Abstract
Haemophilus ducreyi is the etiologic agent of the sexually transmitted genital ulcer disease chancroid. Neither naturally occurring chancroid nor experimental infection with H. ducreyi results in protective immunity. Likewise, a single inoculation of H. ducreyi does not protect pigs against subsequent infection. Accordingly, we used the swine model of chancroid infection to examine the impact of multiple inoculations on a host's immune response. After three successive inoculations with H. ducreyi, pigs developed a modestly protective immune response evidenced by the decreased recovery of viable bacteria from lesions. All lesions biopsied 2 days after the first and second inoculations contained viable H. ducreyi cells, yet only 55% of the lesions biopsied 2 days after the third inoculation did. Nearly 90% of the lesions biopsied 7 days after the first inoculation contained viable H. ducreyi cells, but this percentage dropped to only 16% after the third inoculation. Between the first and third inoculations, the average recovery of CFU from lesions decreased approximately 100-fold. The reduced recovery of bacteria corresponded directly with a fivefold increase in H. ducreyi-specific antibody titers and the emergence of bactericidal activity. These immune sera were protective when administered to naïve pigs prior to challenge with H. ducreyi. These data suggest that pigs mount an effective humoral immune response to H. ducreyi after multiple exposures to the organism.
Chancroid is a sexually transmitted infection often seen in resource-poor tropical areas (34) and is a prevalent genital ulcer disease in certain areas of Asia, Africa, and Latin America (31, 34, 35). As both cross-sectional cohort studies and prospective longitudinal studies indicate that all genital ulcer disease (13, 23, 41, 51) and chancroid in particular (31, 33) increase the risk for sexual transmission and acquisition of human immunodeficiency virus type 1 (HIV-1), control of chancroid could provide an effective intervention strategy against the spread of human immunodeficiency virus type 1 (18, 21, 27, 34, 37, 41, 44, 52). Control of chancroid is complicated by the fact that natural chancroid infection does not appear to protect against subsequent infection (6, 25, 43). Concordantly, a single experimental infection with Haemophilus ducreyi does not protect human volunteers against subsequent experimental challenge (4, 49).
Humans mount what appears to be a delayed-type (type IV) hypersensitivity reaction in response to H. ducreyi (22, 32, 38, 49). This response is neither protective against future infection nor effective at clearing chancroid infections as lesions can persist for weeks or months and ulcer resolution is often incomplete in the absence of antibiotic therapy (34). One possible reason for the ineffective nature of this response is that cell-mediated immunity is highly effective at killing intracellular bacteria and viruses (30), yet the majority of H. ducreyi present in chancroid lesions are extracellular (5).
While the delayed-type (type IV) hypersensitivity response appears ineffective at preventing future chancroid infections, it is unclear what sort of response would be protective. The extracellular existence of the bacteria suggests that a humoral immune response could be protective against infection. We repeatedly exposed pigs to H. ducreyi in an attempt to elicit and identify a protective immune response in the swine model of chancroid. Pigs, like humans, are not protected from subsequent infection by a single exposure to H. ducreyi. However, after three inoculations at 14-day intervals, pigs developed a modest but significant level of protective immunity against H. ducreyi. Protection was defined not as an absolute block of infection but rather as a reduction in disease severity as indicated by reduced recovery of viable H. ducreyi cells. Passive transfer of immune serum protected naïve animals against challenge with H. ducreyi. These results from the swine model support the idea that humoral immunity to H. ducreyi could provide protection against infection by this organism.
MATERIALS AND METHODS
Bacterial inoculum preparation.
The inoculum was prepared and quantified as previously described (29, 45). Briefly, H. ducreyi strain 35000HP (gift from Stanley Spinola) was grown from a freezer stock and passed once on chocolate agar plates containing 1.5% (wt/vol) Bacto agar, 2.5% brain heart infusion (BHI), 1% hemoglobin, 1% IsoVitaleX (all from Becton Dickinson, Cockeysville, Md.), 5% newborn calf serum, and 5% fetal bovine serum (Life Technologies, Inc., Rockville, Md.) at 35°C in a humidified incubator with 5% CO2. Vancomycin, when used, was added to growth media at a concentration of 3 μg/ml.
Animals.
Juvenile female crossbred (Yorkshire-Landrace crossed with Hampshire-Duroc [hereafter, Yorkshire cross]) pigs were employed as previously described (29). Pigs were housed in individual enclosures at North Carolina State University College of Veterinary Medicine in a P2 containment facility accredited by the American Association for Accreditation of Laboratory Animal Care. All pigs were 6 weeks old at the beginning of the study. Animals were sedated for procedures with 0.3 ml of a TKX cocktail per 22.7 kg of body weight. The cocktail consisted of tiletamine HCl-zolazepam HCl (each, 50 mg/ml), (Telazol; Fort Dodge Laboratories, Fort Dodge, Iowa), ketamine HCl (50 mg/ml) (Fort Dodge Laboratories), and xylazine (50 mg/ml) (Miles Laboratories, Shawnee Mission, Kans.). Atropine sulfate (Phoenix Scientific Inc., Joseph, Mo.) was given in the amount of 0.5 ml to 1 ml, depending on the size of the animal, in order to slow bronchial secretion and prevent aspiration.
Inoculation.
Eight sites on the dorsal side of each ear for a total of 16 sites per pig were inoculated with Multi-Test multiple skin test applicators (Lincoln Diagnostics, Decatur, Ill.) as previously described (29, 49). Two sites per ear were inoculated with the Multi-Test applicators loaded with each of the following: 106 CFU of H. ducreyi 35000HP, 107 CFU of H. ducreyi 35000HP, 106 CFU of heat-killed H. ducreyi 35000HP, or 10 μl of sterile phosphate-buffered saline (PBS). This inoculation scheme resulted in an estimated delivered dose of 4 × 103 to 4 × 104 CFU to the dermis and epidermis of the skin (46). Inoculations were repeated at 14-day intervals (Fig. 1). Inoculations were carefully placed such that no two successive sites overlapped. In addition to the pigs that received all three inoculations, one pig received no inoculations, and two pigs received only the first inoculation.
