Abstract
Specific antibody opsonization significantly enhances the level of phagocytosis of Candida in the absence of complement. Furthermore, we have described a system using a recombinant human antibody single-chain variable fragment that allows a comparative study of phagocytosis of multiple Candida species opsonized via a common antigen.
Candidiasis, the most common systemic fungal infection, is increasing in frequency (18). Antibody can play an important role in the host response to Candida (8, 9); however, polyclonal antibody responses may include antibodies detrimental to the host (3). While phagocytes are considered the primary anti-Candida effector cells (13, 27), previous studies on the effects of antibody opsonization on phagocytosis were performed in the presence of complement (4, 10, 15, 19). Specifically targeting organisms to phagocyte Fc receptors (FcR), however, has been shown to benefit the host by augmenting phagocytosis, organism killing, and stimulation of the adaptive immune system (1, 5, 6, 26), whereas phagocytosis via complement has been associated with suppressed immune responses (6). Furthermore, although infections caused by Candida species other than Candida albicans are now in the majority (16, 20, 21, 28), little is known about the effects of antibody on the host response to non-albicans Candida.
We previously described a human single-chain variable fragment (scFv), scFvλ2-18, that recognizes both C. albicans and non-albicans species (2, 7). Herein, we investigated the ability of scFvλ2-18 to target FcR and mediate phagocytosis. scFvλ2-18, which recognizes cell surface mannans (data not shown) on blastoconidia and filamentous forms of Candida, and a control scFv, scFv5 (2), which recognizes a protein antigen expressed only on filamentous Candida, were prepared using the method of Kipriyanov et al. (11). Because scFv does not contain an Fc region, but does contain the FLAG epitope tag (14), organisms were opsonized with a three-layer complex consisting of 10 μg of scFvλ2-18 per ml, 10 μg of M2 anti-FLAG mouse monoclonal antibody per ml (Sigma-Aldrich, St. Louis, Mo.), and a 1:250 dilution of an immunoglobulin G (IgG) fraction of rabbit anti-mouse polyclonal antiserum (Jackson ImmunoResearch, West Grove, Pa.). For comparison, organisms were opsonized with a 1:250 dilution of an IgG fraction of rabbit anti-C. albicans polyclonal antiserum (pIgG; Accurate Chemical, Inc., Westbury, N.Y.).
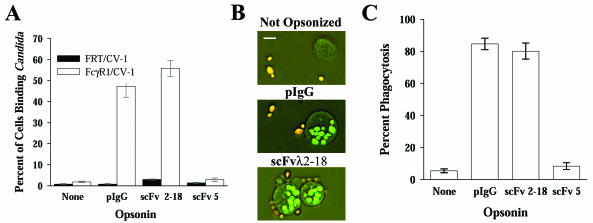
To demonstrate that the scFvλ2-18 complex could target Candida to FcR, opsonized blastoconidia (laboratory strain 3153A) were incubated for 30 min at 37°C with either an FcγR1-expressing epithelial cell line, FcγR1/CV-1, or its parental line, FRT/CV-1 (Gibco Invitrogen, Carlsbad, Calif.), at a blastoconidia/host cell ratio of 10:1. FcγR1/CV-1 cells bound live Candida blastoconidia opsonized with either pIgG or scFvλ2-18, but not unopsonized or scFv5-opsonized organisms (Fig. 1A). Because there was no difference in binding levels of unopsonized and scFv5-opsonized organisms, potential contaminants in the scFv preparations did not affect binding. No differences in binding were observed with live and heat-killed blastoconidia (data not shown), suggesting that C. albicans does not actively evade opsonization or degrade the opsonizing complex.
FIG. 1.
scFvλ2-18 targets C. albicans to FcR and mediates phagocytosis. (A) FcγR1-expressing epithelial cells (FcγR1/CV-1) or the parental cells (FRT/CV-1) were incubated with C. albicans strain 3153A blastoconidia opsonized as indicated. Cells that bound three or more blastoconidia were scored as positive (rosette formation). The mean percentage of cells forming rosettes ± standard error is plotted. scFv5, which does not recognize blastoconidia, was included as a mock opsonin. Anti-C. albicans pIgG- or scFvλ2-18-opsonized organisms were bound at a significantly higher level (P < 0.001 for all comparisons) than unopsonized or mock-opsonized organisms. There was no significant difference between the levels of binding of unopsonized or mock-opsonized organisms (P = 0.212). (B) Photomicrographs of THP-1 cells incubated with C. albicans strain 3153A blastoconidia, opsonized as indicated, demonstrating extracellular (orange) and intracellular (green) blastoconidia. The white bar represents 10 μm. (C) Mean percentage of THP-1 cells completing phagocytosis ± standard error of C. albicans strain 3153A blastoconidia, opsonized as indicated. The levels of phagocytosis of pIgG- or scFvλ2-18-opsonized organisms were not significantly different (P = 0.271); however, the levels of phagocytosis of either scFvλ2-18-or pIgG-opsonized organisms, compared to those of unopsonized or mock-opsonized organisms (P < 0.001 for all comparisons), were significantly different.
