Abstract
A solution additive has been discovered that can be used to measure the number of basic sites in a peptide or protein using electrospray ionization (ESI) mass spectrometry. Addition of millimolar amounts of perchloric acid (HClO4) to aqueous solutions that contain peptides or proteins results in the noncovalent adduction of HClO4 molecules to the multiply charged ions formed by ESI. For 18 oligopeptides and proteins, ranging in molecular weight from 0.5 to 18.3 kDa, the sum of the number of protons plus maximum number of HClO4 molecules adducted to the lower charge state ions is equal to the number of basic sites in the molecule. This method provides a rapid means of obtaining information about the composition of a peptide or protein and does not require high-resolution measurements, or any instrumental or experimental modifications.
Introduction
Accurate molecular weights of a wide range of molecules, even those present in complex mixtures1-9 or at trace levels,3,4 can be obtained using mass spectrometry. Information about elemental composition can be determined from isotope ratios or from exact mass measurements.1-13 Marshall and coworkers were able to assign unique elemental compositions for up to 20,000 compounds in petroleum samples with a mass measuring accuracy of ~400 ppb.2 Because the possible elemental compositions at a given nominal mass increases rapidly with molecular size, obtaining the elemental composition of larger molecules with just accurate mass measurements alone becomes more challenging.
Because biopolymers are comprised of a limited set of molecular sub-units, the number of possible elemental compositions in a given m/z range are constrained. More than 99% of peptides with a nominal mass of 1,000 Da can be excluded with a mass measuring accuracy of ± 1 ppm.12 However, even with an unequivocal elemental composition of a peptide, an unambiguous amino acid composition cannot always be obtained because different combinations of amino acids can have the same elemental composition. The mass limit for unambiguously determining the amino acid composition of a peptide by accurate mass measurements alone is ~500-600 Da.12 For larger peptides and proteins for which the amino acid composition cannot be determined directly from mass alone, additional information that further restricts the possible compositions is needed.7-9 The amino acid composition of peptides formed by proteolysis of intact proteins is limited both by the enzyme used and by the organism. The number of peptides formed by proteolysis that can be used to uniquely identify a protein increases with mass measuring accuracy and protein size assuming that the peptides are not post-translationally modified.9 The percentage of unique tryptic peptides at ~2,000 Da formed by proteolysis of C. elegans proteins that can be identified increases from 5% to 60% at mass measuring accuracies of 10 and 0.1 ppm, respectively.9
Information about the amino acid composition of a peptide can also be obtained by modifying specific residues.4-6,14,15 Many amino acids can be chemically modified selectively, such as conversion of lysine to homo-arginine14 and cysteine thiol to thialamine.15 Residue specific chemical modifications can provide constraints on the possible amino acid composition.4-6 Cysteine modification using an alkylating reagent that contains chlorine has been used to determine the number of cysteine residues in a peptide based on the distinctive isotopic distributions of ions containing chlorine.4 Knowledge of the number of cysteine residues constrains the possible peptide composition, which increases the effectiveness of the accurate mass measurement approach.4 For C. elegans, this additional constraint increases the number of unique tryptic peptides at ~2,000 Da that can be identified from 60% to 80% at a 0.1 ppm mass measuring accuracy.9