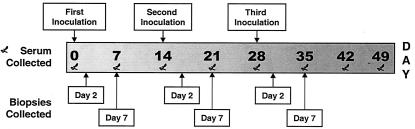
FIG. 1.
Timeline of repeat inoculation study with Yorkshire cross pigs. Inoculations occurred at 14-day intervals. The ears of the pigs were inoculated with live H. ducreyi at four sites per ear per inoculation. Lesion biopsies were collected either 2 or 7 days after each inoculation. Five pigs were inoculated three times, two pigs were inoculated once, and one pig was never inoculated. Serum was collected from all pigs on a weekly basis.
Biopsy of samples.
Inoculation sites were excised 2 or 7 days following each inoculation with 6-mm-diameter skin punches (Acuderm, Ft. Lauderdale, Fla.). Tissues were bisected with sterile scalpels (Acuderm). One half of each biopsy was fixed in 4% paraformaldehyde in PBS, while the other half was minced and plated on chocolate agar plates with 3 μg of vancomycin per ml for recovery and enumeration of viable H. ducreyi. If recovery plates were excessively contaminated, the recovery data were not included in our analyses. Fixed sample halves were embedded in paraffin, sectioned, stained with hematoxylin and eosin (Histopathology Reference Laboratory, Richmond, Calif.), and blindly scored for histological severity according to our previously developed scoring system (46) (data not shown).
Sera and complement collection.
Sera were collected weekly from all animals. One of the animals receiving a single inoculation suffered a leg injury and was euthanized prior to the completion of the study. This animal had serum collected for the final time on day 42 instead of day 49.
Collected blood was allowed to clot overnight at 4°C. Blood was centrifuged at room temperature in a bench top centrifuge at 408 × g for 10 min prior to the removal of serum. Serum was centrifuged again at room temperature in a bench top centrifuge at 408 × g for 10 min before aliquots were frozen at −80°C.
Pig complement was collected from separate naïve Yorkshire cross pigs. Blood was drawn from animals and clotted at 37°C for 30 min. Serum was removed and centrifuged at 4°C at 2,000 × g for 10 min. Serum was passed through a 0.45-μm syringe filter, aliquoted, and frozen immediately at −80°C. Aliquots were used only once as an active complement source.
Immune bactericidal assays.
H. ducreyi 35000HP was grown for 16.5 h from a freezer stock on chocolate agar at 35°C with 5% CO2. Cells were harvested and suspended in 2 ml of BHI broth (Becton Dickinson, Cockeysville, Md.). Cells were vortexed for 5 s and allowed to settle for 5 min in order to remove large aggregates of H. ducreyi. After settling, the top 1 ml of the bacterial solution was removed and the cell density was adjusted such that the final concentration of bacteria was 100 to 500 CFU per 80 μl of media.
Bactericidal assays were performed as described (15, 16). Briefly, assays were performed in sterile 96-well plates (Falcon microtest tissue culture plate; Becton Dickinson, Franklin Lakes, N.J.). Each test well received 80 μl of cells and 10 μl (or 10%) of heat-inactivated test serum. Plates were incubated for 15 min at 35°C with 5% CO2 after which 10 μl (or 10%) of either heat-inactivated or active fresh pig complement serum was added. Plates were mixed by tapping and then incubated for an additional 45 min. Bacteria were quantified by plating 60 μl from each well onto chocolate agar. Percent survival was determined for each immune serum sample tested by dividing the number of colonies that survived exposure to fresh serum complement by the number of colonies that survived with heat-inactivated serum complement and multiplying by 100. To ensure that the serum complement did not kill the bacteria in an antibody-independent fashion, we used BHI media in place of the serum antibody source in bactericidal assays. The average percent survival in the BHI medium was consistently greater than 100% (data not shown), indicating that complement containing serum, in the absence of antibody, did not kill the bacteria. Samples were assayed in triplicate on three separate days.
Serum IgG determination.
Total serum immunoglobulin G (IgG) was measured following the manufacturer's protocol with a pig serum IgG enzyme-linked immunosorbent assay (ELISA) kit (Bethyl Laboratories, Montgomery, Tex.). H. ducreyi-specific IgG was measured by a similar method; however, instead of coating microplate wells (96-well MaxiSorp; Nalge Nunc, Rochester, N.Y.) with goat anti-pig IgG capture monoclonal antibody, wells were coated with whole-cell H. ducreyi lysate at a concentration of 10 μg/ml in 100 mM sodium carbonate, pH 9.6. The lysate was made by sonicating plate-grown H. ducreyi 35000HP on ice in a mixture of 50 mM sodium phosphate (monobasic) and 300 mM sodium chloride, pH 7.8. Insoluble particles and unbroken whole cells were removed from the sonicate mixture by centrifugation at 800 × g for 5 min. The sonicate was filtered (pore size, 0.2 μm) and assayed for total protein content (DC protein assay; Bio-Rad Laboratories, Hercules, Calif.). Aside from the coating, both ELISA formats were performed identically and in tandem. Coated wells were blocked with 1% bovine serum albumin in Tris-buffered saline (50 mM Tris, 0.15 M sodium chloride, pH 8), then washed with TBST (Tris-buffered saline plus 0.05% Tween 20). Sera were serially diluted twofold in TBST containing 1% bovine serum albumin, and a calibrator serum included with the ELISA kit was run to assess the performance of each assay. Sera were incubated in the coated plates and washed with TBST before being incubated with a goat anti-pig IgG secondary antibody conjugated to horseradish peroxidase. After sera were washed again with TBST, tetramethyl benzidine detection substrate (KPL, Gaithersburg, Md.) was added to each well, allowed to develop, and stopped by the addition of 2 M H2SO4. The optical density at 450 nm (OD450) was measured with a SpectraMax 340PC microplate spectrophotometer, and response curves were calculated with SOFTmax PRO software (Molecular Devices, Sunnyvale, Calif.) This software was also used to perform four-parameter curve fitting for the data. Titers were calculated by solving the four-parameter equation at an OD450 value of 15 times the assay background. The assay background was defined as the mean OD450 of all assay blank wells (containing all reagents except serum), plus three times the standard deviation. Response curves for general visual comparison were graphed with Microsoft Excel.
