β norepinephrine receptor antagonists immediately prior to cocaine-induced taste aversion training displayed stronger cocaine-induced taste aversions, i.e., both antagonists potentiated the cocaine-induced CTA. That NE antagonists potentiated cocaine-induced aversions is clearly at odds with suggestions that such aversions are mediated by NE activity. It is difficult to reconcile the apparent inconsistencies in the various assessments of the nature of cocaine's aversive effects. Such differences are not simply due to differences between rats and mice in that the results from the present experiments with mice replicate the work with rats assessing parallel dose-response functions in aversions induced by the monoamine reuptake inhibitors (Freeman et al., 2007) and the effects of US preexposure (Serafine and Riley, 2009). The differences may be a function instead of the specific assays used in the various assessments. Both the dose-response assessments (comparing parallel dose-response functions) and the assessments of the effects of drug preexposure are indirect assays of drug mechanism. Consequently, conclusions regarding mechanisms of drug action based on these assays are dependent on specific assumptions, i.e., that parallel dose-response functions and the attenuating effects of drug preexposure are dependent on common mechanisms of action. Although such assays have each been used in this way, it is important to note that parallel dose-response functions could be produced by drugs working via very different mechanisms (compare LiCl with amphetamine; see Parker, 1993). Further, even if exposure to one drug attenuates aversions induced by another, this attenuation could be a function of systems (e.g., stress, sickness, novelty) other than the immediate and direct effect of the drug (increases in NE levels). The fact that the attenuating effects of drug preexposure have been observed between drugs with few, or no, obvious overlapping pharmacological targets (Kunin et al., 1999; Kunin et al., 2001; Ford and Riley, 1984). It should be noted that such nonspecific systems could mediate the attenuating effects of drug preexposure.
On the other hand, the administration of specific antagonists are thought to more directly assay the involvement of specific neurotransmitters and more directly implicate that neurotransmitter should behavioral effects be altered. The fact that in the Freeman et al. report, both prazosin and propranolol potentiated cocaine-induced aversions would appear to be stronger evidence against NE mediation of such aversions. Although more direct, such assessments also have their interpretational limitations in that while antagonists may immediately block neurotransmitter function, their administration (even short term) can sensitize the system, resulting in greater activity (Holtzman, 1986). In the case of Freeman et al. (2008), sensitization of noradrenergic receptors resulting from the pretreatment procedure could result in stronger aversive noradrenergic activation during subsequent cocaine conditioning. Noradrenergic neuron sensitization has been observed in response to repeated treatment with cocaine and amphetamine (Lanteri et al., 2008), and research in our own laboratory has observed the possible behavioral consequences of noradrenergic sensitization. In the Serafine and Riley (2009) study previously mentioned, exposure to cocaine prior to aversion conditioning with a NET inhibitor (desipramine), potentiated desipramine-induced aversions. This observation is also best explained by compensatory changes in noradrenergic functioning.
Independent of the specific biochemical mechanism(s) mediating the effects of cocaine and the monoamine reuptake inhibitors in the CTA procedure, it is important to note that the present work assumes that the suppression of consumption induced by these compounds reflects something about their aversive effects. It should be, however, noted that others have argued that such avoidance could be a result of a number of other effects of drugs, effects that do not assume aversiveness or toxicity. For example, Hunt and Amit (1987) argued that the avoidance of tastes that had been paired with psychoactive drugs was a function of the novelty of the drug state, i.e., drug shyness (see also Parker, 1993; 1995). The fact that exposure to a drug prior to its pairing with a taste attenuated subsequent conditioning (and avoidance) supported the role of novelty in such effects. Although supportive, with repeated conditioning trials taste aversions can be conditioned following such preexposure, suggesting that drug novelty alone cannot for the suppression seen in this preparation (for a review, see Riley and Simpson, 2001). A more recent position has argued that the avoidance of drug-paired tastes is actually a function of the drug's rewarding effects (see Grigson, 1996). According to this position, the reward comparison hypothesis, the avoidance of solutions associated with drugs such as cocaine are a function of the taste of the solution paling in comparison to the subsequently administered cocaine. As a result of this comparison, the taste itself is avoided (see also Grigson and Freet, 2001; Grigson et al., 2000a,b; though see Broadbent et al., 2002; Huang and Hsiao, 2008). Although the present study did not specifically address these other positions, it is important to note that alternative accounts of aversions induced by drugs of abuse do not require any assumptions about underlying aversive effects.