For phagocytosis assays, cells of a human monocytic cell line, THP-1 (American Type Culture Collection, Manassas, Va.) (23-25) were incubated with Candida as described above, except that the sample was incubated for 30 min at 4°C prior to the 37°C incubation to develop a synchronized pool of host cells with membrane-bound organisms. Surface-bound organisms were distinguished by labeling blastoconidia with fluorescein isothiocyanate (FITC) prior to opsonization and subsequently adding ethidium bromide (EtBr) to the sample prior to microscopic analysis. EtBr, which is excluded from live host cells and, therefore, internalized organisms, stains external organisms, causing them to fluoresce orange (Fig. 1B). Heat-killed organisms were used because live organisms concentrate FITC in their vacuole, which is subsequently protected from EtBr staining. No difference in the level of phagocytosis of live versus heat-killed organisms was observed in pilot studies (data not shown).
The efficiency of phagocytosis was significantly enhanced by antibody opsonization (Fig. 1C). The scFvλ2-18 complex mediated phagocytosis equivalently to mature antibody. The low level of phagocytosis seen with unopsonized or mock-opsonized organisms suggests that neither alternative receptors, such as mannose receptors, nor contaminants from the scFv preparation enhance phagocytosis under these conditions. This is consistent with a recent study in which mannose receptor-deficient mice were not found to have increased mortality from Candida infections (12).
Phagocytosis assays were conducted with seven C. albicans isolates and four non-albicans species (Table 1). Except for one clinical strain of C. albicans (MRO4-O), there was significant enhancement of phagocytosis when organisms were opsonized. Although differences in the level of phagocytosis of various organisms were observed, these differences were relatively small compared to the large increase in the level of phagocytosis associated with opsonization.
TABLE 1.
scFv λ2-18 mediates phagocytosis of various C. albicans strains and multiple Candida species by THP-1 cells
| Organism | Strain | Sourcea | Opsonin | % Phagocytosisb
|
n | |
|---|---|---|---|---|---|---|
| Not opsonizedc | Opsonizedd | |||||
| C. albicans | 3153A | L | pIgG | 4 ± 4 | 81 ± 12 | 12 |
| scFvλ2-18 | 14 ± 9 | 83 ± 10 | 12 | |||
| MRO4-O | C | pIgG | 2 ± 1 | 69 ± 2 | 3 | |
| scFvλ2-18 | 14 ± 12 | 20 ± 7 | 12 | |||
| 613p | L | scFvλ2-18 | 10 ± 2 | 61 ± 7 | 6 | |
| MRO2-O | C | scFvλ2-18 | 10 ± 6 | 73 ± 9 | 6 | |
| MRO9-R | C | scFvλ2-18 | 35 ± 8 | 80 ± 5 | 6 | |
| MRO17-O | C | scFvλ2-18 | 35 ± 10 | 71 ± 6 | 6 | |
| CAH 7-1A | M | scFvλ2-18 | 19 ± 4 | 59 ± 11 | 6 | |
| C. dubliniensis | CBS8500 | R | scFvλ2-18 | 9 ± 11 | 81 ± 7 | 6 |
| C. tropicalis | MRO84-O | C | scFvλ2-18 | 15 ± 7 | 95 ± 3 | 6 |
| C. glabrata | MRO84-R | C | scFvλ2-18 | 14 ± 5 | 96 ± 2 | 6 |
| C. krusei | ATCC 6258 | R | scFvλ2-18 | 4 ± 1 | 68 ± 6 | 6 |
Source of strains used: L, laboratory; C, clinical; R, reference; M, mutant (CAH 7-1A is hwp−/hwp−). All strains were obtained as previously described (2), except ATCC 6248, which was provided by the Clinical Microbiology Laboratory at Strong Memorial Hospital, Rochester, N.Y.
Percentages of cells with ingested organisms. Values are means ± standard deviation.