Passive transfer.
Pigs were infused with 25 ml of serum with a 30-ml syringe (Becton Dickinson, Franklin Lakes, N.J.) attached to a 6-in. male luer lock adapter extension set (Baxter Healthcare Corporation, Deerfield, Ill.) and an 18-gauge needle (Becton Dickinson, Franklin Lakes, N.J.). Two pigs were infused with normal pig serum, and five pigs were infused with serum from a repeatedly inoculated pig. One day after infusion, pigs were inoculated according to the procedure described above. Each pig was inoculated with live bacteria at eight sites per ear, for a total of sixteen live inoculations, and the entire biopsy of each lesion was minced and plated for recovery. Day 7 recovery data from one pig infused with bactericidal serum were not included in the Results section due to overwhelming bacterial and fungal contamination on the recovery plates.
Statistical methods.
Statistical analysis was performed with Sigma Stat version 2.0 (Jandel Scientific, San Rafael, Calif.) and SAS software (SAS Institute Inc., Cary, North Carolina). Bacterial recovery counts were modeled with gamma regression modeling as a function of the day of the biopsy, and generalized estimation equations were used to adjust the data for random effects. The percentages of positive biopsies were analyzed via logistic regression analysis. This hierarchical model was used to accommodate the effect of collecting multiple biopsies from single animals. Data analysis with t tests was used to compare two groups for bactericidal assays, H. ducreyi-specific IgG titers, and recovery data from serum-infused animals.
RESULTS
Recovery of viable bacteria decreased with repeated exposure to H. ducreyi.
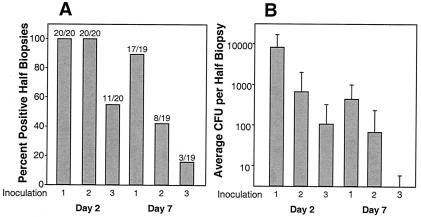
Five pigs were inoculated with H. ducreyi three times at 2-week intervals (Fig. 1). Two and seven days following each inoculation, punch biopsies of inoculation sites were collected and evaluated for lesion severity and recovery of live H. ducreyi. Biopsy results from both inoculation dilutions are presented together (Fig. 2), since analysis revealed that percentages of culture-positive lesion biopsies were identical for all time points (data not shown). All biopsies collected 2 days following the first and second inoculations contained live H. ducreyi, whereas only 55% of total biopsies taken 2 days after the third inoculation were culture positive. We were significantly more likely to recover bacteria from lesions biopsied 7 days after the first inoculation than after the third (89 versus 16% recovery; odds ratio, 45.33; 95% confidence interval, 4.89 to 420.48; P = 0.0008) (Fig. 2).
FIG. 2.
Percentage of all half-biopsies which were positive for recovery (A) and average recovery of CFU per half-biopsy (B). Data are from five pigs inoculated three times at 14-day intervals. Four lesions from live H. ducreyi inoculations were collected from each animal 2 and 7 days after each inoculation. CFU per half-biopsy were enumerated by mincing and plating half of each biopsy on chocolate agar. Recovery of one bacterium was sufficient to mark the lesion biopsy as positive for recovery. (A) Fraction numerators are the number of lesion biopsies positive for recovery, whereas denominators are the total number of lesion biopsies analyzed at that time point. Bars represent percentages of culture-positive lesion biopsies. (B) Average CFU per half-biopsy was calculated for each time point by dividing the total CFU recovered by the number of biopsies analyzed. This graph displays means and standard deviations.
The average number of bacteria recovered from culture-positive lesions also dropped with repeated inoculations (Fig. 2). Gamma regression modeling revealed that significantly more bacteria were recovered after the first versus the third inoculation on both day 2 (odds ratio, 77.138; 95% confidence interval, 14.86 to 400.41; P = <0.0001) and day 7 (odds ratio, 310.00; 95% confidence interval, 61.30 to 1,567.75; P = <0.0001). Decreases in both the number of culture-positive lesions and in CFU recovered per lesion suggest that after three successive inoculations, pigs developed a modest but significant level of protection against H. ducreyi infection.
H. ducreyi-specific serum IgG levels increased over the course of multiple H. ducreyi inoculations.
We measured total and H. ducreyi-specific serum IgG levels at three time points, preinoculation (day 0), 1 week after the second inoculation (day 21), and 2 weeks after the final inoculation (day 42).
H. ducreyi-specific serum IgG titers were greater in the day 42 sera of pigs inoculated three times than in the day 21 or day 0 sera of the same animals (Table 1). Day 42 sera from these pigs had 4.8-fold more H. ducreyi-specific IgG than sera collected from the same animals prior to inoculation (Table 1) (P = 0.019, t test). To determine if the elevated antibody titers in this group of pigs was due to multiple inoculations rather than to an age-dependent effect, we evaluated serum from an animal that was inoculated once on day 0. While there was an initial increase in H. ducreyi-specific IgG, this elevation did not persist over the course of the experiment (Table 1). This result indicated that the increase in H. ducreyi-specific IgG resulted from repeated exposure to H. ducreyi and not from a nonspecific change in the pigs' immune responses. There were no appreciable changes in the levels of total IgG in these sera over the course of the experiment (data not shown).
TABLE 1.