Clearly, additional work must be performed in order to characterize the neurochemical mediation of cocaine-induced aversions. Yet despite the challenges this type of research presents, there is considerable potential for studies attempting to discover the specific biological mechanisms underlying the different aspects of a drug's affective experience. Given that the use and abuse of a drug is thought to be a function of the balance of its rewarding and aversive effects (for reviews, see Cunningham et al., 2009; Riley et al., 2009), an understanding of both of these affective properties (their mediation and how they may be affected by a host of factors) may provide insight into its abuse potential.
Acknowledgments
This research was supported, in part, by a grant from the Mellon Foundations to ALR and the Intramural Research Program of the National Institute on Drug Abuse, NIH/DHHS (FSH, GRU). Requests for reprints should be sent to Jermaine Jones, New York State Psychiatric Institute/Columbia University, 1051 Riverside Drive, New York, NY 10032.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony JC, Tien AY, Petronis KR. Epidemiological evidence on cocaine use and panic attack. Am J Epidemiol. 1989;129:543–9. doi: 10.1093/oxfordjournals.aje.a115166. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Phillips JM, Ayestas MA, Ali SF, Rice KC, Rothman RB. Preclinical evaluation of GBR12909 decanoate as a long-acting medication for methamphetamine dependence. Ann N Y Acad Sci. 2002;965:92–108. doi: 10.1111/j.1749-6632.2002.tb04154.x. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Cocaine potentiates the defensive behaviors related to fear and anxiety. Neurosci Biobehav Rev. 1999;23:981–91. doi: 10.1016/s0149-7634(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Braveman NS. What studies on preexposure to pharmacological agents tells us about the nature of aversion-inducing treatment. In: Baker LM, Best MR, Domjan M, editors. Learning Mechanisms in Food Selection. Waco, Texas: Baylor University Press; 1977. pp. 511–30. [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–48. [PubMed] [Google Scholar]
- Cailhol S, Mormède P. Conditioned taste aversion and alcohol drinking: Strain and gender differences. J Stud Alcohol. 2002;63:91–9. [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE. Conditioned aversion by psychoactive drugs: Does it have significance for an understanding of drug dependence. Addiction Behav. 1975;1:55–64. doi: 10.1016/s0306-4603(75)80018-9. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Pawlush N, Kuhar MJ, Pollard GT, Howard JL. Synthesis, monoamine transporter binding properties, and behavioral pharmacology of a series of 3beta-(substituted phenyl)-2beta-(3′-substituted isoxazol-5-yl)tropanes. J Med Chem. 2004;47:296–302. doi: 10.1021/jm030453p. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Genetic influences on conditioned taste aversion. In: Reilly S, Schachtman TD, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York, NY: 2009. pp. 387–421. [Google Scholar]
- Davis CM, Riley AL. The effects of cocaine preexposure on cocaine-induced taste aversion learning in Fischer and Lewis rat strains. Pharmacol Biochem Behav. 2007;87:198–202. doi: 10.1016/j.pbb.2007.04.016. [DOI] [PubMed] [Google Scholar]
- de Brugada I, Hall G, Symonds M. The US-preexposure effect in lithium-induced flavor-aversion conditioning is a consequence of blocking by injection cues. J Exp Psychol Anim Behav Process. 2004;30:58–66. doi: 10.1037/0097-7403.30.1.58. [DOI] [PubMed] [Google Scholar]
- de Ponti F. Pharmacology of serotonin: What a clinician should know. Gut. 2004;53:1520–35. doi: 10.1136/gut.2003.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–41. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Elkins RL. Bait-shyness acquisition and resistance to extinction as functions of US preexposure prior to conditioning. Physiol Psychol. 1974;2:341–3. [Google Scholar]