Noncovalent adduction has also been used to obtain information about the composition or surface accessibility of specific residues.16-26 McLuckey and coworkers found that adduction of acidic molecules, such as HI and HBr, to various peptides and proteins can occur via gas-phase ion/molecule reactions when the gas phase acidity (ΔHacid) of the acid is less than or equal to ~330 kcal•mol−1.16,17 Peptide and protein ions were reacted in an ion trap with HI until changes in the abundance of adducts were imperceptible, in some instances for up to 3 s,16,17 and the sum of the ion charge state and the maximum number of adducted HI molecules was found to equal the number of basic sites on 20 the 21 oligopeptides.16 Adduction of acidic molecules to peptide and protein ions also occurs in matrix-assisted laser desorption ionization (MALDI)-MS.23-26 For small peptides, the total number of protons and maximum number of adducted acid molecules of bis(trifluoromethylsulfonyl)imide was equal to the number of basic sites, but lower adduction was observed for proteins.26 Different sulfonic acid molecules, such as napthalene-disulfonic acid (NDS) and Cibacron Blue F3G-A (CCB), can bind specifically to either arginine and the N-terminus, exclusively, or all basic sites, respectively.23-25 The maximum number of adducts was equal to the number of arginine residues plus the N-terminus for NDS and all basic sites for CCB for small peptides and ubiquitin.23-25
Solution additives in ESI have been used to reduce effects of salts,27,28 increase or decrease charge states,29-31 and to obtain information about protein composition and structure.18-22,32 Addition of 18-crown-6-ether to solutions containing peptides and proteins results in preferential adduction to lysine although some binding to histidine, arginine, and the N-terminus also occurs.20-22 Information about the number of lysine residues can be obtained for small peptides, but not all lysine residues are adducted with larger proteins, a result that has been attributed to solvent inaccessible residues.20-22 Dibenzo-30-crown-10 ether (DB30C10) also forms complexes with peptides containing arginine, but adduction of two DB30C10 molecules was not observed for a peptide with two arginine residues.19
Here, we demonstrate the number of basic sites on a peptide or protein can be accurately determined directly from an ESI mass spectrum by adding 10 mM of perchloric acid (HClO4) to a solution containing these molecules. For the 18 peptides and proteins studied here with molecular weights between 0.5 and 18.3 kDa, the sum of the ion charge state and the number of HClO4 molecules attached to lower charge states is equal to the number of basic residues and the N-terminus when unmodified.
Experimental
All peptides, proteins, and perchloric acid (70% v:v in water, 11.6 M) were obtained from Sigma Aldrich Chemical Co. (St. Louis, MO). Proteins with a molecular weight (MW) greater than 10 kDa were dialyzed against 18.2 MΩ water using Slide-A-Lyzer (Thermo Scientific) cartridges with a MW cutoff of 10 kDa. Final solutions for electrospray were prepared at a concentration of 10 μM peptide or protein and 10 mM HClO4 in water. ESI mass spectra were acquired using a LTQ-orbitrap hybrid mass spectrometer (Thermo Electron, Bremen, Germany) equipped with a liquid chromatography autosampler. 15.0 μL of sample was injected for each analysis. The sample plug was introduced into the mass spectrometer for ~30 s using 18.2 MΩ water at a flow rate of 50 μL•min−1. Mass spectra were acquired continuously, and two minutes of data were averaged for each spectrum.
Additional experiments, including collision activation, were performed using a Waters-Micromass Q-TOF Premier (Waters Corporation, Manchester, UK) equipped with a nanoESI source. A solution containing 10 μM ubiquitin and 10 mM HClO4 in 18.2 MΩ water was introduced to the mass spectrometer through a pulled borosilicate nanoESI emitter with 1.2 kV applied via a platinum wire. The source was maintained at a temperature of 120 °C with a cone gas velocity of 170 L•hr−1. The sample and extraction cones were operated at voltages of 40 and 0 V, respectively. The collision cell gas velocity was 0.6 mL•min−1, and both the cell entrance and exit were operated at −5 V.