Summary of H. ducreyi-specific IgG titers from an uninoculated pig and from pigs inoculated one and three times
| Treatment (n)a | Titer−1 (SD)
|
||
|---|---|---|---|
| Day 0b | Day 21c | Day 42d | |
| Three inoculations (4/5) | 54 (3.2) | 190 (1.8)e | 260 (1.8) |
| One inoculation (1/2) | 24 | 56 | 25 |
| Uninoculated (1/1) | 120 (1.1) | 65 (1.0) | 50 (1.0) |
n, number of pigs assayed/number of pigs treated.
Day 0, preimmune serum.
Day 21 serum was collected at the time corresponding to 1 week after the second set of inoculations (See Fig. 1).
Day 42 serum was collected 2 weeks after the time corresponding to the third inoculation.
Only two pigs were assayed at day 21.
Serum from pigs inoculated multiple times displayed enhanced bactericidal activity against H. ducreyi.
We used an immune bactericidal assay to determine if the observed increases in H. ducreyi-specific antibody titers corresponded with increased bactericidal activity. We compared the bactericidal activity of preimmune sera (day 0), sera collected 2 weeks after the first inoculation (day 14), 2 weeks after the second inoculation (day 28), and 3 weeks after the third inoculation (day 49) (Fig. 1). We also tested sera collected from one uninoculated pig and two pigs that received only one inoculation.
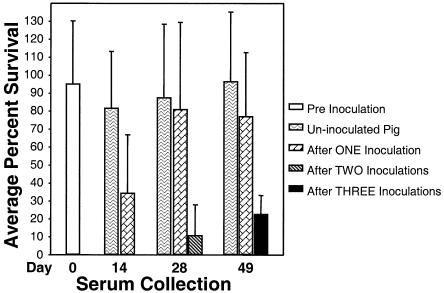
The average percent survival of H. ducreyi in preimmune sera was 95% ± 35%, indicating that none of the preimmune serum samples (n = 8) exhibited bactericidal activity.
The average percent survival of H. ducreyi in day 14 sera of animals receiving only one inoculation was 35% ± 32% (n = 7). The average percent survival of bacteria in day 28 sera from twice-inoculated animals was 11% ± 17%. In sera drawn 3 weeks after the third and final inoculation, the average percent survival of H. ducreyi was 23% ± 10% (Fig. 3). The average percent survival of H. ducreyi in sera from the uninoculated pig was approximately 100% regardless of the day the serum was drawn (Fig. 3). This result indicated that emergence of bactericidal activity was not an age-related phenomenon.
FIG. 3.
Average percent survival of H. ducreyi in immune bactericidal assays with heat-inactivated pig sera collected after three, two, one, or zero inoculations with H. ducreyi. Bars represent average percent survival of H. ducreyi in sera drawn on the day indicated after treatment described. The day 0 data bar represents survival of H. ducreyi in sera drawn from all eight animals prior to any inoculations. Day 14 data bars represent assays done with serum drawn from an uninoculated animal and sera drawn from seven pigs that had received one inoculation on day 0. Day 28 data bars represent assays done with serum drawn from one uninoculated pig, sera drawn from two pigs that received only one inoculation on day 0, and sera drawn from five pigs that had received two prior inoculations on day 0 and day 14. Day 49 data bars represent the percent survival of H. ducreyi in sera drawn on day 49 from three groups of animals: a single uninoculated pig, one pig that had been inoculated once on day 0, and five pigs that were inoculated three times on days 0, 14, and 28. Error bars illustrate standard deviations. Data were subjected to an analysis of variance.
We also wanted to determine if this enhanced bactericidal activity resulted from just the first inoculation or was dependent upon the multiple inoculation protocol. While day 14 sera of animals receiving a single inoculation displayed enhanced bactericidal activity, the average percent survival of H. ducreyi in sera drawn 4 weeks after a single inoculation was 81 ± 48%. A similar 77 ± 35% of bacteria survived in sera collected 7 weeks after a single inoculation (Fig. 3). In fact, sera drawn at the end of the study from pigs that had received a single inoculation did not exhibit a statistically significant difference in bactericidal activity compared to activity in preimmune serum. This finding suggested that while one inoculation with H. ducreyi increased bactericidal antibody titers, this enhancement was transient in the absence of repeated inoculations.
Day 49 sera from pigs inoculated three times had statistically greater bactericidal activity than all preimmune sera (P = <0.001, t test). Day 49 sera from this group of pigs also had much greater bactericidal activity than day 49 sera from animals that received one inoculation or that remained uninoculated (Fig. 3). The increased bactericidal activity of sera collected from the animals receiving three inoculations corresponded with total H. ducreyi-specific IgG titers as measured by ELISA.
The passive transfer of bactericidal serum protected naïve animals against H. ducreyi challenge.
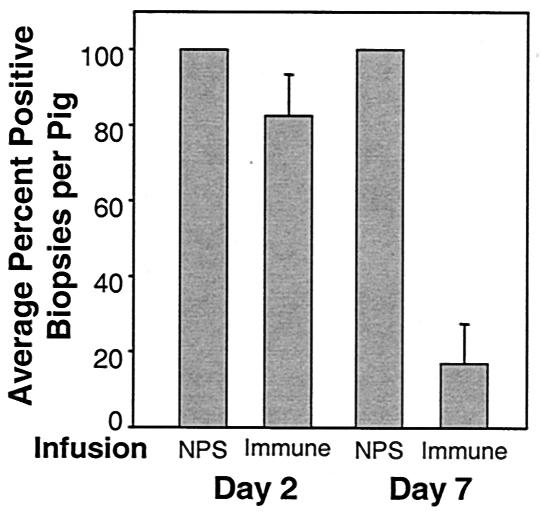
We hypothesized that elevated titers of H. ducreyi-specific antibodies, possibly through the action of complement-mediated killing, were providing the animals that received repeated inoculations with protection against H. ducreyi challenge. To test this idea, we transfused immune serum from a pig that received multiple inoculations into naïve pigs before H. ducreyi challenge. As a control, we also challenged naïve pigs infused with serum from an uninoculated pig (normal pig serum). All lesions collected from pigs infused with normal pig serum contained viable bacteria. Two days after inoculation, 82.5% of the biopsies collected from all immune serum-infused animals contained viable bacteria. Seven days after inoculation, this percentage dropped to 20%. Two of the pigs infused with immune serum yielded exclusively sterile lesions on day 7. Only 25 and 43% of day 7 lesions from the other two animals infused with immune serum contained viable bacteria (Fig. 4). The difference in the percentages of H. ducreyi-positive biopsies per pig for animals infused with the immune versus normal pig serum was statistically significant for day 7 biopsies (P = 0.006, t test).