- Estelles J, Rodriquez-Arias M, Aquilar MA, Minarro J. Social behavioral profile of cocaine in isolated and group male mice. Drug Alcohol Depend. 2004;76:115–23. doi: 10.1016/j.drugalcdep.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology (Berl) 1991;103:455–61. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Hokfelt T. Neuroendocrine anatomy of the hypothalamus. Acta Neurochirurgica. 1990;47:1–15. doi: 10.1007/978-3-7091-9062-3_1. [DOI] [PubMed] [Google Scholar]
- Ferrari CM, O'Connor DA, Riley AL. Cocaine-induced taste aversions: Effects of route of administration. Pharmacol Biochem Behav. 1991;38:267–71. doi: 10.1016/0091-3057(91)90277-9. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Schuster CR. The effects of cocaine in a gustatory avoidance paradigm: A procedural analysis. Pharmacol Biochem Behav. 1982;16:347–52. doi: 10.1016/0091-3057(82)90170-8. [DOI] [PubMed] [Google Scholar]
- Fox MA, Stevenson GW, Rice KC, Riley AL. Naloxone, not proglumide or MK-801, alters effects of morphine preexposure on morphine-induced taste aversion. Pharmacol Biochem Beh. 2006;84:169–77. doi: 10.1016/j.pbb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Rice KC, Riley AL. Assessment of monoamine transporter inhibition in the mediation of cocaine-induced conditioned taste aversion. Pharmacol Biochem Behav. 2005;82:583–9. doi: 10.1016/j.pbb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Verendeev A, Riley AL. Noradrenergic antagonism enhances the conditioned aversive effects of cocaine. Pharmacol Biochem Behav. 2008;88:523–32. doi: 10.1016/j.pbb.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Caron MG. Monoamine transporters: From genes to behavior. Ann Rev Pharmacol Toxicol. 2003;43:261–84. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–8. [PubMed] [Google Scholar]
- Glowa JR, Jeffreys RD, Riley AL. Drug discrimination using a conditioned taste-aversion paradigm in rhesus monkeys. J Exp Anal Behav. 1991;56:303–12. doi: 10.1901/jeab.1991.56-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommas J, Bouwknecht JA, Hijzen TH, Berendsen HH, Broekkamp CL, Maes RA, Olivier B. Stimulus properties of fluvoxamine in a conditioned taste aversion procedure. Psychopharmacology (Berl) 1998;140:496–502. doi: 10.1007/s002130050794. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Dickens DW, Thorton EW. Cocaine-induced conditioned taste aversion in rats. Pharmacol Biochem Behav. 1978;8:757–61. doi: 10.1016/0091-3057(78)90279-4. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Thornton EW. Effects of drug experience on drug induced conditioned taste aversions: Studies with amphetamine and fenfluramine. Psychopharmacologia. 1975;44:77–82. doi: 10.1007/BF00421187. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: A reinterpretation. Behav Neurosci. 1997;111:129–36. [PubMed] [Google Scholar]
- Grigson PS, Freet CS. The suppressive effects of sucrose and cocaine, but not lithium chloride are greater in Lewis than in Fischer rats: evidence for the reward comparison hypothesis. Behav Neurosci. 2000;114:353–63. doi: 10.1037//0735-7044.114.2.353. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Lyuboslavsky P, Tanase D. Bilateral lesions of the gustatory thalamus disrupt morphine- but not lithium chloride-induced intake suppression in rats: evidence against the conditioned taste aversion hypothesis. Brain Res. 2000a;858:327–37. doi: 10.1016/s0006-8993(00)01939-9. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC, Carelli RM. Heroin-induced suppression of saccharin intake in water-deprived and water-replete rats. Pharmacol Biohem Behav. 2000b;66:603–8. doi: 10.1016/s0091-3057(00)00253-7. [DOI] [PubMed] [Google Scholar]
- Grill M, Sanna E, Hanbauer I. Role of corticostriatal nerve projections in the regulation of binding site for dopamine uptake blockers. Society of Neuroscience Abstracts. 1988;14:929. [Google Scholar]