Results and Discussion
Addition of 10 mM HClO4 to aqueous solutions containing either a peptide or protein results in various extents of adduction of HClO4 to the protonated molecules. For example, an ESI mass spectrum of an aqueous solution containing 10 mM HClO4 and 10 μM ubiquitin, which shows various extents of adduction of HClO4 molecules to the different charge states of the protonated protein, is shown in Figure 1a. More HClO4 adduction occurs for lower charge state ions, as has been reported previously for adducts of other ions and molecules.27,33 The 4+ charge state is the lowest one observed for ubiqutin under these conditions, and up to nine HClO4 molecules are adducted, resulting in (ubiquitin + 4H + 9HClO4)4+. The number of protons (4) plus maximum number of HClO4 adducts (9) for the 4+ charge state is 13, which is equal to the number of basic sites on the protein (Arg, His, Lys, and the N-terminus). Similarly, the number of protons (5) and maximum number of HClO4 adducts (8) observed for the 5+ charge state is 13. Interestingly, the abundances of (ubiquitin + 4H + 9HClO4)4+ and (ubiquitin + 5H + 8HClO4)5+ are significantly greater, by a factor of approximately 10 and 2, respectively, than other ions in the same charge state with different numbers of attached HClO4 molecules. No further adduction of HClO4 to (ubiquitin + 4H)4+ and (ubiquitin + 5H)5+ is observed beyond the ninth and eighth molecule, respectively, although additional adduction of NaClO4 does occur. Non-specific adduction of salts and salt clusters to protein ions has been observed previously with ESI-MS.27,34 That no more adducts of HClO4 to the protein ion are observed suggests that there are no more favorable sites to which HClO4 can bind. In contrast, the maximum number of protons and adducted HClO4 molecules for the 6+ to 12+ charge states of ubiquitin is lower than the total number of basic sites. For these ions, the maximum number of protons plus HClO4 adducts is 12. A 13+ charge state is formed in low abundance but no attachment of HClO4 is observed.
Figure 1.
ESI mass spectra of solutions containing 10 mM HClO4 and 10 μM (a) ubiquitin or (b) bovine cytochrome c. Insets show various extents of adduction of HClO4 molecules to lower charge state ions.
Similar results were obtained for bovine cytochrome c. An ESI mass spectrum of an aqueous solution containing 10 μM bovine cytochrome c and 10 mM HClO4 is shown in Figure 1b. The maximum number of HClO4 adducts observed for the 6+, 7+, and 8+ charge states are 17, 16, and 15, respectively, corresponding to the number of protons plus maximum number of attached HClO4 molecules of 23 for each of these ions. This value is equal to the combined total of 23 arginine, histidine, and lysine residues. The abundance of (cytochrome c + 6H + 17HClO4)6+ is ~5× greater than all the other adducts for this charge state, indicating that there are 17 favorable binding sites that are predominantly occupied. Similar results were obtained for equine cytochrome c, which has one additional basic residue. The maximum number of protons plus HClO4 adducts for the lowest charge states (6+ and 7+) is 24, which is equal to the number of basic residues in this protein. Both bovine and equine cytochrome c are acetylated at the N-terminus, which apparently makes this site unfavorable for HClO4 adduction. Acetylation of the N-terminus lowers the gas-phase basicity of this site which is the likely origin of the significantly lower affinity of this site for HClO4 attachment. As was the case for ubiquitin, the number of protons plus the maximum number of HClO4 adducts is lower than the number basic sites for the higher (10+ to 18+) charge states.
For all the peptides and proteins investigated, the maximum number of observed HClO4 adducts to the higher charge state ions were between 1 to 3 molecules lower than the number of basic sites, but this depends on instrument conditions (vide infra). Even with the relatively “harsh” source conditions used here, the sum of the number of protons and maximum number of HClO4 adducts for the lowest charge states of the 18 peptides and proteins investigated is equal to the number of basic sites (Figure 2; slope and correlation coefficient equals 1.00).
Figure 2.
The sum of the number of protons and maximum number of HClO4 molecules adducted to the lowest charge states formed by ESI from solutions containing 10 mM HClO4 as a function of the number of basic sites (Arg, Lys, His, and unmodified N-terminus) for 18 peptides and proteins. These data are fit to a straight line (slope and correlation coefficient is equal to 1).