FIG. 4.
Average percentages of H. ducreyi-positive biopsies per pig after passive transfer of immune or normal pig serum (NPS) to naïve animals. Pigs were inoculated with H. ducreyi 1 day after infusion with either NPS or immune serum. Lesion biopsies were collected either 2 or 7 days after inoculation. Entire lesion biopsies were minced and plated for recovery. Bars represent the average percentage of culture-positive biopsies per pig. Error bars represent standard errors of the means. Analysis via t test revealed that while there was not a statistically significant difference in the percentage of culture-positive biopsies per pig on day 2, there was on day 7 (P = 0.006).
DISCUSSION
After three successive experimental chancroid infections, pigs developed a modestly protective immune response against H. ducreyi. The recovery of viable organisms from lesion biopsies as well as the number of culture-positive lesions decreased throughout the experiment. This decreased recovery corresponded directly with increased serum levels of H. ducreyi-specific IgG and enhanced bactericidal activity. This finding suggested that the humoral component of the immune response was involved in mediating protection against H. ducreyi. Because naïve pigs infused with serum from a pig that received repeated inoculations were protected against H. ducreyi challenge, we concluded that the humoral component of the immune response was playing a major role in mediating protection.
The successful transfer of protection along with the transfer of immune serum distinguishes our findings from similar experiments performed with the temperature-dependent rabbit model of chancroid (40). In the temperature-dependent rabbit model, a single previous experimental infection with H. ducreyi (26), immunization with cell wall components (26), a pilus preparation (11, 12), or purified hemolysin (14) protected rabbits against future experimental challenge. However, passive transfer of whole-cell H. ducreyi-specific or pilus-specific IgG fractions did not confer protection (12). Passive transfer was not successful despite the fact that passively immunized rabbits displayed sustained, titratable antibody levels throughout the experiment (12). Perhaps this result was due to the fact that immune rabbit sera do not possess bactericidal activity against H. ducreyi (20, 28).
The protection observed in the pig model may initially appear to contradict conclusions drawn from the human challenge model of chancroid. Previous experimental infection neither prohibits nor inhibits the development of a second experimental infection in human volunteers (4, 49). However, careful comparison of recovery data from the human and swine models of chancroid revealed similarities. Human challenge studies with a single infection reported that 57 to 100% of lesion biopsies contain viable H. ducreyi (2, 3, 7, 8, 19, 39, 48, 50, 53, 54). In a human challenge reinfection study, viable H. ducreyi was recovered from 83% of biopsies from previously infected people and 67% of biopsies from individuals infected for the first time (4). These percentages are consistent with the percentages of culture-positive biopsies observed in pigs after the first and second inoculations. Two and seven days after the first inoculation, 100 and 89% of the swine biopsies were culture positive, respectively. All 20 of the biopsies collected 2 days after the second inoculation were positive, and 42% of the biopsies collected 7 days after the second inoculation were also culture positive. The significant drops in both the recovery and the number of culture-positive lesions did not occur until after the third inoculation.
It was concluded that experimental human infection to the pustular stage of disease did not protect people against a subsequent chancroid infection (4) because a naïve control group and a group of individuals that had been previously experimentally infected exhibited equivalent abilities to form both pustules and papules. However, after a single previous inoculation, pigs also developed papules and pustules in response to inoculation with H. ducreyi. Pig lesion histology scores dropped significantly only after the third inoculation (data not shown). After the third inoculation, the average histology scores for the PBS and live inoculations and the heat-killed bacteria inoculations were virtually identical. While we saw the development of protective immunity in the swine model of chancroid infection, the results from the first two rounds of pig inoculations are similar to results from the human challenge model of chancroid infection.
Pigs developed antibodies to H. ducreyi after single (29) and repeated inoculations. In contrast, initial (38, 48) and repeated (4) experimental human infection up to 14 days or the pustular stage does not evoke an antibody response to bacterial proteins or lipooligosaccharides. Unlike experimental infection, naturally occurring chancroid results in the development of a humoral response to H. ducreyi (1, 9, 10, 17, 36, 42, 47). Once chancroid develops, the likelihood of producing H. ducreyi-specific antibodies increases along with ulcer duration (10, 42). Patients with genital ulcers persisting in excess of 4 weeks have the strongest humoral response (10).
Antibodies may mediate protection through enhanced opsonization, increased bactericidal activity, blockage of attachment, or some combination of all three effects. While people with naturally occurring chancroid infection develop antibodies against H. ducreyi, these antibodies are not bactericidal (20). In contrast, antibodies produced by pigs that received three inoculations were bactericidal against H. ducreyi, and serum containing these antibodies conferred enhanced bacterial clearance when transferred to naïve animals. This outcome strongly suggested that the bactericidal ability of the H. ducreyi-specific swine antibodies provided protection against chancroid.