- Grupp LA. Effects of pimozide on the acquisition, maintenance, and extinction of an amphetamine-induced taste aversion. Psychopharmacology (Berl) 1977;53:235–42. doi: 10.1007/BF00492357. [DOI] [PubMed] [Google Scholar]
- Hall FS, Li X, Sora I, Xu F, Caron M, Lesch KP, Murphy DL, Uhl GR. Cocaine mechanisms: Enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neurosci. 2002;115:153–61. doi: 10.1016/s0306-4522(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Hall FS, Sora I, Drgonova J, Li XF, Goeb M, Uhl GR. Molecular mechanism underling the rewarding effects of cocaine. Ann N Y Acad Sci. 2004;1025:47–56. doi: 10.1196/annals.1316.006. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Britton KT, Koob GF. Both conditioned taste preference and aversion induced by corticotropin-releasing factor. Phamacol Biochem Behav. 1991;40:717–21. doi: 10.1016/0091-3057(91)90075-d. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Discriminative stimulus properties of caffeine in the rat: Noradrenergic mediation. J Pharmacol Exp Ther. 1986;239:706–14. [PubMed] [Google Scholar]
- Howell LL, Bryd LD. Characterization of the effects of cocaine and GBR 12909, a dopamine uptake inhibitor, on behavior in squirrel monkeys. J Pharmacol Exp Ther. 1991;258:178–85. [PubMed] [Google Scholar]
- Huang AC, Hsiao S. Re-examination of amphetamine-induced conditioned suppression of tastant intake in rats: the task-dependent drug effects hypothesis. Behav Neurosci. 2008;122:1207–16. doi: 10.1037/a0013511. [DOI] [PubMed] [Google Scholar]
- Hunt T, Amit Z. Conditioned taste aversion induced by self-administered drugs: Paradox revisited. Neurosci Biobehav Rev. 1987;11:107–30. doi: 10.1016/s0149-7634(87)80005-2. [DOI] [PubMed] [Google Scholar]
- Hunt WA, Spivak K, Amit Z. Aversive stimulus properties of morphine: Evaluation using the drug preexposure conditioned taste aversion paradigm. Behav Neural Biol. 1985;44:60–73. doi: 10.1016/s0163-1047(85)91181-1. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET, Williamson EC. Nicotine-induced taste aversion: Characterization and perexposure effects in rats. Pharmacol Biochem Behav. 1984;21:527–32. doi: 10.1016/s0091-3057(84)80034-9. [DOI] [PubMed] [Google Scholar]
- June HL, June PL, Domanque KR, Hicks LH, Lummis GH, Lewis M. Failure of Ro15-4513 to alter ethanol-induced taste aversion. Pharmacol Biochem Behav. 1992;41:455–60. doi: 10.1016/0091-3057(92)90126-z. [DOI] [PubMed] [Google Scholar]
- Jones JD, Busse GD, Riley AL. Strain-dependent sex differences in the effects of alcohol on cocaine-induced taste aversions. Pharmacol Biochem Behav. 2006;83:554–60. doi: 10.1016/j.pbb.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–91. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Kunin D, Smith BR, Amit Z. Cocaine and ethanol interaction in the conditioned taste aversion paradigm. Physiol Behav. 1991;67:627–30. doi: 10.1016/s0031-9384(99)00105-5. [DOI] [PubMed] [Google Scholar]
- Kunin D, Bloch RT, Smith BR, Amit Z. Caffeine, nicotine and mecamylamine share stimulus properties in the preexposure conditioned taste aversion procedure. Psychopharmacology (Berl) 2001;159:70–6. doi: 10.1007/s002130100888. [DOI] [PubMed] [Google Scholar]
- Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP. Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a non-dopaminergic mechanism. Neuropsychopharmacology. 2008;33:1724–34. doi: 10.1038/sj.npp.1301548. [DOI] [PubMed] [Google Scholar]
- LeBlanc AE, Cappell H. Attenuation of punishing effects of morphine and amphetamine by chronic prior pretreatment. J Comp Physiol Psychol. 1974;87:691–8. doi: 10.1037/h0036978. [DOI] [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, Rice KC, Howell LL. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: Relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther. 2004;309:959–66. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacology. 2001;9:131–43. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Martin GM, Bechara A, van der Kooy D. Morphine preexposure attenuates the aversive properties of opiates without preexposure to the aversive properties. Pharmacol Biochem Behav. 1988;30:687–92. doi: 10.1016/0091-3057(88)90085-8. [DOI] [PubMed] [Google Scholar]