For scFvλ2-18, secondary and tertiary antibody ligand only; for IgG, no opsonin.
For all organisms except MRO4-O (scFvλ2-18), comparison of opsonized to not opsonized was statistically significant (P < 0.001). For MRO4-O, P = 0.185.
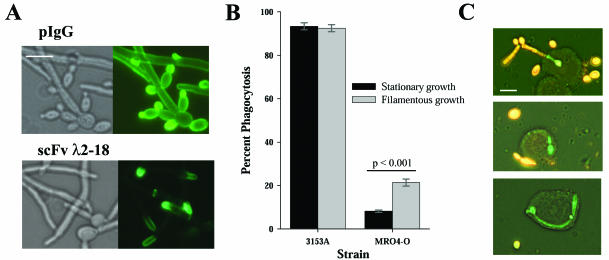
Because strain MRO4-O was efficiently ingested when opsonized with pIgG, but not scFvλ2-18 (Table 1), we performed an immunofluorescence assay to investigate scFvλ2-18 cognate antigen expression in this strain (Fig. 2A). Both pIgG and scFvλ2-18 recognize Candida blastoconidia and filaments of strain 3153A with a diffuse, bright staining pattern (2, 7). In strain MRO4-O, this pattern was observed with pIgG but not scFvλ2-18, which recognized only the point of elongation (Fig. 2A). Because stationary-growth-phase blastoconidia were used in the phagocytosis assays, strain MRO4-O blastoconidia would not have been effectively opsonized. When the phagocytosis assay was repeated by using blastoconidia that had been cultured for 1 h in medium M199 (Gibco), which allows development of nascent filaments, a significantly higher level of phagocytosis was observed (Fig. 2B and C). Furthermore, host cells that partially ingested filaments, causing exclusion of EtBr (Fig. 2C, top), or that ingested relatively large filaments (Fig. 2C, bottom) were observed.
FIG. 2.
scFvλ2-18 recognizes C. albicans strain MR04-O at the point of elongation and mediates phagocytosis of organisms with nascent germ tube growth. (A) Immunofluorescence assays of C. albicans strain MRO4-O performed with pIgG (detected by BODIPY FL-conjugated goat anti-rabbit antibody [Molecular Probes, Eugene, Oreg.]) or scFvλ2-18 (detected by M2 anti-FLAG antibody and BODIPY FL-conjugated goat anti-mouse antibody [Molecular Probes]) demonstrate that scFvλ2-18 recognizes only the area at the point of elongation. (B) Phagocytosis assays using scFvλ2-18-opsonized blastoconidia (stationary growth) or scFvλ2-18-opsonized nascent germ tubes were performed with C. albicans strains 3153A and MRO4-O. The mean percentage of phagocytosis ± standard error is plotted in panel B. (C) Fluorescence photomicrographs of THP-1 cells that had ingested organisms from the nascent growth sample of strain MRO4-O. The white bars in panels A and C represent 10 μm.
To verify that antibody opsonization could enhance phagocytosis in native human phagocytes, human monocytes and neutrophils that were obtained through an institutionally approved protocol were assayed as described above. Blood was drawn from healthy adult volunteers; monocytes were isolated with the RosetteSep monocyte enrichment system (StemCell Technologies, Vancouver, British Columbia, Canada), and neutrophils were isolated with the 1-Step Polymorphs system (Accurate Chemical, Inc.). Monocyte preparations contained 98% monocytes, and neutrophil preparations contained >99% neutrophils with no cross-contamination of cell types.
Phagocytosis by ex vivo monocytes obtained from two different donors (Fig. 3A) occurred in 40 to 50% of cells. The level of phagocytosis was significantly enhanced by opsonization in monocytes from donor A; however, cells from donor B demonstrated a high level of phagocytosis of both opsonized and unopsonized organisms. This donor-related discrepancy could be due to the monocyte activation state: Marodi et al. demonstrated that activated monocyte-derived macrophages did not require opsonization for phagocytosis, whereas unactivated monocytes did (15).
FIG. 3.
Ex vivo human phagocytes ingest C. albicans blastoconidia opsonized with scFv λ2-18. (A) Peripheral blood monocytes were obtained from two different donors and assayed for phagocytosis with blastoconidia opsonized with scFv λ2-18 or scFv 5, as indicated. THP-1 cells were assayed in parallel for comparison. The mean percentage of phagocytosis ± standard error is plotted. There was a significant increase in the level of phagocytosis of opsonized versus mock-opsonized blastoconidia for donor A (P < 0.001) but not for donor B (P = 0.127). (B) Neutrophils (PMN) were obtained from a single donor (donor B) on 2 different days (indicated as PMN-A and PMN-B) and assayed for phagocytic activity. For both samples, there was a significant increase in the level of phagocytosis of opsonized versus mock-opsonized organisms (P < 0.001).