The development of bactericidal activity in pigs was specifically dependent on the multiple inoculation protocol as the uninoculated animal did not display enhanced bactericidal ability and animals receiving single inoculations displayed only transient increases in bactericidal activity. This greatly enhanced bactericidal activity was accomplished with only a fivefold increase in H. ducreyi-specific antibody titer. While this increase is not a large elevation in titer, protective antibody titers following natural infections are often lower than the titers seen postvaccination. For example, both previous natural infection and vaccination against hepatitis B infection are protective, but after natural seroconversion, the average geometric mean titers of anti-hepatitis B antibodies are 41-fold lower than titers at the peak of response in vaccinees (24).
We examined the longevity of the protection seen in the three-times inoculated animals by inoculating a single pig from this group a fourth time with H. ducreyi 70 days after the third inoculation. While 25% of the lesions biopsied 2 days after the fourth inoculation yielded live organisms, none of the lesions biopsied 7 days after the fourth inoculation yielded live organisms (data not shown). Reduced bacterial recovery once again corresponded with increased bactericidal ability as 66 ± 18% of H. ducreyi cells survived in serum drawn just prior to the fourth inoculation, while only 15 ± 4% of the cells survived in serum drawn 7 days after the fourth inoculation (data not shown). The rapid development of bactericidal activity suggests the existence of antigen-specific memory B cells and implies that memory B cell generation will be an important component of any successful human H. ducreyi vaccine.
We have begun to identify targets of swine antibodies produced in response to repeated H. ducreyi exposure. All pigs receiving repeated inoculations appear to develop antibodies to the same distinct set of H. ducreyi antigens (data not shown). We hope to identify both the specific antigens and their roles as bactericidal antibody targets. The comparison of proteins eliciting antibody responses could also be important in understanding how a humoral response is protective in pigs but not in people.
Acknowledgments
This work was supported by National Institutes of Health (NIH), National Institute of Allergy and Infectious Disease grant AI42824 to T.H.K., National Research Service Award 1 F31 AI09565-01 to L.R.S.M., National Science Foundation predoctoral fellowship and University of North Carolina (UNC) Chapel Hill Division of Infectious Diseases Training Grant (NIH 5-T32 AI07001) to L.E.C., and UNC Chapel Hill Graduate School Royster Fellowship to R.A.F..
We are grateful for the technical assistance of Patty Routh; her expertise with pigs enables the swine model of chancroid to continue. We also thank Drew Reinbold for his technical assistance.
Editor: T. R. Kozel
REFERENCES
- 1.Alfa, M. J., N. Olson, P. Degagne, L. Slaney, F. Plummer, W. Namaara, and A. R. Ronald. 1992. Use of an adsorption enzyme immunoassay to evaluate the Haemophilus ducreyi specific and cross-reactive humoral immune response of humans. Sex. Transm. Dis. 19:309-314. [PubMed] [Google Scholar]
- 2.Al-Tawfiq, J. A., M. E. Bauer, K. R. Fortney, B. P. Katz, A. F. Hood, M. Ketterer, M. A. Apicella, and S. M. Spinola. 2000. A pilus-deficient mutant of Haemophilus ducreyi is virulent in the human model of experimental infection. J. Infect. Dis. 181:1176-1179. [DOI] [PubMed] [Google Scholar]
- 3.Al-Tawfiq, J. A., K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2000. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J. Infect. Dis. 181:1049-1054. [DOI] [PubMed] [Google Scholar]
- 4.Al-Tawfiq, J. A., K. L. Palmer, C. Y. Chen, J. C. Haley, B. P. Katz, A. F. Hood, and S. M. Spinola. 1999. Experimental infection of human volunteers with Haemophilus ducreyi does not confer protection against subsequent challenge. J. Infect. Dis. 179:1283-1287. [DOI] [PubMed] [Google Scholar]
- 5.Bauer, M. E., M. P. Goheen, C. A. Townsend, and S. M. Spinola. 2001. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 69:2549-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackmore, C. A., K. Limpakarnjanarat, J. G. Rigau-Perez, W. L. Albritton, and J. R. Greenwood. 1985. An outbreak of chancroid in Orange County, California: descriptive epidemiology and disease-control measures. J. Infect. Dis. 151:840-844. [DOI] [PubMed] [Google Scholar]
- 7.Bong, C. T., K. R. Fortney, B. P. Katz, A. F. Hood, L. R. San Mateo, T. H. Kawula, and S. M. Spinola. 2002. A superoxide dismutase C mutant of Haemophilus ducreyi is virulent in human volunteers. Infect. Immun. 70:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bong, C. T., R. E. Throm, K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2001. DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect. Immun. 69:1488-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, T. J., R. C. Ballard, and C. A. Ison. 1995. Specificity of the immune response to Haemophilus ducreyi. Microb. Pathog. 19:31-38. [DOI] [PubMed] [Google Scholar]