- Mokrani MC, Duval F, Crocq MA, Bailey P, Macher JP. HPA axis dysfunction in depression: Correlation with monoamine system abnormalities. Psychoneuroendocrinology. 1997;22 1:S63–8. doi: 10.1016/s0306-4530(97)00012-7. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the case and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- National Research Council. Guide for the case and use of mammals in behavioral and neuroscience research. Washington, DC: National Academy Press; 2003. [Google Scholar]
- Olivier B, Gommans J, van der Gugten J, Bouwknecht JA, Herremans AH, Patty T, Hijzen TH. Stimulus properties of the selective 5-HT reuptake inhibitor fluvoxamine in conditioned taste aversion procedures. Pharmacol Biochem Behav. 1999;64:213–20. doi: 10.1016/s0091-3057(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste reactivity responses elicited by cocaine-, phencyclidine-, and methamphetamine-paired sucrose solutions. Behav Neurosci. 1993;107:118–29. doi: 10.1037//0735-7044.107.1.118. [DOI] [PubMed] [Google Scholar]
- Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Rev. 1995;19:143–57. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Cappell H. An associative analysis of the pretreatment effects in gustatory conditioning by amphetamine. Psychopharmacology. 1979;64:201–07. doi: 10.1007/BF00496063. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Hendricks SE, Yells DP, Balogh S. Conditioned taste aversions induced by fluoxetine. Physiol Behav. 1996;60:311–5. doi: 10.1016/0031-9384(95)02234-1. [DOI] [PubMed] [Google Scholar]
- Pruitt Dl, Bolanos CA, McDougall SA. Effects of D1 and D2 receptor antagonists on cocaine-induced place preference in preweaning rats. European J Pharmacol. 1995;283:125–31. doi: 10.1016/0014-2999(95)00309-9. [DOI] [PubMed] [Google Scholar]
- Randall-Thompson JF. Dissertation Abstracts International. 2005. Characterization of dopamine and serotonin in concurrently conditioned taste aversion and place preferences. [Google Scholar]
- Randich A, LoLordo VM. Associative and nonassociative theories of the UCS preexposure phenomenon: Implications for Pavlovian conditioning. Psychol Bull. 1979;86:523–48. [PubMed] [Google Scholar]
- Refahi-Lyman F, Saadauni S, Costentin J, Bonnet J. Interaction of two sulfydryl reagents with a cation recognition site of the neuronal dopamine carrier evidences small differences between (H) GBR 12783 and (H) cocaine binding sites. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:136–45. doi: 10.1007/BF00169327. [DOI] [PubMed] [Google Scholar]
- Riley AL, Diamond HF. The effects of cocaine preexposure on the acquisition of cocaine-induced taste aversions. Pharmacol Biochem Behav. 1998;60:739–45. doi: 10.1016/s0091-3057(98)00052-5. [DOI] [PubMed] [Google Scholar]
- Riley AL, Simpson GR. The attenuating effects of drug preexposure on taste aversion conditioning: Generality, experimental parameters, underlying mechanism and implications for drug use and abuse. In: Mower RR, Klein SB, editors. Contemporary Learning Theory. 2nd. Hillsdale, NJ: Lawrence Erlbaum Associates; 2001. pp. 505–59. [Google Scholar]
- Riley AL, Tuck DL. Conditioned taste aversions: A behavioral index of toxicity. Ann N Y Acad Sci. 1985;443:381–437. doi: 10.1111/j.1749-6632.1985.tb27079.x. [DOI] [PubMed] [Google Scholar]
- Riley AL, Davis CM, Roma PG. Strain differences in taste aversion learning: implications for animal models of drug abuse. In: Reilly S, Schachtman TD, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York, NY: 2009. pp. 226–61. [Google Scholar]