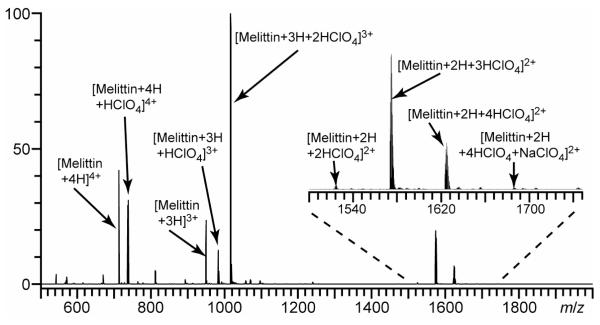
For each peptide or protein except melittin, the abundance of the most extensively adducted ion was significantly greater, by at least a factor of 2, than other ions with the same charge state but with lower numbers of HClO4 molecules attached. The high abundance indicates that all the available binding sites in these molecules have high affinity for HClO4, and makes it possible to unambiguously identify the number of binding sites. In contrast, the abundance of the most highly adducted ion in MALDI experiments with NDS23 and CCB25 was lower than other ions for the same charge state, suggesting a lower affinity for these molecules to basic sites. For melittin, the lowest charge state is 2+, and a maximum of four HClO4 molecules are observed to adduct to form (melittin + 2H + 4HClO4)2+ (Figure 3). Melittin has six basic sites including an unmodified N-terminus. Thus, the maximum number of protons plus adducts of HClO4 for this ion is equal to the number of basic sites. But unlike the other peptides and proteins investigated, the relative intensity of (melittin + 2H + 4HClO4)2+ is ~3× less than that for (melittin + 2H + 3HClO4)2+. Four of the six basic residues in melittin are adjacent to one another, which may result in steric hindrance and a lowering of the binding affinity of the fourth HClO4 molecule at these four adjacent sites.
Figure 3.
ESI mass spectrum of a solution containing 10 μM melittin and 10 mM HClO4. Inset shows adduction of HClO4 observed for the 2+ charge state.
The relative intensity of the most adducted ion decreases at lower HClO4 concentrations indicating a significant excess of HClO4 is required to provide an unambiguous determination of the number of basic sites on a protein. Under these conditions, the optimum ratio of acid:protein was ~1000:1 to produce high intensities for the most adducted ions. At lower concentrations of acid, adduction was either incomplete (100:1) or not observed (10:1 and lower), whereas cluster ions became the most intense features in the mass spectra at higher (10000:1 and greater) acid concentrations.
To determine the extent to which the abundances of the adducts depend on instrumental conditions, a mass spectrum of an aqueous solution containing 10 μM ubiquitin and 10 mM HClO4 was obtained using a nanoESI emitter (~25 nL/min flow rate) and a Q-TOF mass spectrometer (Figure 4a). A comparison of the mass spectra in Figure 1a and 4a, obtained from the same solution, but with two different instruments operated with significantly different source conditions, shows that although the charge state distributions can differ significantly, formation of the ion for which the maximum number of adducts plus the number of protons is equal to the number basic sites is remarkably robust for the low charge states. The abundances of these maximally adducted ions are much greater in the nanoESI spectrum (Figure 4a) where they are observed for all the charge states produced (4+ to 12+). The (ubiquitin + 4H + 9HClO4)4+ is the only 4+ ion observed in Figure 4a, and the (ubiquitin + 5H + 8HClO4)5+ is ~10× more intense than the (ubiquitin + 5H + 7HClO4)5+ in Figure 4a, compared to 2× in Figure 1a.
Figure 4.
(a) A nanoESI mass spectrum of 10 μM ubiquitin and 10 mM HClO4 acquired with a Q-TOF mass spectrometer, with inset showing adduction to the 11+ charge state, and (b) The normalized precursor abundance of mass-selected (ubiquitin + 11H + 2HClO4)11+ (squares), (ubiquitin + 7H + 6HClO4)7+ (triangles), and (ubiquitin + 4H + 9HClO4)4+ (circles) ions as a function of collisional activation voltage.
More highly charged ions are observed with the orbitrap for which high solution flow rates were used. The presence of HClO4 at high concentration necessitated high source temperatures under these conditions to obtain consistent ion signal. Thermal denaturation can occur at high source temperatures and could explain the more abundant high charge states. In contrast, the flow rates with nanospray are many of orders of magnitude lower, and more reproducible signals were obtained under “softer” source conditions. The higher charge states in Figure 4a are likely the result of denaturation owing to the solution pH ~2.