The ex vivo neutrophils, which were obtained in independent experiments from the same donor, demonstrated a significant increase in the level of phagocytosis of opsonized organisms (Fig. 3B). The ex vivo phagocytes did not undergo phagocytosis at the level of the THP-1 cells, although the observed level of 25 to 50% is similar to those in other investigations with human phagocytes (17, 22). The ex vivo phagocytes are likely to be heterogeneous in FcR expression and activation state, which may have contributed to the lower level of phagocytosis compared with THP-1 cells.
The majority of studies investigating the phagocytosis of Candida have used fresh serum as an opsonizing agent (10, 15, 17, 19, 22). In their study on the protective monoclonal antibody B6.1, Caesar-TonThat and Cutler found that fresh serum was necessary for optimal organism killing (4). Herein, we demonstrated that specific antibody opsonization dramatically increases the level of phagocytosis in the absence of complement. Furthermore, scFvλ2-18 functions as a Candida binding ligand equivalent to specific pIgG. Thus, scFvλ2-18 is an important tool for studying the phagocyte response to Candida because it functions as an opsonin for many Candida species. Native antibody responses, which are complex and may not confer protection (3), may not adequately enhance phagocytosis. Because the antibody fragments tested in these studies are of human origin, they may provide the basis for future antibody prophylaxis or therapy for Candida infections.
Acknowledgments
We thank Jacob J. Schlesinger and Clark L. Anderson for for the FcγR1/CV-1 cell line and Lani A. Sherrill for technical expertise.
This work was supported by the Wilmot Cancer Research Fellowship (M.W.), University of Rochester Medical Center, Rochester, N.Y., and by National Institutes of Health grant T32 AI07464 (M.W. and J.B.).
The studies presented in this article will be submitted as a partial fulfillment of the requirements for the Doctor of Philosophy degree in Microbiology and Immunology for M.W.
Editor: T. R. Kozel
REFERENCES
- 1.Bazzoni, F., M. A. Cassatella, C. Laudanna, and F. Rossi. 1991. Phagocytosis of opsonized yeast induces tumor necrosis factor-alpha mRNA accumulation and protein release by human polymorphonuclear leukocytes. J. Leukoc. Biol. 50:223-228. [DOI] [PubMed] [Google Scholar]
- 2.Bliss, J. M., M. A. Sullivan, J. Malone, and C. G. Haidaris. 2003. Differentiation of Candida albicans and Candida dubliniensis by using recombinant human antibody single-chain variable fragments specific for hyphae. J. Clin. Microbiol. 41:1152-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bromuro, C., A. Torosantucci, P. Chiani, S. Conti, L. Polonelli, and A. Cassone. 2002. Interplay between protective and inhibitory antibodies dictates the outcome of experimentally disseminated candidiasis in recipients of a Candida albicans vaccine. Infect. Immun. 70:5462-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caesar-TonThat, T.-C., and J. E. Cutler. 1997. A monoclonal antibody to Candida albicans enhances mouse neutrophil candidacidal activity. Infect. Immun. 65:5354-5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caron, E., and A. Hall. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717-1721. [DOI] [PubMed] [Google Scholar]
- 6.Ehlers, M. R. 2000. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2:289-294. [DOI] [PubMed] [Google Scholar]
- 7.Haidaris, C. G., J. Malone, L. A. Sherrill, J. M. Bliss, A. A. Gaspari, R. A. Insel, and M. A. Sullivan. 2001. Recombinant human antibody single chain variable fragments reactive with Candida albicans surface antigens. J. Immunol. Methods 257:185-202. [DOI] [PubMed] [Google Scholar]
- 8.Han, Y., and J. E. Cutler. 1995. Antibody response that protects against disseminated candidiasis. Infect. Immun. 63:2714-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han, Y., and J. E. Cutler. 1997. Assessment of a mouse model of neutropenia and the effect of an anti-candidiasis monoclonal antibody in these animals. J. Infect. Dis. 175:1169-1175. [DOI] [PubMed] [Google Scholar]