- Roberts DC. Self Administration of GBR 12909 on a fixed ratio and progressive ratio schedule in rats. Psychopharmacology. 1993;111:202–6. doi: 10.1007/BF02245524. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, Davies H. Self-administration of cocaine analogs by rats. Psychopharmacology (Berl) 1999;144:389–97. doi: 10.1007/s002130051022. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Goulding EH, O'Dell LE, Mead AN, Coufal NG, Parsons LH, Tecott LH. Enhanced locomotor, reinforcing and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. J Neurosci. 2005;2:10039–45. doi: 10.1523/JNEUROSCI.22-22-10039.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MI, Kosten TA. Cocaine-associated panic attacks in methadone-maintained patients. Am J Drug Alcohol Abuse. 1992;18:57–62. doi: 10.3109/00952999209001611. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, Roth B, Baumann MH. Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther. 2005;313:1361–9. doi: 10.1124/jpet.104.082503. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Glowa JR. A review of the effects of dopaminergic agents on humans, animals, and drug-seeking behavior, and its implications for medication development: Focus on GBR 12909. Mol Neurobiol. 1995;11:1–19. doi: 10.1007/BF02740680. [DOI] [PubMed] [Google Scholar]
- Serafine KM, Riley AL. Possible role of norepinephrine in cocaine-induced taste aversion. Pharmacol Biochem Behav. 2009;92:111–16. doi: 10.1016/j.pbb.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: Conditioned place preference can be established in dopamine and serotonin transporter knockout mice. Proc Natl Acad Sci USA. 1998;95:7699–704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: Combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA. 2001;98:5003–5. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, D'Mello GD. Role of training conditions in discrimination of central nervous system stimulants by rats. Psychopharmacology (Berl) 1981;73:295–303. doi: 10.1007/BF00422421. [DOI] [PubMed] [Google Scholar]
- Taylor D, Ho BT. Comparison of inhibition of monoamine uptake by cocaine, methylphenidate and amphetamine. Res Commun Chem Pathol Pharmacol. 1978;21:67–75. [PubMed] [Google Scholar]
- Tella SR. Effects of monoamine reuptake inhibitors on cocaine self-administration in rats. Pharmacol Biochem Behav. 1995;5:687–92. doi: 10.1016/0091-3057(94)00438-o. [DOI] [PubMed] [Google Scholar]
- Wellman Cl, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin knockout transporter knock-out mice. J Neurosci. 2007;27:684–91. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Norbrega JN, Carrol ME, Niznik HB, Shannak K, Lac ST, Pristupa ZB, Dixon LM, Kish SJ. Heterogenous subregional binding patterns of H-WIN 35,328 and H-GBR 12,935 are differentially regulated by chronic cocaine self-administration. J Neurosci. 1994;14:2966–79. doi: 10.1523/JNEUROSCI.14-05-02966.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL. Evaluation of the role of norepinephrine in the reinforcing effects of psychomotor stimulants in rhesus monkeys. Pharmacol Biochem Behav. 1987;26:835–9. doi: 10.1016/0091-3057(87)90618-6. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Kleven MS. Multiple dopamine receptors and the behavioral effects of cocaine. NIDA Research Monograph. 1988;88:160–84. [PubMed] [Google Scholar]
- Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine administration in mice lacking the dopamine D1 receptor. Brain Res. 2000;852:198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Fukushima S, Shen HW, Hall FS, Uhl GR, Numachi Y, Kobayashi H, Sora I. Norepinephrine transporter blockade can normalize the prepulse inhibition deficits found in dopamine transporter knockout mice. Neuropsychopharmacology. 2006;31:2132–9. doi: 10.1038/sj.npp.1301009. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman Al, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: Neurochemical and behavioral studies of. Pharmacol Biochem Behav. 1992;41:643–50. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]
- Zhou J. Norepinephrine transporter inhibitors and their therapeutic potential. Drugs Future. 2004;29:1235–44. doi: 10.1358/dof.2004.029.12.855246. [DOI] [PMC free article] [PubMed] [Google Scholar]