The significantly greater abundance of the maximally adducted ions with nanoESI and “soft” source conditions suggests that the absence of these ions for the higher charge states under “harsher” source conditions is the result of gas-phase dissociation of these adducts. The stabilities of these maximally adducted ions were investigated by measuring collisional activation spectra of mass selected (ubiquitin + 4H + 9HClO4)4+, (ubiquitin + 7H + 6HClO4)7+, and (ubiquitin + 11H + 2HClO4)11+ ions as a function of collision voltage. These ions dissociate by sequential loss of HClO4 molecules, and the abundance of these precursor ions as a function of collision voltage is shown in Figure 4b. These data show a clear trend in decreasing ion stability with increasing charge state. The collision voltage necessary to dissociate half of the precursor occurs at ~8.0, 12, and 29 V for the (ubiquitin + 11H + 2HClO4)11+, (ubiquitin + 7H + 6HClO4)7+, and (ubiquitin + 4H + 9HClO4)4+ ions, respectively. These results indicate that ion activation in any region of the mass spectrometer should be minimized in order to best preserve the adducts for all charge states.
The inverse correspondence between HClO4 adduction and protonation extent indicates that HClO4 adduction occurs at basic sites by displacing a net proton and forming an ion pair. Previous results from gas-phase ion/molecule reactions indicate that acid adduction to proteins and peptides occurs for acids with a ΔHacid of 330 kcal•mol−1 or less,17 consistent with our observation that adduction occurs for HClO4 which has a ΔHacid of 287 kcal•mol−1.35 Based on McLuckey’s findings that HI adduction to peptide and protein ions can occur as a result of gas-phase ion/molecule reactions and the maximum number of these adducts plus the ion charge state is equal to the number of basic sites,16 HI was added to solutions at a concentration of 10 mM for 10 of the 18 proteins to test whether HI was equally as effective as HClO4 as a solution-phase additive for this analysis. The resulting ESI mass spectra show lower extents of adduction of HI compared to HClO4. HI adducts may be more weakly bound to these ions than HClO4, and may be lost more readily by gas-phase dissociation.
In separate experiments, the extents of sodium and acid molecule adduction to molecular ions formed by ESI from aqueous solutions individually containing 11 different sodium salts and four different proteins was investigated. Adduction depends strongly on the gas-phase acidity of the counter ion of the salt, with acid adduction observed for acids with ΔHacid ≤ 315 kcal•mol−1. Based on these results, HSbF6 or HPF6 may also be effective for determining the number of basic sites in a peptide or protein.
Conclusions
These results demonstrate that the number of basic sites (Arg, Lys, His, and unmodified N-terminus) in peptides and proteins can be accurately determined from the number of HClO4 molecules adducted to lower charge state ions formed by ESI from solutions that contain millimolar concentrations of HClO4. This method has the advantage that it is rapid, and no covalent modifications, proteolytic digestion, gas-phase ion/molecule reactions, instrumental or other experimental modifications are required to obtain the number of basic sites in a peptide or protein. Because adduction of HClO4 results in a 100 Da increase, this method does not require high resolution measurements and can be performed on any mass spectrometer with an ESI source. Addition of HClO4 does reduce the overall ion signal but by less than a factor of two in these experiments. Any post-translational modification to a basic site that reduces basicity will also likely reduce binding affinity of HClO4 and these sites will not likely be detected by this method. However, the simplicity and accuracy of this method could make it useful for determining peptide composition from accurate mass measurements by providing an additional constraint that would reduce the mass accuracy required to uniquely determine the peptide composition. Conversely, this information could be used to extend the molecular weight range of this method to larger peptides. Even if used for trypic peptide analysis for which cleavages occur at Lys and Arg, this method provides additional information about the number of His residues or missed cleavages due to incomplete digestion or when Pro is on the C-terminal end of Lys or Arg. Because the elemental composition of the adducts are known, the m/z spacing between adducts could potentially be used as an internal mass calibrant to further increase the mass measuring accuracy in these experiments.