- 10.Kagaya, K., and Y. Fukazawa. 1981. Murine defense mechanism against Candida albicans infection. II. Opsonization, phagocytosis, and intracellular killing of C. albicans. Microbiol. Immunol. 25:807-818. [DOI] [PubMed] [Google Scholar]
- 11.Kipriyanov, S. M., G. Moldenhauer, and M. Little. 1997. High level production of soluble single chain antibodies in small-scale Escherichia coli cultures. J. Immunol. Methods 200:69-77. [DOI] [PubMed] [Google Scholar]
- 12.Lee, S. J., N.-Y. Zheng, M. Clavijo, and M. C. Nussenzweig. 2003. Normal host defense during systemic candidiasis in mannose receptor-deficient mice. Infect. Immun. 71:437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitz, S. M. 1992. Overview of host defenses in fungal infections. Clin. Infect. Dis. 14(Suppl. 1):S37-S42. [DOI] [PubMed] [Google Scholar]
- 14.Malone, J., and M. A. Sullivan. 1996. Analysis of antibody selection by phage display utilizing anti-phenobarbital antibodies. J. Mol. Recognit. 9:738-745. [DOI] [PubMed] [Google Scholar]
- 15.Marodi, L., H. M. Korchak, and R. B. Johnston, Jr. 1991. Mechanisms of host defense against Candida species. I. Phagocytosis by monocytes and monocyte-derived macrophages. J. Immunol. 146:2783-2789. [PubMed] [Google Scholar]
- 16.Marr, K. A. 2000. The changing spectrum of candidemia in oncology patients: therapeutic implications. Curr. Opin. Infect. Dis. 13:615-620. [DOI] [PubMed] [Google Scholar]
- 17.Newman, S. L., and A. Holly. 2001. Candida albicans is phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect. Immun. 69:6813-6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odds, F. C. 1988. Candida and candidosis, 2nd ed. Baillière Tindall, London, United Kingdom.
- 19.Pereira, H. A., and C. S. Hosking. 1984. The role of complement and antibody in opsonization and intracellular killing of Candida albicans. Clin. Exp. Immunol. 57:307-314. [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller, M. A., R. N. Jones, G. V. Doern, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, R. J. Hollis, and The SENTRY Participant Group. 2000. Bloodstream infections due to Candida species: SENTRY Antimicrobial Surveillance Program in North America and Latin America, 1997-1998. Antimicrob. Agents Chemother. 44:747-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaller, M. A., R. N. Jones, S. A. Messer, M. B. Edmond, and R. P. Wenzel. 1998. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. SCOPE Participant Group. Surveillance and Control of Pathogens of Epidemiologic. Diagn. Microbiol. Infect. Dis. 30:121-129. [DOI] [PubMed] [Google Scholar]
- 22.Richardson, M. D., and F. Donaldson. 1994. Interaction of Candida krusei with human neutrophils in vitro. J. Med. Microbiol. 41:384-388. [DOI] [PubMed] [Google Scholar]
- 23.Stokes, R. W., and D. Doxsee. 1999. The receptor-mediated uptake, survival, replication, and drug sensitivity of Mycobacterium tuberculosis within the macrophage-like cell line THP-1: a comparison with human monocyte-derived macrophages. Cell Immunol. 197:1-9. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki, T., N. Ohno, Y. Ohshima, and T. Yadomae. 1998. Soluble mannan and beta-glucan inhibit the uptake of Malassezia furfur by human monocytic cell line, THP-1. FEMS Immunol. Med. Microbiol. 21:223-230. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26:171-176. [DOI] [PubMed] [Google Scholar]
- 26.van Spriel, A. B., I. E. van den Herik-Oudijk, N. M. van Sorge, H. A. Vile, J. A. van Strijp, and J. G. van de Winkel. 1999. Effective phagocytosis and killing of Candida albicans via targeting FcgammaRI (CD64) or FcalphaRI (CD89) on neutrophils. J. Infect. Dis. 179:661-669. [DOI] [PubMed] [Google Scholar]
- 27.Vázquez-Torres, A., and E. Balish. 1997. Macrophages in resistance to candidiasis. Microbiol. Mol. Biol. Rev. 61:170-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh, T. J., C. Gonzalez, E. Roilides, B. U. Mueller, N. Ali, L. L. Lewis, T. O. Whitcomb, D. J. Marshall, and P. A. Pizzo. 1995. Fungemia in children infected with the human immunodeficiency virus: new epidemiologic patterns, emerging pathogens, and improved outcome with antifungal therapy. Clin. Infect. Dis. 20:900-906. [DOI] [PubMed] [Google Scholar]