- 10.Chen, C. Y., K. J. Mertz, S. M. Spinola, and S. A. Morse. 1997. Comparison of enzyme immunoassays for antibodies to Haemophilus ducreyi in a community outbreak of chancroid in the United States. J. Infect. Dis. 175:1390-1395. [DOI] [PubMed] [Google Scholar]
- 11.Desjardins, M., L. G. Filion, S. Robertson, and D. W. Cameron. 1995. Inducible immunity with a pilus preparation booster vaccination in an animal model of Haemophilus ducreyi infection and disease. Infect. Immun. 63:2012-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desjardins, M., L. G. Filion, S. Robertson, L. Kobylinski, and D. W. Cameron. 1996. Evaluation of humoral and cell-mediated inducible immunity to Haemophilus ducreyi in an animal model of chancroid. Infect. Immun. 64:1778-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickerson, M. C., J. Johnston, T. E. Delea, A. White, and E. Andrews. 1996. The causal role for genital ulcer disease as a risk factor for transmission of human immunodeficiency virus: an application of the Bradford Hill criteria. Sex. Transm. Dis. 23:429-440. [DOI] [PubMed] [Google Scholar]
- 14.Dutro, S. M., G. E. Wood, and P. A. Totten. 1999. Prevalence of, antibody response to, and immunity induced by Haemophilus ducreyi hemolysin. Infect. Immun. 67:3317-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkins, C., K. B. Barkley, N. H. Carbonetti, A. J. Coimbre, and P. F. Sparling. 1994. Immunobiology of purified recombinant outer membrane porin protein I of Neisseria gonorrhoeae. Mol. Microbiol. 14:1059-1075. [DOI] [PubMed] [Google Scholar]
- 16.Elkins, C., N. H. Carbonetti, V. A. Varela, D. Stirewalt, D. G. Klapper, and P. F. Sparling. 1992. Antibodies to N-terminal peptides of gonococcal porin are bactericidal when gonococcal lipopolysaccharide is not sialylated. Mol. Microbiol. 6:2617-2628. [DOI] [PubMed] [Google Scholar]
- 17.Elkins, C., K. Yi, B. Olsen, C. Thomas, K. Thomas, and S. Morse. 2000. Development of a serological test for Haemophilus ducreyi for seroprevalence studies. J. Clin. Microbiol. 38:1520-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming, D. T., and J. N. Wasserheit. 1999. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex. Transm. Infect. 75:3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortney, K. R., R. S. Young, M. E. Bauer, B. P. Katz, A. F. Hood, R. S. Munson, Jr., and S. M. Spinola. 2000. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect. Immun. 68:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisk, A., H. J. Ahmed, E. Van Dyck, and T. Lagergard. 1998. Antibodies specific to surface antigens are not effective in complement-mediated killing of Haemophilus ducreyi. Microb. Pathog. 25:67-75. [DOI] [PubMed] [Google Scholar]
- 21.Gadkari, D. A., T. C. Quinn, R. R. Gangakhedkar, S. M. Mehendale, A. D. Divekar, A. R. Risbud, K. Chan-Tack, M. Shepherd, C. Gaydos, and R. C. Bollinger. 1998. HIV-1 DNA shedding in genital ulcers and its associated risk factors in Pune, India. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 18:277-281. [DOI] [PubMed] [Google Scholar]
- 22.Gelfanova, V., T. L. Humphreys, and S. M. Spinola. 2001. Characterization of Haemophilus ducreyi-specific T-cell lines from lesions of experimentally infected human subjects. Infect. Immun. 69:4224-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray, R. H., M. J. Wawer, N. K. Sewankambo, D. Serwadda, C. Li, L. H. Moulton, T. Lutalo, F. Wabwire-Mangen, M. P. Meehan, S. Ahmed, L. A. Paxton, N. Kiwanuka, F. Nalugoda, E. L. Korenromp, and T. C. Quinn. 1999. Relative risks and population attributable fraction of incident HIV associated with symptoms of sexually transmitted diseases and treatable symptomatic sexually transmitted diseases in Rakai District, Uganda. AIDS 13:2113-2123. [DOI] [PubMed] [Google Scholar]
- 24.Gregorek, H., K. Madalinski, M. Woynarowski, J. Mikolajewicz, M. Syczewska, and J. Socha. 2000. The IgG subclass profile of anti-HBs response in vaccinated children and children seroconverted after natural infection. Vaccine 18:1210-1217. [DOI] [PubMed] [Google Scholar]
- 25.Hammond, G. W., M. Slutchuk, J. Scatliff, E. Sherman, J. C. Wilt, and A. R. Ronald. 1980. Epidemiologic, clinical, laboratory, and therapeutic features of an urban outbreak of chancroid in North America. Rev. Infect. Dis. 2:867-879. [DOI] [PubMed] [Google Scholar]
- 26.Hansen, E. J., S. R. Lumbley, J. A. Richardson, B. K. Purcell, M. K. Stevens, L. D. Cope, J. Datte, and J. D. Radolf. 1994. Induction of protective immunity to Haemophilus ducreyi in the temperature-dependent rabbit model of experimental chancroid. J. Immunol. 152:184-192. [PubMed] [Google Scholar]
- 27.Hayes, R. J., K. F. Schulz, and F. A. Plummer. 1995. The cofactor effect of genital ulcers on the per-exposure risk of HIV transmission in sub-Saharan Africa. J. Trop. Med. Hyg. 98:1-8. [PubMed] [Google Scholar]
- 28.Hiltke, T. J., M. E. Bauer, J. Klesney-Tait, E. J. Hansen, R. S. Munson, Jr., and S. M. Spinola. 1999. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb. Pathog. 26:93-102. [DOI] [PubMed] [Google Scholar]
- 29.Hobbs, M. M., L. R. San Mateo, P. E. Orndorff, G. Almond, and T. H. Kawula. 1995. Swine model of Haemophilus ducreyi infection. Infect. Immun. 63:3094-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janeway, C. 2001. Immunobiology: the immune system in health and disease, 5th ed. Garland Publishing, New York, N.Y.