Determining the number of basic sites from HClO4 adduction to low charge states is remarkably robust even when vastly different source conditions or instruments are used. Collisional activation of these maximally adducted ions results in sequential loss of HClO4 molecules and the stabilities of these adducts increase with decreasing charge state. Thus, adducts will be best preserved using “soft” source conditions and nanospray. To insure maximum reliability of this method, either instrumental conditions for which the sum of protons and maximum HClO4 adducts is the same for all charge states should be found, or proteins with a known number of basic residues could be used to determine the extent to which these adducts are preserved under a given set of experimental conditions. Addition of HClO4 to solutions prior to ESI as done here does increase mass spectral complexity owing to the formation of a large number of adducts. For more complex samples, this reagent could be added in either a split flow36,37 or a dual spray38 in order to alternately acquire spectra with and without the reagent to aid spectral interpretation.
Acknowledgements
The authors acknowledge financial support from the National Science Foundation (Grant CHE-1012833) and National Institutes of Health (5F32GM093593-02 for fellowship support for SIM).
References
- 1.Hughey CA, Rodgers RP, Marshall AG. Anal. Chem. 2002;74:4145–4149. doi: 10.1021/ac020146b. [DOI] [PubMed] [Google Scholar]
- 2.McKenna AM, Purcell JM, Rodgers RP, Marshall AG. Energy Fuels. 2010;24:2929–2938. [Google Scholar]
- 3.Haskins NJ, Eckers C, Organ AJ, Dunk MF, Winger BE. Rapid Commun. Mass Spectrom. 1995;9:1027–1030. [Google Scholar]
- 4.Goodlett DR, Bruce JE, Anderson GA, Rist B, Pasa-Tolic L, Fiehn O, Smith RD, Aebersold R. Anal. Chem. 2000;72:1112–1118. doi: 10.1021/ac9913210. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez H, Niehauser S, Boltz SA, Gawandi V, Phillips RS, Amster I. J. Anal. Chem. 2006;78:3417–3423. doi: 10.1021/ac0600407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leitner A, Lindner W. J. Mass Spectrom. 2003;38:891–899. doi: 10.1002/jms.477. [DOI] [PubMed] [Google Scholar]
- 7.Smith RD, Anderson GA, Lipton MS, Pasa-Tolic L, Shen YF, Conrads TP, Veenstra TD, Udseth HR. Proteomics. 2002;2:513–523. doi: 10.1002/1615-9861(200205)2:5<513::AID-PROT513>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 8.Smith RD, Anderson GA, Lipton MS, Masselon C, Pasa-Tolic L, Shen Y, Udseth HR. OMICS. 2002;6:61–90. doi: 10.1089/15362310252780843. [DOI] [PubMed] [Google Scholar]
- 9.Conrads TP, Anderson GA, Veenstra TD, Pasa-Tolic L, Smith RD. Anal. Chem. 2000;72:3349–3354. doi: 10.1021/ac0002386. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers RP, Blumer EN, Hendrickson CL, Marshall AG. J. Am. Soc. Mass Spectrom. 2000;11:835–840. doi: 10.1016/S1044-0305(00)00158-6. [DOI] [PubMed] [Google Scholar]
- 11.He F, Hendrickson CL, Marshall AG. Anal. Chem. 2001;73:647–650. doi: 10.1021/ac000973h. [DOI] [PubMed] [Google Scholar]
- 12.Zubarev RA, Hakansson P, Sundqvist B. Anal. Chem. 1996;68:4060–4063. [Google Scholar]
- 13.Kim S, Rodgers RP, Marshall AG. Int. J. Mass Spectrom. 2006;251:260–265. [Google Scholar]
- 14.Kimmel JR. Method Enzymol. 1967;11:584–589. [Google Scholar]
- 15.Itano HA, Robinson EA. J. Biol. Chem. 1972;247:4819–4824. [PubMed] [Google Scholar]
- 16.Stephenson JL, McLuckey SA. Anal. Chem. 1997;69:281–285. doi: 10.1021/ac961119m. [DOI] [PubMed] [Google Scholar]
- 17.Stephenson JL, McLuckey SA. J. Am. Chem. Soc. 1997;119:1688–1696. [Google Scholar]
- 18.Julian RR, Beauchamp JL. J. Am. Soc. Mass Spectrom. 2004;15:616–624. doi: 10.1016/j.jasms.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Julian RR, Akin M, May JA, Stoltz BM, Beauchamp JL. Int. J. Mass Spectrom. 2002;220:87–96. [Google Scholar]
- 20.Julian RR, Beauchamp JL. Int. J. Mass Spectrom. 2001;210:613–623. [Google Scholar]
- 21.Ly T, Julian RR. J. Am. Soc. Mass Spectrom. 2006;17:1209–1215. doi: 10.1016/j.jasms.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Ly T, Julian RR. J. Am. Soc. Mass Spectrom. 2008;19:1663–1672. doi: 10.1016/j.jasms.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Friess SD, Zenobi R. J. Am. Soc. Mass Spectrom. 2001;12:810–818. doi: 10.1016/S1044-0305(01)00257-4. [DOI] [PubMed] [Google Scholar]
- 24.Friess SD, Daniel JM, Hartmann R, Zenobi R. Int. J. Mass Spectrom. 2002;219:269–281. [Google Scholar]
- 25.Salih B, Zenobi R. Anal. Chem. 1998;70:1536–1543. doi: 10.1021/ac9708506. [DOI] [PubMed] [Google Scholar]
- 26.Kruger R, Karas M. J. Am. Soc. Mass Spectrom. 2002;13:1218–1226. doi: 10.1016/S1044-0305(02)00450-6. [DOI] [PubMed] [Google Scholar]
- 27.Iavarone AT, Udekwu OA, Williams ER. Anal. Chem. 2004;76:3944–3950. doi: 10.1021/ac049724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan JX, Xu K, Yang XD, Choy WY, Konermann L. Anal. Chem. 2009;81:5008–5015. doi: 10.1021/ac900423x. [DOI] [PubMed] [Google Scholar]
- 29.Mirza UA, Chait BT. Anal. Chem. 1994;66:2898–2904. doi: 10.1021/ac00090a017. [DOI] [PubMed] [Google Scholar]
- 30.Iavarone AT, Jurchen JC, Williams ER. J. Am. Soc. Mass Spectrom. 2000;11:976–985. doi: 10.1016/S1044-0305(00)00169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iavarone AT, Williams ER. J. Am. Chem. Soc. 2003;125:2319–2327. doi: 10.1021/ja021202t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang ZQ, Smith DL. Protein Sci. 1993;2:522–531. doi: 10.1002/pro.5560020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan P, Gunawardena HP, Xia Y, McLuckey SA. Anal. Chem. 2004;76:1165–1174. doi: 10.1021/ac035209k. [DOI] [PubMed] [Google Scholar]
- 34.Juraschek R, Dulcks T, Karas M. J. Am. Soc. Mass Spectrom. 1999;10:300–308. doi: 10.1016/S1044-0305(98)00157-3. [DOI] [PubMed] [Google Scholar]
- 35.Marcus Y. J. Chem. Soc. Farad. Trans. I. 1987;83:339–349. [Google Scholar]
- 36.Konermann L, Collings BA, Douglas DJ. Biochemistry. 1997;36:5554–5559. doi: 10.1021/bi970046d. [DOI] [PubMed] [Google Scholar]
- 37.Yang HJ, Smith DL. Biochemistry. 1997;36:14992–14999. doi: 10.1021/bi9717183. [DOI] [PubMed] [Google Scholar]
- 38.Xia Y, Liang XR, McLuckey SA. J. Am. Soc. Mass Spectrom. 2005;16:1750–1756. doi: 10.1016/j.jasms.2005.07.013. [DOI] [PubMed] [Google Scholar]