- 31.Jessamine, P. G., and A. R. Ronald. 1990. Chancroid and the role of genital ulcer disease in the spread of human retroviruses. Med. Clin. North Am. 74:1417-1431. [DOI] [PubMed] [Google Scholar]
- 32.King, R., J. Gough, A. Ronald, J. Nasio, J. O. Ndinya-Achola, F. Plummer, and J. A. Wilkins. 1996. An immunohistochemical analysis of naturally occurring chancroid. J. Infect. Dis. 174:427-430. [DOI] [PubMed] [Google Scholar]
- 33.Langley, C. 1996. Update on chancroid: an important cause of genital ulcer disease. AIDS Patient Care STDS 10:221-226. [DOI] [PubMed] [Google Scholar]
- 34.Lewis, D. A. 2000. Chancroid: from clinical practice to basic science. AIDS Patient Care STDS 14:19-36. [DOI] [PubMed] [Google Scholar]
- 35.Mroczkowski, T. F., and D. H. Martin. 1994. Genital ulcer disease. Dermatol. Clin. 12:753-764. [PubMed] [Google Scholar]
- 36.Museyi, K., E. Van Dyck, T. Vervoort, D. Taylor, C. Hoge, and P. Piot. 1988. Use of an enzyme immunoassay to detect serum IgG antibodies to Haemophilus ducreyi. J. Infect. Dis. 157:1039-1043. [DOI] [PubMed] [Google Scholar]
- 37.O'Farrell, N. 1996. Genital ulcers associated with human immunodeficiency virus-related immunosuppression. J. Infect. Dis. 174:445-447. [DOI] [PubMed] [Google Scholar]
- 38.Palmer, K. L., C. T. Schnizlein-Bick, A. Orazi, K. John, C. Y. Chen, A. F. Hood, and S. M. Spinola. 1998. The immune response to Haemophilus ducreyi resembles a delayed-type hypersensitivity reaction throughout experimental infection of human subjects. J. Infect. Dis. 178:1688-1697. [DOI] [PubMed] [Google Scholar]
- 39.Palmer, K. L., A. C. Thornton, K. R. Fortney, A. F. Hood, R. S. Munson, Jr., and S. M. Spinola. 1998. Evaluation of an isogenic hemolysin-deficient mutant in the human model of Haemophilus ducreyi infection. J. Infect. Dis. 178:191-199. [DOI] [PubMed] [Google Scholar]
- 40.Purcell, B. K., J. A. Richardson, J. D. Radolf, and E. J. Hansen. 1991. A temperature-dependent rabbit model for production of dermal lesions by Haemophilus ducreyi. J. Infect. Dis. 164:359-367. [DOI] [PubMed] [Google Scholar]
- 41.Robinson, N. J., D. W. Mulder, B. Auvert, and R. J. Hayes. 1997. Proportion of HIV infections attributable to other sexually transmitted diseases in a rural Ugandan population: simulation model estimates. Int. J. Epidemiol. 26:180-189. [DOI] [PubMed] [Google Scholar]
- 42.Roggen, E. L., G. Hoofd, E. Van Dyck, and P. Piot. 1994. Enzyme immunoassays (EIAs) for the detection of anti-Haemophilus ducreyi serum IgA, IgG, and IgM antibodies. Sex. Transm. Dis. 21:36-42. [DOI] [PubMed] [Google Scholar]
- 43.Ronald, A. R. a. W. A. 1990. Chancroid and Haemophilus ducreyi, p. 263-271. In P.-A. M. K. K. Holmes, P. F. Sparling, and P. J. Wiesner (ed.), Sexually transmitted diseases, 2nd ed. McGraw-Hill, New York, N.Y.
- 44.Rothenberg, R. B., J. N. Wasserheit, M. E. St Louis, J. M. Douglas, et al. 2000. The effect of treating sexually transmitted diseases on the transmission of HIV in dually infected persons: a clinic-based estimate. Sex. Transm. Dis. 27:411-416. [DOI] [PubMed] [Google Scholar]
- 45.San Mateo, L. R., K. L. Toffer, P. E. Orndorff, and T. H. Kawula. 1999. Immune cells are required for cutaneous ulceration in a swine model of chancroid. Infect. Immun. 67:4963-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.San Mateo, L. R., K. L. Toffer, P. E. Orndorff, and T. H. Kawula. 1999. Neutropenia restores virulence to an attenuated Cu,Zn superoxide dismutase-deficient Haemophilus ducreyi strain in the swine model of chancroid. Infect. Immun. 67:5345-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schalla, W. O., L. L. Sanders, G. P. Schmid, M. R. Tam, and S. A. Morse. 1986. Use of dot-immunobinding and immunofluorescence assays to investigate clinically suspected cases of chancroid. J. Infect. Dis. 153:879-887. [DOI] [PubMed] [Google Scholar]
- 48.Spinola, S. M., A. Orazi, J. N. Arno, K. Fortney, P. Kotylo, C. Y. Chen, A. A. Campagnari, and A. F. Hood. 1996. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J. Infect. Dis. 173:394-402. [DOI] [PubMed] [Google Scholar]
- 49.Spinola, S. M., L. M. Wild, M. A. Apicella, A. A. Gaspari, and A. A. Campagnari. 1994. Experimental human infection with Haemophilus ducreyi. J. Infect. Dis. 169:1146-1150. [DOI] [PubMed] [Google Scholar]
- 50.Throm, R. E., J. A. Al-Tawfiq, K. R. Fortney, B. P. Katz, A. F. Hood, C. A. Slaughter, E. J. Hansen, and S. M. Spinola. 2000. Evaluation of an isogenic major outer membrane protein-deficient mutant in the human model of Haemophilus ducreyi infection. Infect. Immun. 68:2602-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torian, L. V., I. B. Weisfuse, H. A. Makki, D. A. Benson, L. M. DiCamillo, and F. E. Toribio. 1995. Increasing HIV-1 seroprevalence associated with genital ulcer disease, New York City, 1990-1992. AIDS 9:177-181. [PubMed] [Google Scholar]
- 52.Wasserheit, J. N. 1992. Epidemiological synergy: interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex. Transm. Dis. 19:61-77. [PubMed] [Google Scholar]
- 53.Young, R. S., K. Fortney, J. C. Haley, A. F. Hood, A. A. Campagnari, J. Wang, J. A. Bozue, R. S. Munson, Jr., and S. M. Spinola. 1999. Expression of sialylated or paragloboside-like lipooligosaccharides are not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect. Immun. 67:6335-6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young, R. S., K. R. Fortney, V. Gelfanova, C. L. Phillips, B. P. Katz, A. F. Hood, J. L. Latimer, R. S. Munson, Jr., E. J. Hansen, and S. M. Spinola. 2001. Expression of cytolethal distending toxin and hemolysin is not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect. Immun. 69:1938-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]